Abstract
Objective:
To describe the detection frequency and clinical associations of immunoglobulin G (IgG) targeting dipeptidyl-peptidase-like protein-6 (DPPX), a regulatory subunit of neuronal Kv4.2 potassium channels.
Methods:
Specimens from 20 patients evaluated on a service basis by tissue-based immunofluorescence yielded a synaptic immunostaining pattern consistent with DPPX-IgG (serum, 20; CSF, all 7 available). Transfected HEK293 cell-based assay confirmed DPPX specificity in all specimens. Sixty-nine patients with stiff-person syndrome and related disorders were also evaluated by DPPX-IgG cell-based assay.
Results:
Of 20 seropositive patients, 12 were men; median symptom onset age was 53 years (range, 13–75). Symptom onset was insidious in 15 and subacute in 5. Twelve patients reported prodromal weight loss. Neurologic disorders were multifocal. All had one or more brain or brainstem manifestations: amnesia (16), delirium (8), psychosis (4), depression (4), seizures (2), and brainstem disorders (15; eye movement disturbances [8], ataxia [7], dysphagia [6], dysarthria [4], respiratory failure [3]). Nine patients reported sleep disturbance. Manifestations of central hyperexcitability included myoclonus (8), exaggerated startle (6), diffuse rigidity (6), and hyperreflexia (6). Dysautonomia involved the gastrointestinal tract (9; diarrhea [6], gastroparesis, and constipation [3]), bladder (7), cardiac conduction system (3), and thermoregulation (1). Two patients had B-cell neoplasms: gastrointestinal lymphoma (1), and chronic lymphocytic leukemia (1). Substantial neurologic improvements followed immunotherapy in 7 of 11 patients with available treatment data. DPPX-IgG was not detected in any of the stiff-person syndrome patients.
Conclusions:
DPPX-IgG is a biomarker for an immunotherapy-responsive multifocal neurologic disorder of the central and autonomic nervous systems.
Antigen-specific autoimmune disorders targeting central glycinergic and GABAergic pathways are characterized by diffuse or focal stiffness, spasms, and exaggerated startle.1–3 A novel autoimmune encephalitis with prominent seizures, myoclonus, agitation, and diarrhea was recently described in 4 patients with serum immunoglobulin G (IgG) specific for dipeptidyl-peptidase-like protein-6 (DPP6, also known as DPPX),4 and a further 3 patients with progressive encephalomyelitis, rigidity, and myoclonus (PERM).5
DPPX6 is a regulatory subunit of the voltage-gated A-type (rapidly inactivating) Kv4.2 potassium channel complex expressed in neuronal dendrites and soma.6,7 Kv4.2 is the principal channel responsible for transient, inhibitory currents in the central and peripheral nervous systems. These currents regulate repetitive firing rates and back-propagation of action potentials into neuronal dendrites. DPPX is critical also for the normal generation of Kv4.3-dependent cardiac rhythms.8
The widespread CNS distribution of Kv4.2 complexes predicts a multifocal neurologic phenotype for DPPX autoimmunity. Herein, we report the frequency of DPPX-IgG detection among neurologic patients undergoing autoimmune serologic evaluation in the Mayo Clinic Neuroimmunology Laboratory and the clinical correlations of this autoantibody.
METHODS
Standard protocol approvals, registrations, and patient consents.
The Mayo Clinic institutional review board approved this study (08-007846). Written consent was obtained to publish video material (patients 14 and 15).
Serologic testing.
DPPX-IgG was identified by the characteristic indirect immunofluorescence pattern visualized on a composite substrate of mouse hippocampus, cerebral cortex, cerebellum, basal ganglia, thalamus, kidney, and gut (figure 1). DPPX specificity was confirmed molecularly by indirect immunofluorescence on HEK293 cells transfected with the DPPX complementary DNA. Control cells were transfected with empty vector. Cells were grown on glass coverslips, fixed with 1% formalin, prepared as millimeter-sized biochip fragments on microscope slides as a mosaic of DPPX-expressing and control cells (Euroimmun, Lübeck, Germany), and stored at −20°C until use.
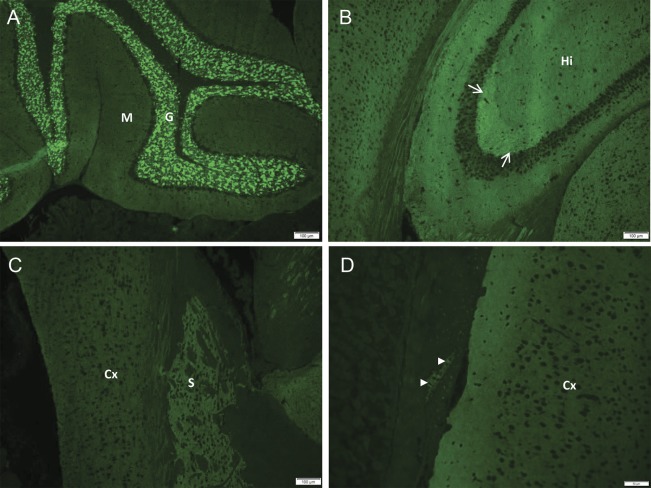
Figure 1. Synaptic pattern of DPPX immunoreactivity in mouse central and enteric nervous system revealed by IgG in serum or CSF of patients by tissue immunofluorescence assay.
(A) IgG binds more prominently to the cerebellar granular layer (G) than molecular layer (M); Purkinje neurons are not reactive. (B) In hippocampus (Hi), the mossy fibers of the stratum lucidum (arrows) stain most brightly. In the cerebrum (C), the cortex (Cx) and striatum (S) are reactive. (D) IgG binds to ganglionic neurons in the myenteric plexus of the gut wall (arrowheads). DPPX = dipeptidyl-peptidase-like protein-6; IgG = immunoglobulin G.
DPPX-IgG seropositivity in patient 1 was confirmed by Dr. J. Dalmau, University of Barcelona. Testing for coexisting neuronal or glial nuclear, cytoplasmic, or plasma membrane-reactive IgGs also was performed (appendix e-1 on the Neurology® Web site at Neurology.org).9
Patients.
Group 1 consisted of stored specimens from 83 patients referred for evaluation of paraneoplastic neurologic autoimmunity (1991–2012, before the first DPPX-IgG report4) for whom tissue-based immunofluorescence screening had revealed an unclassified IgG autoantibody reactive with CNS and enteric synapses. Available specimens were serum only (64), serum and CSF (19). Each specimen was reevaluated by tissue-based immunofluorescence and by cell-based assay for DPPX-IgG.
Group 2 consisted of 8 patients identified prospectively (January 2013 to March 2014) through service testing (tissue-based immunofluorescence) to have DPPX-IgG among 41,812 patients' specimens submitted for evaluation of neural autoantibodies (0.02%). Serum was available for all 8 patients, and paired CSF specimens were available for 3. Medical records were reviewed.
Group 3 consisted of 69 patients with the following: classic stiff-person syndrome (also known as stiff-man syndrome) (34) (glutamic acid decarboxylase 65 [GAD65]-IgG positive [30], glycine receptor-IgG positive [3], seronegative [1]); limited stiff-person syndrome variants, including stiff-limb (30) (GAD65-IgG positive [15], glycine receptor-IgG positive [3], seronegative [14]); PERM (3) (GAD65-IgG positive [1], glycine receptor-IgG positive [1], seronegative [1]); and hyperekplexia (2). Specimens available for testing were serum (all patients) and CSF (8).
RESULTS
DPPX-IgG was detected by tissue-based immunofluorescence in 20 patients (20 serums and 7 paired CSF specimens; figure 1). Twelve patients were identified retrospectively (group 1), and 8 were identified prospectively after 2012 (group 2). These patients (and none others) yielded DPPX-IgG seropositivity by cell-based assay. All 69 in group 3 (stiff-person syndrome and related disorders) were DPPX-IgG seronegative by both assays. Where both specimen types were available for testing, no patient was positive in serum only or CSF only. Median serum titer was 1:15,360 (range, 1,920–245,760) and median CSF titer was 1:64 (range, 2–128).
Summary of demographic and clinical findings.
Twelve of the 20 patients were male. The median age at symptom onset was 53 years (range, 13–75). The median follow-up period was 6 months (range, 0–68 months). Symptom onset was insidious in 12, subacute in 5, subacute on insidious in 2, and stepwise in 1. The median time from symptom onset to initial evaluation was 12 months (range, 0–177 months). Clinical details are summarized in tables 1, 2, and e-1. Prominent clinical manifestations of DPPX autoimmunity were encephalopathy (with cortical, cerebellar, or brainstem manifestations), myelopathy, weight loss, and autonomic dysfunction.
Table 1.
Neurologic manifestations of 20 DPPX-IgG seropositive patients
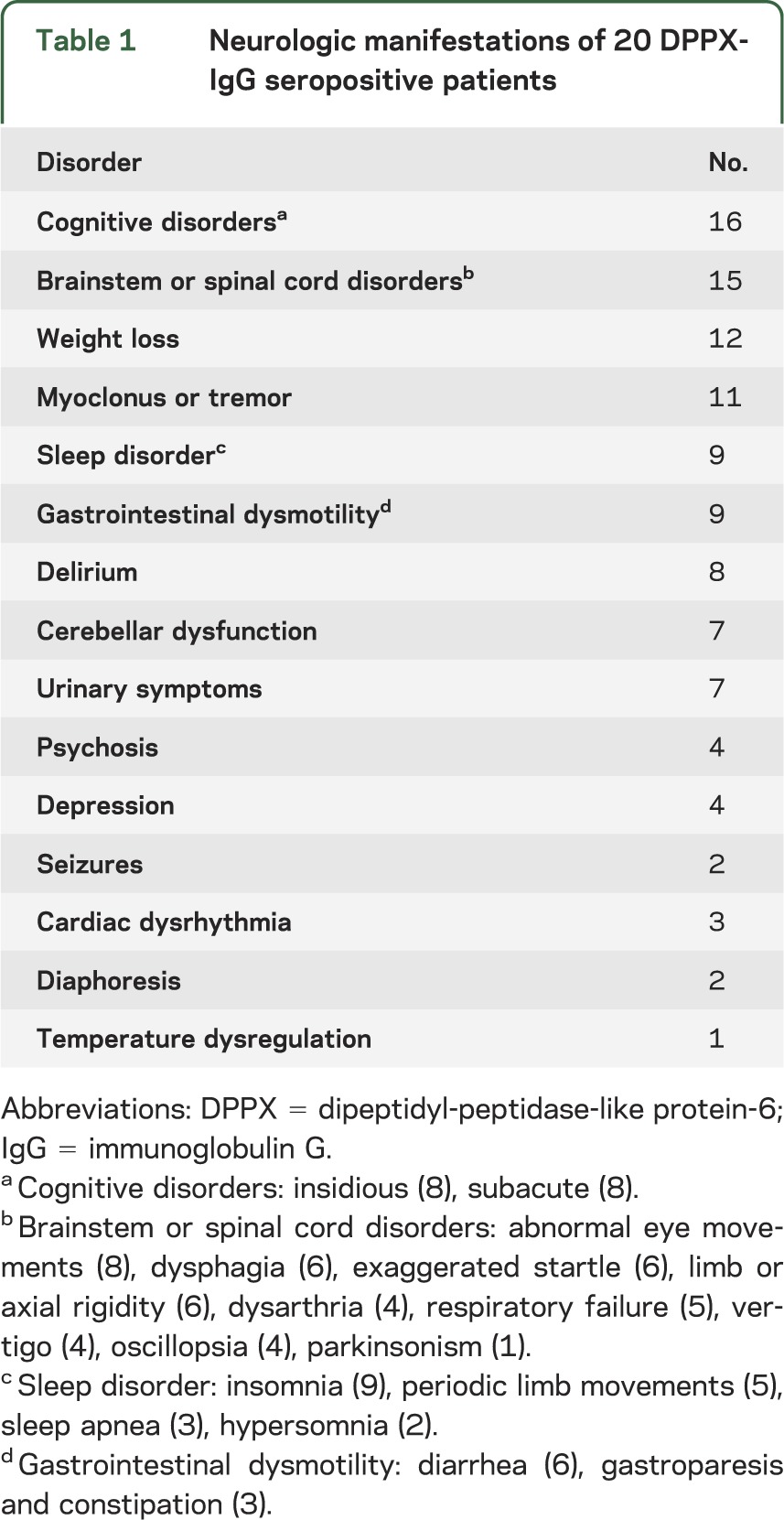
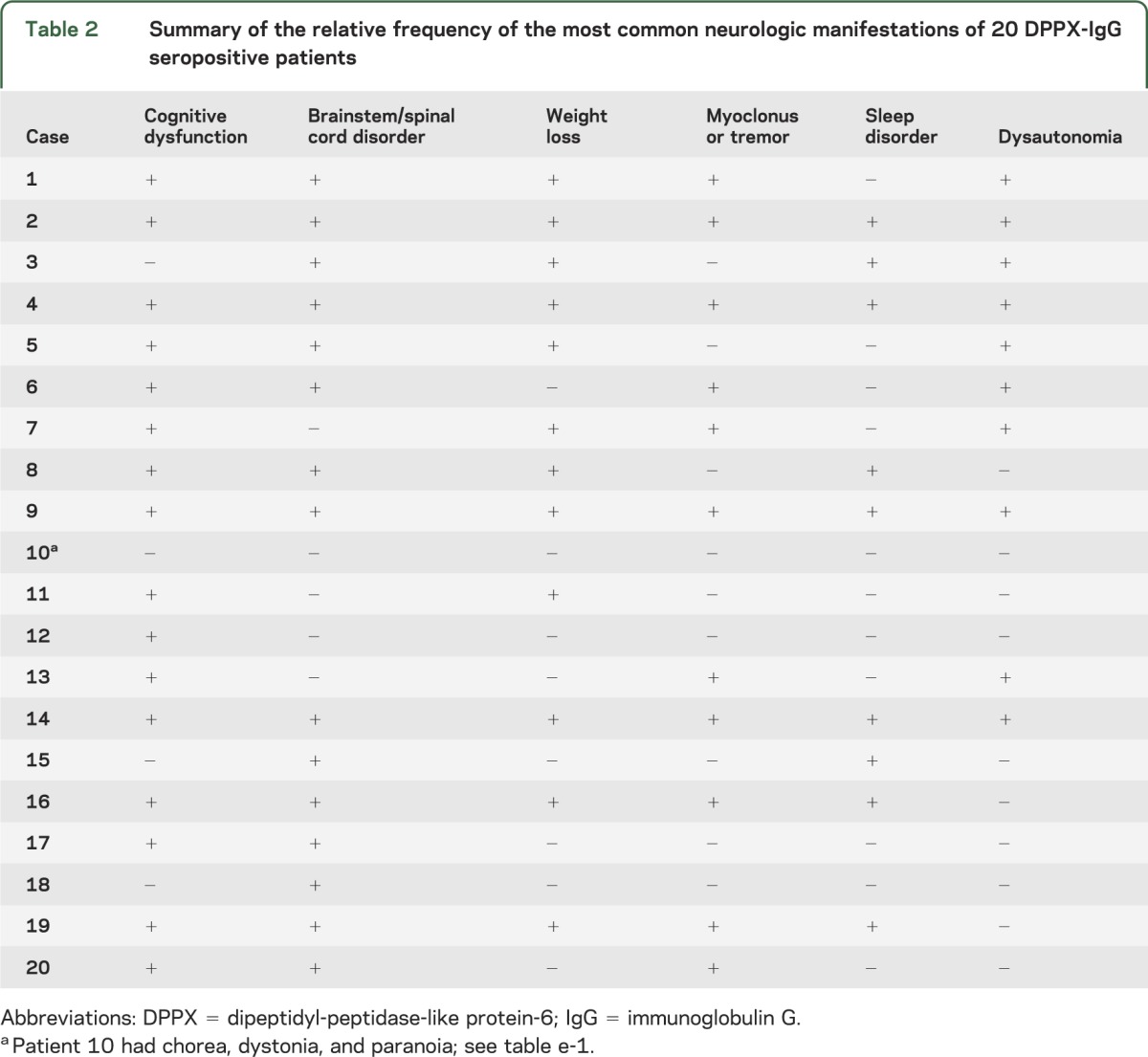
Table 2.
Summary of the relative frequency of the most common neurologic manifestations of 20 DPPX-IgG seropositive patients
Weight loss was the initial symptom in 7 patients (median loss, 34 kg; range, 23–52 kg); 5 patients reported anorexia. Weight loss and neurologic symptoms began simultaneously in 5 patients, and in 5 patients, weight loss began a median of 30 months before the neurologic symptoms (range, 3–180 months).
Ten patients reported autonomic symptoms: gastrointestinal (9) (diarrhea [6 patients], gastroparesis, and constipation [3 patients, figure 2]); urinary symptoms (7); and fluctuating hypothermia/hyperthermia (1). Holter monitoring revealed asymptomatic ventricular tachycardia (3–4 beats) in patients 1, 4, and 14. Patient 14 (aged 27 years) had a cardiac arrest within approximately 4 days of neurologic relapse.
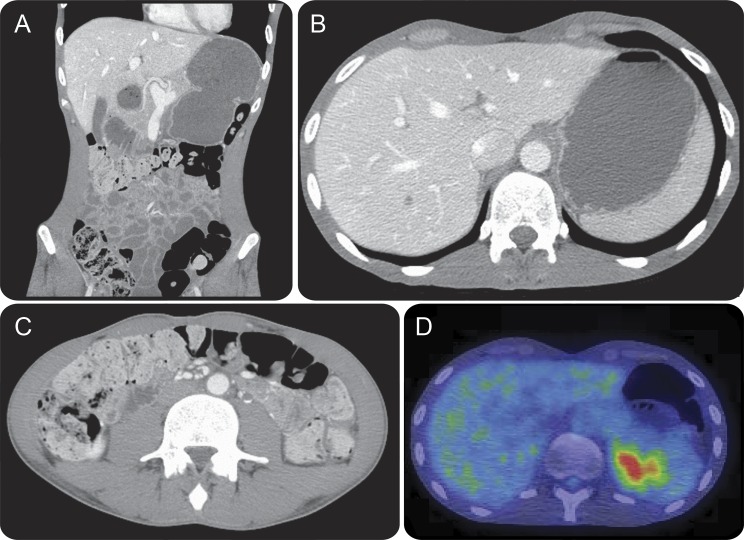
Figure 2. Patient 14: Radiologic evidence of gastrointestinal hypomotility.
The patient defecated at 3-week intervals. Coronal (A), and axial (B and C) CT of the abdomen demonstrates marked gastric distension (A and B), and gross colonic loading with feces (A and C). Fluorodeoxyglucose (FDG)-PET demonstrates retention of food bolus in the gastric fundus secondary to gastroparesis, as well as normal excretion of FDG by the kidneys into the bladder (D).
Neuropsychiatric symptoms included amnesia (16), delirium (8), psychosis (4), and depression (4). Patients 1 and 16 had seizures. EEG revealed focal or diffuse slowing in 5 of 7 patients evaluated, but no epileptiform discharges were reported.
Brainstem symptoms included diplopia, oscillopsia or blurred vision (8), dysphagia (6), and respiratory failure (3). Cerebellar ataxia was noted on neurologic examination in 7 patients. Video 1 demonstrates abnormal eye movements in patient 15 who complained of diplopia, episodic ataxia, nausea, and vomiting.
Disrupted sleep was reported for 9 patients: insomnia (6), periodic limb movements (5), sleep apnea (3), and hypersomnia (2). Sleep studies (performed in 3 patients) demonstrated periodic limb movements (2), obstructive sleep apnea (1), and ambiguous sleep (1, patient 14). Ambiguous sleep is characterized polysomnographically by REM (EEG sawtooth waves) and non-REM sleep (EEG sleep spindles, K-complexes) occurring simultaneously. This patient fell into sleep multiple times during consultation, and immediately commenced talking and moving his arms and legs.
Clinical findings in 11 patients were consistent with central hyperexcitability, but only 2 (patients 9 and 13) had both stiffness/rigidity and myoclonus, considered typical of PERM. Myoclonus (video 2) was evident in 8 patients (documented by surface EMG to be of likely cortical origin in 2 [burst durations <50 milliseconds]). Six had exaggerated startle, 6 had limb or truncal stiffness/rigidity, and 6 had brisk deep tendon reflexes. Patients 1 and 4 reported startle, and in detailed neurophysiologic studies, trunk and limb responses to repetitive acoustic startle stimulation were diffuse, nonhabituating, and exaggerated (reflex latency <80 milliseconds). Exteroceptive reflex stimulation revealed widespread exaggerated responses below the reflex latency of 100 milliseconds. Patient 20 had cortical myoclonus, but did not report abnormal startle; acoustic startle and exteroceptive neurophysiology, and monopolar EMG of paraspinal muscles were normal.
In no patient was the neurologic disorder unifocal, but abnormalities in 5 patients were somewhat limited (patients 3, 10, 11, 15, and 18). Early neurologic misdiagnoses included progressive supranuclear palsy (patient 4) and idiopathic Parkinson disease (patient 6). Two patients with contrasting phenotypes (patients 3 and 14) are described in appendix e-2.
Associated B-cell neoplasms were detected in patients 5 and 19 (gastrointestinal follicular lymphoma and chronic lymphocytic leukemia). In both patients, the neoplasm and neurologic disorders remitted after rituximab treatment.
Additional findings consistent with neurologic autoimmunity.
In addition to DPPX-IgG autoantibody, other findings supporting an autoimmune neurologic diagnosis included a coexisting serum autoantibody in 6 and a coexisting autoimmune disease in 4 (table e-1). Abnormalities were reported in brain MRIs of 4 patients (data available for 13), but none had abnormalities specific for encephalitis. CSF data were available for 10 patients; 7 were normal. Protein was elevated in 3 patients (55 mg/dL, 75 mg/dL, and unspecified value; normal value, 0–35 mg/dL) and white cell count was elevated in 2 (6 and 73 leukocytes/µL; normal range, 0–5 leukocytes/µL).
Treatments and outcomes.
Physicians reported neurologic improvements in 8 of 12 patients who received immunotherapy (table 1): 6 of 11 receiving corticosteroids; 4 of 5 receiving IV immune globulin; 3 of 5 receiving plasmapheresis; 4 of 6 receiving rituximab; and 2 of 3 receiving cyclophosphamide. Among 18 patients with adequate follow-up data, 4 completely remitted or had mild disability, 5 had a partial response to treatment, 6 remained unchanged, and 3 worsened progressively.
DISCUSSION
DPPX-IgG unifies patients with an unusual multifocal autoimmune neurologic disorder that is often insidious in onset, severe, responsive to early immunotherapy, and tends to relapse. Consistent with the widespread expression of Kv4.2 channels in the nervous system, prominent clinical manifestations of DPPX autoimmunity were encephalopathy (with cortical, cerebellar, or brainstem manifestations), myelopathy, and autonomic dysfunction.10
DPPX autoimmunity is a new addition to the differential diagnosis for acquired neurologic disorders with prominent (but not exclusive) symptoms of central hyperexcitability (stiffness, spasms, tremulousness, and exaggerated startle).2,3 Myoclonus, stiffness/rigidity exaggerated startle, and brainstem or spinal cord dysfunction were common but not universal in our cohort, and only 2 had classic findings of PERM.5 Diarrhea was earlier recognized as a prominent feature of DPPX autoimmunity.4 Here, we have documented profound gastrointestinal hypomotility as an occasional manifestation.
Sleep dysfunction has been described in the context of autoimmunity targeting Ma1 and Ma2, voltage-gated Kv1 potassium channel complexes, and most recently IgLON5.11–13 The varied sleep symptoms (including insomnia, limb movements, and disrupted REM sleep) among our DPPX-IgG seropositive patients adds to the growing literature of autoimmune sleep disorders.
Detection of DPPX-IgG was critical to the neurologic diagnosis of the patients and led to an oncologic diagnosis (B-cell neoplasia) in 2. The neurologic findings varied in onset and anatomical distribution, and most patients lacked other historical and laboratory testing clues to an autoimmune neurologic diagnosis.
In this report and in the original report,4 most patients receiving early and sustained immunotherapies improved neurologically, an observation consistent with an autoimmune disorder that is IgG-mediated. Proof of the pathogenicity of DPPX-IgG awaits experimental studies in cell culture and animal model systems, such as have been described for aquaporin-4 and NMDA receptor autoantibodies.14,15
Of our 20 patients, 8 were identified prospectively over 15 months, making this disorder considerably less common than autoimmune NMDA receptor encephalitis (93 patients identified in 2013) but more common than autoimmune AMPA receptor encephalitis (1 patient identified in 2013). At this stage of our experience, serum and CSF seem to be equally sensitive specimens for DPPX-IgG testing. Comprehensive evaluation for all autoimmune encephalitis-pertinent antibodies is assured by testing both serum and CSF.16,17 Tissue-based immunofluorescence staining by DPPX-IgG has a distinctive synaptic pattern in both the central and enteric nervous systems. Reactivity was most prominent in the cerebellar granular layer and mossy fibers within the stratum lucidum of the hippocampus, both known to be DPPX-enriched.18–20
The dysautonomic manifestations of DPPX potassium channel autoimmunity appear to extend to cardiac dysrhythmia. Kv4.3 complexes are abundant in intercalated disc regions connecting cardiac myocytes, and are responsible for modulating early repolarization of cardiac action potentials.10 Gain-of-function mutations in the DPPX gene causing ventricular fibrillation but not neurologic dysfunction has been reported.21,22
The neurologic manifestations of DPPX potassium channel autoimmunity are diverse, multifocal, and sometimes intermittent. Common manifestations include weight loss, neuropsychiatric and brainstem disorders, CNS hyperexcitability, and dysautonomia. Patients appear to respond well to early-initiated immunotherapy. Optimal neurologic outcomes may require long-term immunosuppressant therapy.
Supplementary Material
GLOSSARY
- DPPX
dipeptidyl-peptidase-like protein-6
- GAD65
glutamic acid decarboxylase 65
- IgG
immunoglobulin G
- PERM
progressive encephalomyelitis, rigidity, and myoclonus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Tobin designed the study, collected and analyzed the data, wrote the first draft of the manuscript. Dr. Lennon supplied critical reagents and testing, collected and analyzed data, reviewed the manuscript for important intellectual content. Dr. Komorowski supplied critical reagents and reviewed the manuscript for important intellectual content. Dr. Probst supplied critical reagents and reviewed the manuscript for important intellectual content. Dr. Clardy collected data and reviewed the manuscript for important intellectual content. Dr. Aksamit collected data and reviewed the manuscript for important intellectual content. Dr. Appendino collected data and reviewed the manuscript for important intellectual content. Dr. Lucchinetti collected data and reviewed the manuscript for important intellectual content. Dr. Matsumoto collected and analyzed data and reviewed the manuscript for important intellectual content. Dr. Pittock collected data and reviewed the manuscript for important intellectual content. Dr. Sandroni contributed and analyzed data and reviewed the manuscript for important intellectual content. Dr. Tippmann-Peikert collected and analyzed data and reviewed the manuscript for important intellectual content. Dr. Wirrell collected and analyzed data and reviewed the manuscript for important intellectual content. Dr. McKeon conceived and designed the study, collected and analyzed the data, and reviewed the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
W. Tobin reports no disclosures relevant to the manuscript. V. Lennon is a named inventor on 2 patent applications filed by Mayo Foundation for Medical Education and Research that relate to aquaporin-4 (AQP4) autoantibody and its application to cancer and functional assays for its detection. She shares in royalties from marketing of kits for detecting AQP4-IgG. Royalties received to date by Dr. Lennon and Mayo Clinic exceed the federal threshold for significant financial interest. Serologic testing for neural autoantibodies is offered on a service basis by Mayo Collaborative Service, Inc., an agency of Mayo Foundation. Neither Dr. Lennon nor her laboratory benefit financially from this testing. Dr. Lennon receives research support from the NIH (RO1 NS065829). L. Komorowski is an employee of Euroimmun Inc., which manufactures and markets assay kits for detecting DPPX antibody. C. Probst is an employee of Euroimmun Inc., which manufactures and markets assay kits for detecting DPPX antibody. S. Clardy and A. Aksamit report no disclosures relevant to the manuscript. J. Appendino receives research support from Children's Hospital Foundation of Manitoba. C. Lucchinetti shares in royalties from marketing of kits for detecting AQP4 autoantibody and from the sale of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); she receives research support from the NIH (NS49577-R01 [PI]), the Guthy-Jackson Charitable Foundation (PI), and the National Multiple Sclerosis Society (RG 3185B3 [PI]). J. Matsumoto reports no disclosures relevant to the manuscript. S. Pittock has received no royalties to date but may accrue revenue for patents relating to AQP4 antibodies for diagnosis of neuromyelitis optica and AQP4 autoantibody as a cancer marker. He receives research support from the Guthy-Jackson Charitable Foundation, Alexion Pharmaceuticals, Inc., and the NIH (RO1 NS065829). P. Sandroni, M. Tippmann-Peikert, and E. Wirrell report no disclosures relevant to the manuscript. A. McKeon receives research support from the Guthy-Jackson Charitable Foundation and MedImmun, Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hutchinson M, Waters P, McHugh J, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology 2008;71:1291–1292. [DOI] [PubMed] [Google Scholar]
- 2.McKeon A, Robinson MT, McEvoy KM, et al. Stiff-man syndrome and variants: clinical course, treatments, and outcomes. Arch Neurol 2012;69:230–238. [DOI] [PubMed] [Google Scholar]
- 3.McKeon A, Martinez-Hernandez E, Lancaster E, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol 2013;70:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 2013;73:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balint B, Jarius S, Nagel S, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology 2014;82:1521–1528. [DOI] [PubMed] [Google Scholar]
- 6.Wada K, Yokotani N, Hunter C, Doi K, Wenthold RJ, Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family. Proc Natl Acad Sci USA 1992;89:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadal MS, Ozaita A, Amarillo Y, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 2003;37:449–461. [DOI] [PubMed] [Google Scholar]
- 8.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (I(to)) expression and regulation. J Mol Cell Cardiol 2010;48:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Toole O, Lennon VA, Ahlskog JE, et al. Autoimmune chorea in adults. Neurology 2013;80:1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev 2004;84:803–833. [DOI] [PubMed] [Google Scholar]
- 11.Adams C, McKeon A, Silber MH, Kumar R. Narcolepsy, REM sleep behavior disorder, and supranuclear gaze palsy associated with Ma1 and Ma2 antibodies and tonsillar carcinoma. Arch Neurol 2011;68:521–524. [DOI] [PubMed] [Google Scholar]
- 12.Cornelius JR, Pittock SJ, McKeon A, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol 2011;68:733–738. [DOI] [PubMed] [Google Scholar]
- 13.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 2014;13:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci USA 2012;109:1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology 2011;76:1108–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadin BM, Pfaffinger PJ. A new TASK for dipeptidyl peptidase-like protein 6. PLoS One 2013;8:e60831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadows HJ, Randall AD. Functional characterisation of human TASK-3, an acid-sensitive two-pore domain potassium channel. Neuropharmacology 2001;40:551–559. [DOI] [PubMed] [Google Scholar]
- 20.Clark BD, Kwon E, Maffie J, et al. DPP6 localization in brain supports function as a Kv4 channel associated protein. Front Mol Neurosci 2008;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alders M, Koopmann TT, Christiaans I, et al. Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am J Hum Genet 2009;84:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, Koopmann TT, Ordog B, et al. Unique cardiac Purkinje fiber transient outward current beta-subunit composition: a potential molecular link to idiopathic ventricular fibrillation. Circ Res 2013;112:1310–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.