REM sleep behavior disorder (RBD) is characterized by dream enactment behaviors (DEB) and REM sleep without atonia (RSWA). A key structure in REM sleep muscle tone regulation in the rodent model is the sublateral dorsal (SLD) tegmental nucleus in the dorsomedial pons.1 Lesions of an analogous structure, the subceruleus (SC) nucleus are thought to mediate RSWA in humans, enabling DEB.1,2 However, in vivo human studies of discrete dorsomedial pontine lesions causing RBD in isolation have been limited.2–4 We report such a case of lesional RBD due to vasculitis. The Mayo Clinic Institutional Review Board approved this study.
Case report.
A 47-year-old man presented with initial neurologic symptoms and signs of multiple peripheral mononeuropathies 3 years prior to evolution of violent DEB. Sural nerve biopsy demonstrated microvasculitis, and progression was ameliorated by oral prednisone. Two months before DEB onset, he developed painless vertical diplopia. Examination showed left fourth cranial nerve palsy, and brain MRI with contrast was unremarkable. The patient began citalopram for anxiety. One month later, he developed dysarthria, dysphagia, and right facial weakness, with right III and IV and bilateral VI, VII, X, and XII cranial neuropathies, and required hospitalization for dysphagia. Brain MRI (figure) revealed right dorsomedial pontine T2 and fluid-attenuated inversion recovery signal hyperintensity. CSF and paraneoplastic antibody testing had normal results. Cyclophosphamide improved his symptoms.
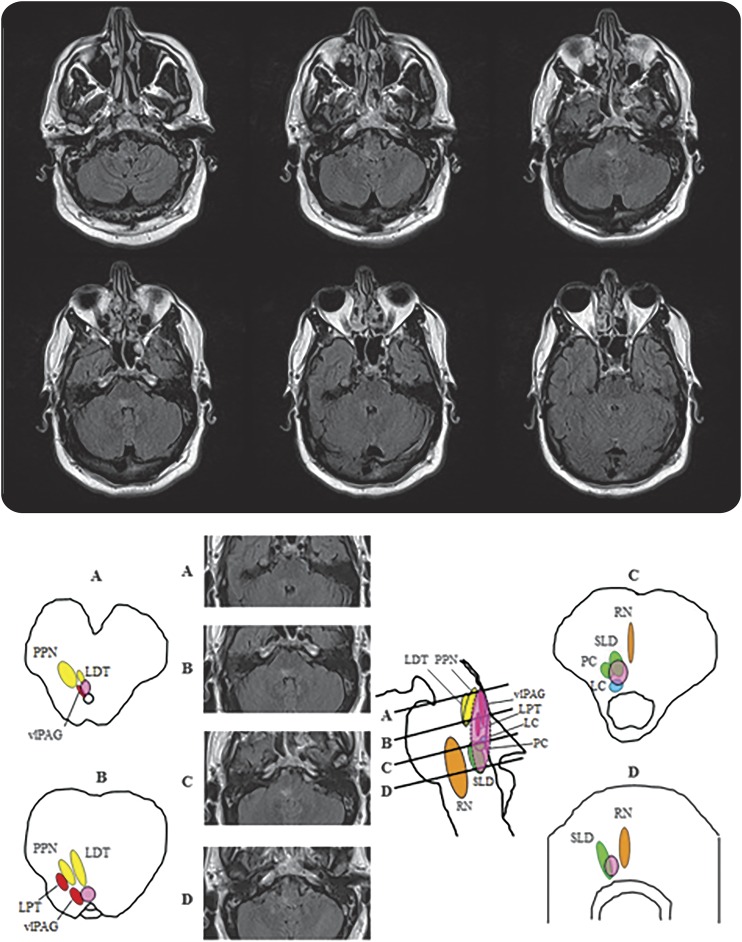
Figure. Lesional REM sleep behavior disorder in the dorsal pontomedullary junction resulting from CNS vasculitis.
(A) Coronal fluid-attenuated inversion recovery (FLAIR) intensity MRI sections at the level of the medulla and pons show a discrete longitudinally extensive hyperintense lesion at the level of the dorsomedial pons extending rostrally to the right superior pons ventral to the superior cerebellar peduncle. (B) The brainstem nuclei thought to be involved in REM sleep atonia regulation are shown on human brainstem templates. Letters for each template and corresponding MRI FLAIR sections selected from our case represent cross-sectional views through the brainstem as shown in the midsagittal figure, with sections representing (A) the pontomesencephalic junction, (B) the upper/mid pons, (C) the lower/mid pons, and (D) the pontomedullary junction. The approximate location of the lesion is shown in the superimposed pink oval. LC = locus ceruleus; LDT = laterodorsal tegmental nucleus; LPT = lateral pontine tegmentum; PC = precoeruleus; PPN = pedunculopontine nucleus; RN = raphe nucleus; SLD = sublateral dorsal nucleus; vlPAG = ventrolateral part of the periaqueductal gray matter. Modified from Boeve BF, Silber MH, Saper C, et al. Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease. Brain 2007;130:2770–2788. Reprinted with permission from Oxford University Press.
Within a few weeks following hospital discharge, the patient's wife reported that he began having nightly violent arm and leg flailing, corresponding to violent dreams of attack by fire ants or having fallen through ice into a freezing lake. He inadvertently punched his wife and fell out of bed several times. Symptoms of sleepwalking, daytime sleepiness, hypnogogic hallucinations, sleep paralysis, and cataplexy were absent. Video polysomnography captured DEB with repeated punching of the bedrail (video on the Neurology® Web site at www.neurology.org), and quantitative RSWA analysis by established methods5 demonstrated overall phasic muscle activity percentage of 90.9% and tonic percentage of 38.6% (male controls aged 49 years, phasic 22.5%, tonic 0%). Moderate obstructive sleep apnea (OSA) (apnea-hypopnea index = 21 per hour) was identified, and the patient was adherent to positive airway pressure therapy during follow-up, demonstrating 72% 4-hour or greater use days for an average of 6 hours 38 minutes nightly. Thirty-eight months later, DEB persisted twice weekly despite treatment with melatonin 12 mg nightly and discontinuation of citalopram. Treatment for anxiety with buspirone 15 mg TID also improved DEB frequency.
Discussion.
This case provides evidence for a discrete dorsomedial pontine lesion causing RSWA and RBD. In distinction to several previous reports of lesional RBD, our patient had isolated RBD and RSWA without overlap parasomnia disorder, narcolepsy, or cataplexy. RBD pathophysiology has been elucidated from studies in the cat, rat, and human pathologic and imaging studies.1–4,6 Pontomedullary structures including the magnocellular reticular formation (MCRF) and the SLD, locus ceruleus (LC)/SC, laterodorsal tegmental nuclei, and pedunculopontine nuclei are responsible for REM sleep atonia control.1,2 Lesions in SLD, LC, and MCRF may cause RSWA, and the size and location of lesions determine the complexity of motor behavior.1–4
Increased REM phasic muscle activity was recently reported in caudal mesopontine junction-lesioned cats,6 corresponding approximately to the upper extent of the lesion in our case, which maximally involved the right dorsomedial pons near the proposed location of the SLD/SC, adding to a growing literature implicating lesions of pontine structures causing RBD.1–4,6 We attribute the discrete vasculitic lesion of the dorsomedial pons as the cause of this patient's RBD given both lesion proximity to the SLD/SC or its descending projections to the dorsal MCRF and spinal cord responsible for REM sleep atonia, as well as the strong temporal association of DEB evolving shortly after other focal brainstem deficits, consistent with previously proposed criteria for determining lesional RBD causation.4 There were potentially confounding factors of antidepressant medication and OSA in our case (factors implicated in possibly aggravating or independently mediating RSWA and RBD),2,4 and our 38-month-long follow-up may not yet preclude future evolution of a neurodegenerative disorder as an underlying cause for RBD. However, our patient's DEB began almost immediately after the vasculitic lesion appeared, and RBD persisted despite discontinuing antidepressants and treating OSA, consistent with lesional RBD as the primary cause.4 Our case adds to the growing literature demonstrating a diversity of lesional RBD etiologies including ischemic stroke,3 hemorrhage,4 multiple sclerosis,7 and other structural lesions,2,4 and suggests that the main anatomical region responsible is the dorsomedial pons involving the SLD/SC.1,2 Further neuroimaging and pathologic studies are necessary to confirm key brainstem structures generating RSWA and DEB in RBD.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank Lori Lynn Reinstrom, Department of Neurology, Mayo Clinic, for assistance with copyediting and manuscript submission.
Footnotes
Supplemental data at Neurology.org
Author contributions: Erik K. St. Louis: study concept and design, acquisition of data, data analysis and interpretation, authorship and critical revision of the manuscript, study supervision. Stuart J. McCarter: acquisition of data, analysis, authorship and critical revision of manuscript. Bradley F. Boeve: critical revision of the manuscript for important intellectual content. Michael H. Silber: critical revision of the manuscript for important intellectual content. Kejal Kantarci: critical revision of the manuscript for important intellectual content. Eduardo E. Benarroch: critical revision of the manuscript for important intellectual content. Alora Rando: analysis of data and critical revision of manuscript. Maja Tippmann-Peikert: critical revision of the manuscript for important intellectual content. Eric J. Olson: critical revision of the manuscript for important intellectual content. Michelle Mauermann: critical revision of the manuscript for important intellectual content.
Study funding: Supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant 1 UL1 RR024150-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure: E. St. Louis receives research support from the Mayo Clinic Center for Translational Science Activities (CTSA), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant 1 UL1 RR024150-01, and a Mayo Clinic Alzheimer's Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574). S. McCarter reports no disclosures relevant to the manuscript. B. Boeve is an investigator in clinical trials sponsored by Cephalon, Inc., Allon Pharmaceuticals, and GE Healthcare. He receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge Medicine, 2009). He has received honoraria from the American Academy of Neurology. He serves on the board of the Tau Consortium. He receives research support from the National Institute on Aging (P50 AG16574 [coinvestigator], U01 AG06786 [coinvestigator], RO1 AG32306 [coinvestigator]) and the Mangurian Foundation. M. Silber, K. Kantarci, E. Benarroch, A. Rando, M. Tippmann-Peikert, E. Olson, and M. Mauermann report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature 2006;441:589–594. [DOI] [PubMed] [Google Scholar]
- 2.Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder–neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann NY Acad Sci 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xi Z, Luning W. REM sleep behavior disorder in a patient with pontine stroke. Sleep Med 2009;10:143–146. [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Aparicio J. A lesson from anatomy: focal brain lesions causing REM sleep behavior disorder. Sleep Med 2009;10:9–12. [DOI] [PubMed] [Google Scholar]
- 5.McCarter SJ, St Louis EK, Duwell EJ, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without co-morbid obstructive sleep apnea. Sleep 2014;37:1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai YY, Hsieh KC, Nguyen D, Peever J, Siegel JM. Neurotoxic lesions at the ventral mesopontine junction change sleep time and muscle activity during sleep: an animal model of motor disorders in sleep. Neuroscience 2008;154:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tippmann-Peikert M, Boeve BF, Keegan BM. REM sleep behavior disorder initiated by acute brainstem multiple sclerosis. Neurology 2006;66:1277–1279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.