Abstract
Maneuvering safely through the environment is central to survival of almost all species. The ability to do this depends on learning and remembering locations. This capacity is encoded in the brain by two systems: one using cues outside the organism (distal cues), allocentric navigation, and one using self-movement, internal cues and nearby proximal cues, egocentric navigation. Allocentric navigation involves the hippocampus, entorhinal cortex, and surrounding structures; in humans this system encodes allocentric, semantic, and episodic memory. This form of memory is assessed in laboratory animals in many ways, but the dominant form of assessment is the Morris water maze (MWM). Egocentric navigation involves the dorsal striatum and connected structures; in humans this system encodes routes and integrated paths and, when overlearned, becomes procedural memory. In this article, several allocentric assessment methods for rodents are reviewed and compared with the MWM. MWM advantages (little training required, no food deprivation, ease of testing, rapid and reliable learning, insensitivity to differences in body weight and appetite, absence of nonperformers, control methods for proximal cue learning, and performance effects) and disadvantages (concern about stress, perhaps not as sensitive for working memory) are discussed. Evidence-based design improvements and testing methods are reviewed for both rats and mice. Experimental factors that apply generally to spatial navigation and to MWM specifically are considered. It is concluded that, on balance, the MWM has more advantages than disadvantages and compares favorably with other allocentric navigation tasks.
Navigational Learning and Memory
Navigation is the ability of organisms to learn to find their way through the environment without getting lost, which requires memory for locations and routes. All organisms need to be able to leave their nest, burrow, den, or home and move in the environment to forage for food, find water, avoid predators, locate mates, and return safely to where they started. Without this ability, no organism could survive; hence, this capacity has evolved in almost all species.
Navigation is so vital to survival that it is conserved in phyla as basic as insects, including ants (Wittlinger et al. 2006) and bees (Henry et al. 2012; Menzel et al. 1998), avians, fish, bats (Heys et al. 2013), and all terrestrial mammals (Etienne 1992). In birds, bats, fish, and marine mammals, navigation has evolved to the extent of these animals being able to navigate in three dimensions rather than two.
Types of Navigation
There are at least two distinct types of navigation, and perhaps three, depending on how it is defined. The dichotomous distinction is between allocentric and egocentric navigation. Allocentric wayfinding, also referred to as spatial navigation, is characterized by the ability to navigate using distal cues—that is, cues/landmarks located outside and at some distance from the organism. Egocentric wayfinding is characterized by the ability to navigate using internal cues (i.e., by feedback from limb movements for rate of movement [speed], direction, turns, and sequence of turns), optokinetic flow as the organism moves past surrounding objects, and signposts. Signposts are different than landmarks in that a landmark is farther away and a signpost is close and signposts and landmarks convey different information. A landmark provides relational information as to where the organism is compared with other landmarks, whereas a signpost is a marker of where to change direction along a path but does not give relational information. Egocentric navigation can operate in darkness, indicating that visual cues are not essential for this method of navigating, although in the absence of visual cues egocentric navigational accuracy is reduced. By contrast, allocentric navigation is disabled by the absence of visual cues.
Egocentric navigation and path integration have been used interchangeably by some, whereas others distinguish between them. Egocentric navigation generally refers to the ability to navigate by internal self-movement cues, but a further distinction can be made by dividing egocentric navigation into route-based and path integration. Route-based navigation relies on internal cues of rate of movement, turns, and signposts, whereas path integration relies on these but involves an additional attribute, vector addition. In route-based navigation, an organism follows a path with the order of turns remembered as a set of specific rules, such as straight-left-right-left-left—that is, which direction to turn when it reaches specific signposts or moves in a specific direction for a given number of steps. In humans an easy way of assessing this behavior is to have subjects walk around a circle and then retrace the circle when blindfolded. Patients with striatal perturbations have a difficult time retracing the circle relative to control subjects (Paquette et al. 2011). Interestingly, these memorized operations can be overlearned to such a degree they become habits. When this occurs, the location of the memory shifts within the brain and is reclassified as implicit or procedural memory. In humans, implicit memory is seen as skilled behavior that is relatively automatic and not conscious, such as the sequence of movements involved in driving a car, riding a bicycle, throwing a ball, hitting a baseball, skiing, and so on. By contrast, path integration is seen as the ability of an organism to leave its home-base and move to different locations and then return by a different, more direct, path. For example, an organism could travel from its home (H) to location A, location B, and location C and then return to its base by a more direct path from C to H without retracing its steps in reverse through B and A.
Path integration is assessed in humans in multiple ways, but perhaps the most straightforward way is by blindfolding subjects and leading them in routes through a large open room, then stopping and asking them to point to where they started and estimate how far they are away from where they began. Those with or without damage to their hippocampus and entorhinal cortex do this task with remarkable and essentially equal accuracy to control subjects (Shrager et al. 2008), demonstrating that this ability is distinguishable from spatial navigation. By contrast, subjects with temporal lobe injury are severely impaired on many spatial tasks (Buzsaki and Moser 2013). Given such data, these two navigational systems are clearly mediated by different neural networks. Because these are important in human navigation and are tied to other types of memory, it is important to have tests that differentiate these in rodent model systems. Accomplishing this has not proven to be so easy because the two systems overlap extensively. The result is that treatments aimed at disrupting only one of these systems often end up affecting both to one degree or another. This difficulty does not indicate that distinguishing between the two is impossible, but rather that there are gray areas in the resulting data about how well any given experiment or any given test is able to dissociate the two phenomena.
Phenomenology of Navigation
In natural environments, navigation is characterized by the range or space over which an organism moves to forage for food, find mates, and/or defend territory. Species range can vary widely. For some mammals, it may be just a few acres, but for some predators, the range may be over long distances. Moreover, some migratory species have seasonal ranges that are extremely large—for example, monarch butterflies that migrate from Canada to Mexico to overwinter. Many species of birds, whales, elk, wildebeest, and others also migrate long distances. Long-distance navigation relies on navigational systems not well understood and beyond the definition studied in laboratory animals as allocentric navigation, and these systems are not the subject of this review.
Brain Regions Mediating Navigation
Spatial Navigation
All brain systems consist of complex circuits or networks that span many brain regions. It is an oversimplification to assign a given functional capacity to any one or even several brain regions. Yet it is also the case that different functional abilities have primary regions essential to a given function. In the case of allocentric navigation, the primary regions that are crucial to mediating this ability are the hippocampus and entorhinal cortex. The hippocampus has been identified for many decades as a key structure in forming cognitive maps (O'Keefe and Nadal 1978). Lesions, pharmacological inhibition, long-term potentiation (LTP) saturation, the act of learning itself, and loss-of-function genetic mutations of signaling molecules or receptors within the hippocampus result in impaired spatial learning and memory (Brandeis et al. 1989; Burgess et al. 2002; Buzsaki and Moser 2013; McNamara and Skelton 1993; Moser et al. 1998; Penner and Mizumori 2012; Suh et al. 2011; Whitlock et al. 2006).
Using electrophysiological methods, place cells have been identified in the hippocampus. These cells, located in specific subregions of the hippocampus, respond to different environments and the features within them. Collectively, they form a neural map of the environment, and these cells remap the environment as the organism moves within a given space or moves to a new space. In recent years, the role of the entorhinal cortex in spatial mapping has been elucidated (Leutgeb et al. 2005b). The medial entorhinal cortex, especially in layer 2, has been identified as having place cells and communicating with place cells in the hippocampus (Hafting et al. 2005). Grid cells in the entorhinal cortex that form tiling patterns with response fields that map larger regions of the environment than place cells also occur (Leutgeb et al. 2005b); moreover, these response fields differ in scale topographically, such that smaller response fields are located in the dorsal entorhinal cortex and progressively larger response fields are located in more ventral regions of the entorhinal cortex (Brun et al. 2008; Buzsaki and Moser 2013; Kjelstrup et al. 2008). In addition, the entorhinal cortex, along with the presubiculum and parasubiculum, contain head direction cells (Buzsaki and Moser 2013) that play a key role in orienting the organism to distal cues and contribute to direction of movement. The medial entorhinal cortex has also been found to contain border cells (Solstad et al. 2008). These cells have place response fields that react to boundaries or edges within the environment. In concert, the place, grid, head direction, and border cells of the entorhinal cortex and place cells of the hippocampus form a functional network that maps places in space outside the organism. Such place cells show progressively increased firing rates as rats swimming in a Morris water maze (MWM) approach an escape platform during hidden platform trials but not when approaching a visible platform (Hollup et al. 2001).
The same network that provides spatial information in rodents is implicated in semantic and episodic memory in humans. Semantic memory, which is memory of facts as well as places, is suggested to be an extension of the spatial encoding system that in rodents is tested as allocentric learning and memory (Buzsaki and Moser 2013). Episodic memory captures information about the order of events, but not in the sense of being time-stamped but rather memory for one event happening before or after another, and is linked to spatial encoding (Leutgeb et al. 2005a). It has often been observed in human recall experiments that memory for an event will trigger memories of the order of events and vice versa (Miller et al. 2013). The egocentric network provides sequential information that is thought to contribute to episodic memory because it is self-referenced and uses information about a starting point and time spent moving derived from limb movement cues that combine speed and directional information. These features suggest that the egocentric network also contributes to episodic memory (Buzsaki and Moser 2013) but exactly how is not yet known. Although the hippocampus and surrounding structures are critical for spatial learning and memory in all mammals so far tested, and in humans spatial learning and semantic and episodic memory are intertwined, memory storage and retrieval require the interplay of these structures with the prefrontal cortex and anterior cingulate cortices, and to a lesser degree with parietal and retrosplenial cortices (Maviel et al. 2004).
Nonspatial Navigation
Brain regions that mediate egocentric navigation are less well studied than those for allocentric navigation. Perhaps the most important fact is that the two systems overlap (Sherrill et al. 2013). Head direction cells are especially important for egocentric navigation. First discovered in the presubiculum, head direction cells are found in other regions as well, including in the entorhinal cortex, thalamus, mammillary nucleus, retrosplenial cortex, and dorsal striatum (van Strien et al. 2009). However, under some conditions the egocentric and allocentric systems can be dissociated (e.g., hippocampal lesions result in spatial, but not nonspatial, deficits in the MWM, whereas dorsal striatal lesions result in nonspatial, but not spatial, deficits in the MWM) (Devan et al. 1999; McDonald and White 1994; Packard and McGaugh 1992). At the same time, studies in humans using virtual environments show that egocentric path integration recruits neural activity in the hippocampus and parietal cortex (Sherrill et al. 2013), demonstrating once again that egocentric and allocentric pathways overlap.
Principles of Navigational Assessment
Spatial Navigation
The key distinction in differentiating allocentric from egocentric navigation is the cues on which the animal depends to find its way through the environment. Allocentric navigation depends on distal cues, whereas egocentric navigation depends on proximal and internal cues. Therefore, in developing tests to assess these functions, it is necessary that the test environment for allocentric navigation be designed so that it has ample distal cues but is free of proximal cues to the fullest extent possible. The elimination of proximal cues is challenging, and it is one reason an open pool of water has become a principal device for creating a test environment that meets this essential requirement. By contrast, test environments for assessing egocentric navigation need to be designed to provide ample proximal cues and minimize distal cues, or at the extreme, eliminate distal cues entirely, such that the animal must rely entirely on internal cues. This can be done and it requires navigation in complete darkness or, in humans, by blindfolding. Designing tasks that control or manipulate proximal cues in such a way that they can be accounted for separately from distal cues is another way to determine how an animal is navigating in an environment, as can be done in the star maze (Fouquet et al. 2013; Rondi-Reig et al. 2006).
Nonspatial Navigation
As mentioned, egocentric navigation overlaps with but is distinct from spatial navigation. The principal challenge for egocentric learning is to create an environment with proximal but not distal cues or to create an environment with both distal and proximal cues and arrange the environment in such a way that the subject decides which cues to use during the training phase and then test which cues are dominant when the arrangement of cue positions is changed. This allows the experimenter to determine which cues are most salient. In addition, this technique can be used to show how a treatment affects a group of animals' preferred strategy and, by implication, how the treatment affected one memory system over the other.
Learning-Performance Distinction
Regardless of the navigational task, learning-performance distinctions are critical for all tests of learning and memory. As with any learning and memory assessment, methods that use multiple measures of behavior to provide converging evidence are best at ensuring that results are interpreted as reflecting navigational learning and memory and are not confounded by performance factors such as differences in motivation, the tendency toward thigmotaxis, or motor deficits. In appetitive tasks, this additionally means ensuring that animals are matched for the incentive value of the reinforcement. For shock-motivated tasks, this means equating groups for shock threshold and reactivity. For swimming tasks, this means ensuring that groups are equal in swimming ability, such as swim speed, learning that the platform is the goal/escape, and that there is no escape other than climbing on the platform and waiting to be removed.
Assessing Navigation in Rodents
Spatial Assessment
Many tasks have been developed to assess allocentric navigation, such that it is impractical to review them all; for a review of many tasks of spatial learning adapted for mice, see Sharma and colleagues (2010). The tests most widely used and for which the most data exist are reviewed here. These are the MWM, the radial-arm maze (RAM), and a few other mazes with features that address aspects of spatial learning differently than the MWM and RAM.
Morris Water Maze
The MWM was developed by Richard Morris (Morris 1981). The prevailing test at the time was the RAM (Olton and Samuelson 1976). The problem with the RAM was that once an animal enters an arm there are proximal cues associated with the corridor it enters, a problem extant in all channel-type mazes. This feature of corridor mazes blurs the line when it comes to differentiating navigation that relies on distal versus proximal cues. Even though all the arms of the RAM are intended to be identical, it is difficult in practice to prove there are not unseen cues. Morris used a circular pool of water that was featureless on the inside but had many cues on the outside. Reinforcement was provided by having a small platform located at one position in the pool that was neither close to the wall nor in the center. He made the platform either invisible, by submerging it, or visible, by protruding it above the surface of the water. Morris devised four test conditions to determine how rats use distal versus proximal cues to learn to swim to find the escape platform. In one condition (Place), the platform was painted white, submerged, and camouflaged (by making the water also white) so it could not be seen from water-level; for this test the platform remained in a fixed location but the start positions were randomized on each trial between one of four cardinal start positions (north, south, east, west; not actual compass directions). In the second condition (Cue + Place), he used a platform that was raised above the water and painted black to make it stand-out from the white background; in this condition he again used a fixed platform with randomized start positions. In the third condition (Cue Only), he used the black visible platform but randomized both the start and platform positions on each trial. In the fourth condition (Place-Random), he used the submerged white platform again but randomized the start and platform positions on every trial. He found that the Cue + Place and Cue-Only groups learned rapidly, so fast in fact that they reached asymptotic performance in three trials. The Place group learned at an intermediate rate but still became proficient with no proximal cues available and reached the same level of performance as the Cue groups by trial 5. Importantly, the Place-Random group showed very poor to no learning. If animals had been able to see the platform or had other cues available, they should have been able to learn, but they did not. Instead, this group showed only modest improvement. It has since been demonstrated that animals learn that the platform is not near the wall or the center and therefore swim in search patterns that permit them to hunt for the platform away from the wall; they do this relatively systematically, unlike the disorganized patterns they exhibit on early trials. Regardless of what strategy they use in the random condition, overall the data demonstrate that distal cues alone provide information sufficient for efficient learning whereas the absence of such cues and without proximal cues to compensate, animals are unable to improve their performance beyond learning a generic strategy. Interestingly, rats pass through stages of learning in the MWM. The first is typically thigmotaxis, whereby the animals swim around the perimeter (Dalm et al. 2000). This is followed by swimming at a distance from the edge of the pool accompanied by weaving and circling, strategies that often end with finding the platform. Such nonspatial patterns have been found to persist in animals with damage to the hippocampus (Hodges 1996). To test memory for what had been learned, Morris removed the platform and tested to see if rats remembered where the platform had been. He called this a transfer trial, which is now more commonly called a probe trial. On this trial, one gives the animal a fixed length of time to search for the platform's former location and records the animal's spatial bias for the goal area.
Later Morris developed the test further (Morris 1984). One of the first things he did was increase the pool diameter. The first maze was 132 cm in diameter, but he then enlarged it to 214 cm in diameter. He also introduced curtains around the pool during cued trials to reduce the animal's ability to use distal cues. Several two-platform discrimination procedures were also described, although these have not been widely adopted. The utility of the MWM was shown thereafter, beginning when Morris and colleagues published a series of experiments that lesioned the hippocampus and associated pathways (e.g., Morris et al. 1982); he later used pharmacological agents to disrupt hippocampal function (e.g., Morris, Anderson, et al. 1986) and showed that if the hippocampus was sufficiently disrupted, allocentric navigation was severely impaired.
Within a few years of Morris's original paper, more detailed methods on the test were published (Stewart and Morris 1993). The use of the maze by other laboratories expanded rapidly, especially in mice after the development of pronuclear injection and homologous recombination methods to create transgenic, knockout, and knock-in genetic models, areas that are rapidly expanding with the development of zinc-finger, TALEN, and CRISPR/Cas9 methods of creating new genetic models more efficiently (Park and Telugu 2013; Sampson and Weiss 2014). As the number of genetic models tested in the MWM proliferated, the number of procedural variations also increased. Unfortunately, many users of the maze fail to include all of the procedures that permit interpretation of differences and account for potential performance factors, as has been noted elsewhere (Vorhees and Williams 2006).
There have been a number of reviews on how the MWM has provided insight into the neural basis of allocentric learning and memory (Brandeis et al. 1989; Cain 1997; Cain and Saucier 1996; D'Hooge and De Deyn 2001 McNamara and Skelton 1993; Morris 1993; Morris, Hagan, and Rawlins 1986). There have also been a number of studies showing effects that can alter MWM performance that are not the result of deficits in allocentric learning. For example, N-methyl-D-aspartate (NMDA) antagonists impair sensorimotor function and degrade MWM performance (Cain and Saucier 1996; Cain et al. 1996; Hoh and Cain 1997), but these effects can be eliminated by nonspatial pretraining (Bannerman et al. 1995; Saucier et al. 1996). This is one of the important lessons of using this or any test of learning; careful control procedures are needed to ensure that differences are what they appear to be and not secondary to confounding factors.
A variation of the MWM was introduced later in which lights (beacons) were hung from wires directly above the platform and the other three equivalent positions in the cardinal quadrants of the pool. Control rats with sham lesions used these beacons to find the platform's previous location on probe trials, whereas rats with electrolytic ablation of the hippocampus could not (Clark et al. 2007). These data indicate that more was lost in the hippocampal-lesioned group than spatial navigation alone because the presence of the additional information of the beacons was insufficient for the lesioned group to find the platform efficiently.
Radial-Arm Maze
In contrast with the MWM, the RAM has a central hub and arms radiating out from the center, like spokes of a wheel (Olton and Samuelson 1976). The maze typically has no cover or may have a clear acrylic cover so that distal cues are visible. Typically the entire maze is elevated above the floor. In most instances the task is appetitively motivated. There are two main procedures: (1) the working memory and (2) the working/reference memory versions. The former is the original RAM method and was designed with eight arms. Although the number of arms in use today varies from the original, in all cases the conceptual basis of the test is the same. Arm numbers in the literature range from as few as four, five, or six, to the standard number of eight, and up to 12 or 17. The larger number of arms is intended to make the task more difficult and to improve the method, especially when using the dual working/reference memory version.
The RAM requires training before the assessment of learning. Before training, food restriction is required to induce motivation along with exposure to the rewards so animals are familiar with them before being placed in the maze. Training involves placing baits throughout the maze to encourage searching. Once this is learned, test trials begin by placing an animal in the center and allowing it to explore with a single reward at the end of each arm. Data recorded are which arms the subject visited once versus those visited more than once up to the limit that once all baits are obtained the trial ends. Revisits are scored as errors (i.e., as failures to remember that an arm was already visited on that trial). Because the arms are rebaited for each trial, there is no memory from a previous trial that provides information on which arms to visit and in which order during the next trial; hence, only short-term, trial-dependent memory provides information on which arms remain to be visited and which arms have already been visited. Because the test measures trial-dependent memory, it is an assessment of working memory and, more specifically, spatial working memory because the principal cues to guide arm choices are outside the maze. The problem is that animals may solve the maze in ways other than relying on spatial working memory. One of these is use of chaining or a serial strategy (i.e., entering each arm successively in a systematic order). An example would be to always turn right or always turn left and enter the adjacent arm. This strategy is efficient but circumvents the purpose of the test. Running the test this way, with free access to all arms, is common but may not measure working memory. Experimenters may have an observer watch the animal's performance and report on whether they observe chaining (also called stereotypic patterns). The problem is that irregular chaining patterns can be difficult to distinguish from working memory. For example, an animal may go from arm 1 to arm 3, then arm 4, arm 6, arm 8, arm 2, arm 5, and arm 7. Although not a successive pattern, the animal in this example is using a right-turn strategy.
Another issue in the RAM is that, because food restriction is necessary, one has to ensure that animals in experimental and control groups are equally hungry and hence equally motivated to search for food, which are important issues in neuropharmacological and neurotoxicological experiments. This can be problematic if the treatment reduces body weight or suppresses appetite or palatability of food. Equating the incentive value of the reinforcement is often not tested, leaving questions about how well matched the groups may be. If motivation and reward value are equal, how can one ensure that working memory is being used and not another strategy? The best way to prevent chaining is by interfering with sequential choices. This can be done by imposing a delay between arm choices. This requires that after the subject enters an arm all remaining doors are closed to the other arms to prevent an immediate entry into another arm. Once the animal reenters the center, the door it exited is also closed so that all arms are blocked for a specified confinement period. After the confinement is over, all doors are opened simultaneously, permitting the subject to make a new choice and requiring the animal to hold in working memory the arm it most recently visited. There are data showing that imposing an intertrial confinement interval prevents chaining (Dubreuil et al. 2003). Another issue with the RAM is the one Morris described; the maze has within it proximal and olfactory cues, which the animal may use to guide its choices, thus making it not a clear test of distal cue use compared with the MWM where these two factors are eliminated. Another limitation is that the RAM has a narrow sensitivity range. Although in theory animals could make many errors before obtaining all eight baits, in practice animals do not make large numbers of errors, so the total number of errors, even at the beginning of the test, is often not much greater than the number of arms This limits the range of scores possible, which, in turn, has the effect of compressing the range to demonstrate group differences caused by the independent variable.
The second version of the RAM is the combined working/spatial memory version. In this procedure, some arms are baited on each trial and some are not. Optimally, the animal should visit only the always-baited arms and never the always-unbaited arms. As before, revisits to baited arms after the bait is taken are scored as working memory errors and entries into never-baited arms are scored as reference (long-term) or trial-independent errors. This is an effective procedure to measure both types of memory within the same test and is widely used. One disadvantage is that if one uses three or four of the arms for reference memory, that leaves only four or five arms for the assessment of working memory, which makes the sensitivity range even narrower than it is in the eight-arm working memory version. This has motivated the invention of 12- and 17-arm mazes to allow for four or more unbaited arms while retaining eight or more baited arms for the assessment of spatial working memory.
In a review of the RAM compared with the MWM, it was noted that rats learning the RAM learn both spatial and associative aspects of the task because of the internal structure of the maze and olfactory cues, which provide them with more information than is available in the open pool used in the MWM. It was also noted that learning is much slower in the RAM than in the MWM, which was interpreted as an advantage in one sense because slower learning makes for a more protracted learning curve, which, in turn, makes deviations in the slope of the curve more apparent. But the disadvantage of longer learning times and nonspatial learning components is that interpretation of RAM data can be challenging (Hodges 1996). Hence, Hodges (1996) concluded that the MWM was preferable for assessing spatial navigation and the RAM better for assessing spatial working and associative learning together. The RAM can also assess working and reference memory, along with associative learning, all at once. The RAM also requires more training before conducting test trials than the MWM.
To avoid some of the problems with appetitive tasks mentioned above, swimming versions of the RAM have been developed (i.e., radial-arm water mazes [RWM]) (Figure 1). As with the RAM, there are different versions of the RWM with different numbers of arms. There are also a variety of protocols. One that closely models the appetitive RAM uses an intertrial interval to ensure that chaining does not occur (Bimonte et al. 2000). In this version, one arm serves as the start and the other seven (or more) each have submerged platforms at the ends. On the first trial, the animal can enter any arm and find an escape platform, just as in the appetitive RAM where any choice leads to food. The animal is then removed for a specified length of time and the platform it found is also removed. On the second trial, if it remembers the arm it chose, it should not reenter that arm but choose another one. This process continues until the animal finds all platforms. This can be adapted just like the appetitive RAM such that some arms have platforms at the start of each new trial and others never have platforms. The RWM requires about 10 days for rats or mice to become proficient. There are suggested rapid protocols for the RWM (Alamed et al. 2006), but a word of caution about these is that they are designed as quick tests of reference memory only.
Figure 1.
Radial-arm water maze (RWM). Depiction of the RWM with eight arms radiating from a central hub. One arm is always the start, and hidden platforms are located in each of the remaining seven arms at the start of a new session/day (not depicted). After each hidden platform is found, the rat remains on it for 10 seconds and is then placed in a holding cage while that platform is removed. For trial 2, the animal is placed back in the start arm and allowed to freely choose once again. Unlike the appetitive radial-arm maze, in which the animal has no incentive to return to the last arm visited, in the RWM the animal is reinforced to revisit the arm it just found because it escaped at that location on the previous trial. This creates an initial increase in errors in the water version until the rat learns a win-switch or nonmatching to sample rule to not return to the previously visited arm. In this drawing, there are T-shaped structures attached to the floor so the maze may be used in a different configuration by insertion of a structure that rests against these T-shaped guides that are not relevant to the maze's use as a RWM because the T-guides are far below the water level. Drawing courtesy of AB Plastics, Cincinnati, Ohio; reproduced with permission.
It should be noted, however, that although the RWM and RAM both assess spatial memory they also possess some fundamental differences. In the RAM, once the bait has been consumed from an arm, the animal has no incentive to search that arm again because it has no expectation that food would reappear, whereas in the RWM there is an incentive to return to the last arm visited because it found an escape there. This leads to the probability that the animal will make a last-arm-visited error on the next trail; this is unlikely in the RAM because the last arm visited is disincentivized by consumption of the reinforcement. Hence, in the RWM the animal learns a different rule than in the RAM or is prepared differently for the next step.
Other Mazes
The MWM and RAM are not the only mazes devised to assess spatial learning and memory; many others, including T mazes, have been developed.
T mazes
T mazes were the first mazes and can be used to test spatial working memory. There are many test procedures, but perhaps the simplest for assessing spatial learning and memory is to reward animals for turning in one direction across a series of trials. To test whether the animals learned a position habit, such as always turning right, or a spatial position, the maze is rotated 180o on the test trial such that if the animal is using habit it will turn right regardless of where that arm is within the room, but if it learned a place, it will turn left so as to end up in the same place in relation to distal cues. One can also introduce reversal trials to further test that location and not habit is controlling choices.
Hole-board maze
Another maze-like test of spatial learning and memory is an appetitive hole-board task (Post et al. 2011). Hole-board type tests rely on the same concept as the MWM (i.e., having an open-field design, usually square, that provides distal cues while being uniform within the apparatus to prevent use of proximal cues). In the design described by Post and colleagues (2011), the test arena has 25 uniformly positioned holes in the floor. The procedure is appetitive and therefore requires food restriction and reduction in free-feeding body weight to 80–85% of normal weight, as with the RAM. Five of the holes (in an irregular pattern) are baited on every trial, leaving 20 holes always empty. Over the course of 5 days of training with 6 trials per day, the authors report that C57BL/6 mice become proficient at the task. Moreover, they could distinguish spatial working versus reference memory effects in the experimental group.
Star maze
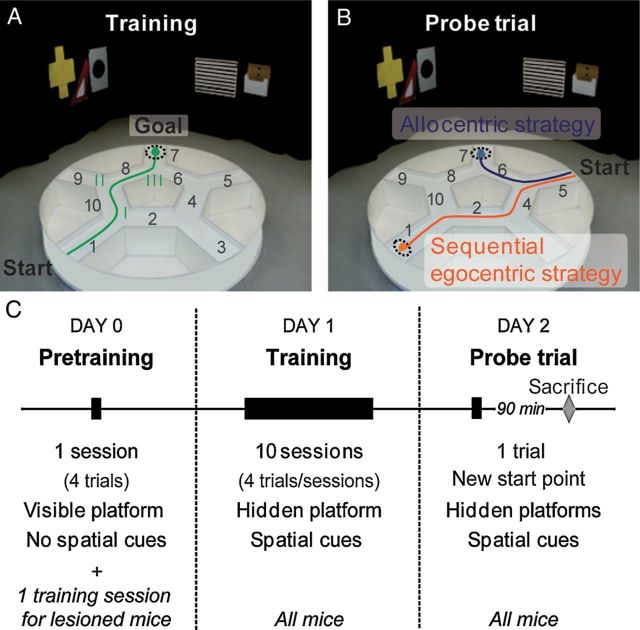
An interesting newer water maze, the star maze, has been introduced that offers some novel features (Fouquet et al. 2013; Rondi-Reig et al. 2006). This cleverly designed swimming maze has five arms and a central pentagonal interior such that animals cannot swim directly from one arm to the next but must swim around the pentagon. One arm is always the start and one arm always the goal (Figure 2). The procedure begins with a training phase in which the goal arm contains a visible platform with distal cues removed. This teaches escape. The learning phase differs from training inasmuch as the platform is submerged. The pentagon is arranged such that each corner faces an arm and each wall is decorated with different designs to provide differential proximal cues with right/left information. Once animals have mastered the hidden platform task, they are given a probe trial starting from a different arm. If, for example, the start arm is designated arm 1, the remaining arms and adjoining segments numbered counterclockwise from 2 to 10, and the goal is in arm 7, the animal in this case would learn that the most efficient path is to turn left at the end of the start arm, turn right around the first corner of the pentagon, and turn left to enter arm 3 (sequence 1-10-8-7 in Figure 2). For the probe trial, the animal is started in arm 5. If the animal is using distal, spatial cues, it should turn right and swim directly to arm 7 (sequence 5-6-7). If it is using egocentric cues, it should go left-right-left (sequence 5-4-2-1), as it did during learning, which would lead it back to arm 1. If it is using what the authors called a guidance strategy, it will follow the pattern displayed on the pentagonal walls. Because the path from arm 1 to arm 7 during the learning phase was a speckled wall at the first turn and a solid black wall at the second turn, if the animal follows the same wall patterns, it should now go 5-6-8-9, ending in arm 9, well off from where it would go using either an allocentric or egocentric strategy. If the animal does not use cues but merely searches every arm in sequence, always going left, it will reach the goal in arm 1 but will enter arm 3 en route, following the sequence 5-4-3-2-1, a chaining strategy.
Figure 2.
Star maze. The Star maze is run in three phases consisting of the following: Day 0 (pretraining): Run with no intentional distal cues present and a visible platform located in arm 7 with the start position always in arm 1. Day 1 (training): Run with intentional distal cues attached to surrounding curtains and the platform located in arm 7 and submerged. This phase continues for 10 sessions, with four trials per session. Day 2 (probe): The animal is now started in arm 5 with hidden platforms in arms 7 and 1 to determine which arm they chose but reinforcing either equally. Reprinted from Fouquet et al. (2013) and reprinted here with permission of the senior author (Dr. Laure Rondi-Reig).
These authors found that the guidance and chaining strategies were seldom used and that mice divided themselves such that some used an allocentric and some an egocentric strategy. By comparing the proportion of wild-type mice using these strategies with the proportion of NMDA receptor knockout mice, they found a shift away from allocentric and toward egocentric strategies in knockout mice but that both types of learning were affected, indicating once again that allocentric and egocentric networks overlap, such that knocking out NMDA receptors in multiple brain regions produces mixed effects on both types of navigation. This is not a surprising finding, but worth bearing in mind when interpreting navigational data.
Nonspatial Assessment
Distinguishing between allocentric and egocentric learning is the subject of ongoing investigation. Given this, it is worth mentioning that labyrinthine mazes, when used in the dark (Vorhees et al. 2008; Vorhees, Schaefer, et al. 2009; Vorhees, Skelton, et al. 2009) or by blindfolding animals to eliminate distal cues (Maaswinkel and Whishaw 1999), have proven effective in dissociating egocentric from allocentric navigation.
However, the neural substrates of egocentric navigation are less well known. Part of this stems from the fact that place cells in the hippocampus, which play a key role in allocentric navigation, were discovered long ago (Bliss and Lomo 1973; Malenka and Nicoll 1999) and have been the subject of great interest. This was followed by experimental evidence that blocking LTP in the hippocampus results in impaired allocentric learning and memory. At the molecular level, NMDA receptors are required for the expression of LTP (Malenka and Nicoll 1999), and NMDA inhibitors suppress LTP and impede allocentric learning and memory, thereby establishing a functional link that associates molecular signals to electrophysiological cellular events to behavioral outcomes. Extensive research on the hippocampus, including its anatomy, interconnections to surrounding structures, electrophysiologic properties of cell types within it, neurotransmitters, receptors, and modulatory molecules involved that mediate LTP (and related phenomena: short-term potentiation and long-term depression) have made this the best understood learning and memory system (van Strien et al. 2009). Through interconnections between the hippocampus, entorhinal cortex, and subiculum, the wider network that collectively maps spatial locations has provided an extensive understanding of spatial information processing (van Strien et al. 2009). A similar network has not, however, been described for egocentric navigation.
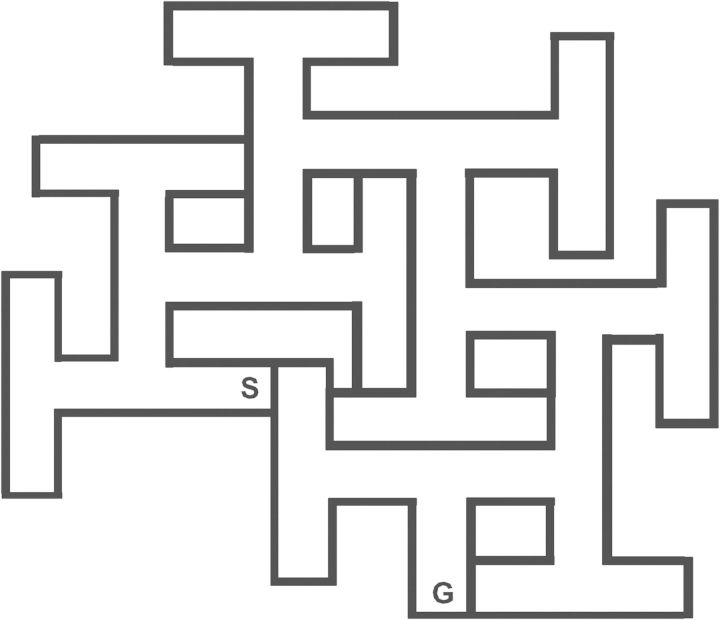
As noted, labyrinthine mazes are one way of testing egocentric navigation because they contain proximal cues and rely on self-motion related to distance, direction, and junctional signposts. There are many types of mazes but the interpretation of what they show is difficult if distal cues are present. As noted, the principal solution to this is to test animals in the dark, as was done in more recent studies using the Cincinnati water maze (CWM) (Vorhees 1987; Vorhees et al. 1991) under infrared light (Vorhees et al. 2008) (Figure 3). A related approach used a circular arena with a home base from which animals could exit, explore, and find food located at distal points. When tested under lighted conditions, animals could find and carry the food back to their home base by allocentric navigation, whereas when distal cues were removed by being tested in the dark, animals could perform the task by switching to egocentric navigation instead (Whishaw et al. 2001). Thus, whether the task uses an open arena or labyrinth, the key feature for separating the two forms of navigation is eliminating distal cues to force animals to use egocentric navigation.
Figure 3.
Cincinnati water maze (CWM). Schematic drawing of the CWM. Channels are 15 cm wide throughout. The maze is constructed of black, high-density polyethylene with chemically welded seams (AB Plastics, Cincinnati, OH). Walls are 51 cm high, and the water is filled to a depth of 22 cm. The maze is water-tight and mounted on leveling legs 25 cm in height. “S” is the start location, and “G” is the goal location. A platform submerged 1 to 2 cm below the water surface is positioned at point G. Errors of commission are defined as when an animal's head and two front legs pass an imaginary line between the main channel into the stem of a T, when its head and front legs cross a line into either arm of a T, or when an animal reenters into the start corridor after having left it. Rats receive two trials per day with a trial limit of 5 minutes. Errors and latency to escape are recorded. Data are analyzed as two-trial (day) blocks. Under white light testing, it typically takes 5 or 6 days for proficient learning. Under infrared light, it takes about 15 days. If a rat fails to find the escape within the time limit, it is placed on the platform for 10 seconds and then given a 5-minute rest before trial 2. If a rat finds the escape in less than 5 minutes, it is given trial 2 after 10 seconds on the platform.
Features of the MWM
As noted, there are several features of the MWM that have proved beneficial for using it to assess allocentric navigation: (1) rodents are natural swimmers but still prefer to be out of water, and thus swimming provides sufficient motivation for animals to actively search for an escape; (2) it is relatively easy to construct a pool that is uniform in shape and featureless on the inside, thereby eliminating proximal cues, but rich with distal cues outside the pool in the surrounding room, the ideal arrangement for the organism to rely on allocentric navigation; (3) the task is amenable to assessing proximal cue navigation by using a visible platform while obscuring distal cues with curtains around the pool; and (4) by assessing swim speed during learning one can determine whether animals in all groups are equal in their ability to swim and motivation to escape. The above features are part of why the MWM is the most widely used test of learning and memory. To illustrate, a search on PubMed (December 2013) for “Morris water maze” returns 5196 citations compared with 1913 for “radial-arm maze” and 1869 for “T maze test.”
Advantages
Water mazes in general and the MWM in particular, have advantages over other learning and memory tasks. First, water is an equal-opportunity motivator that balances motivation to escape over a wide range of body weight differences among groups of animals (Cravens 1974), an effect that does not apply to appetitive tasks that are inherently problematic when a treatment causes differences in body weight, appetite, or the reward value of the reinforcer.
Second, in rats, 100% of the animals complete the task, and in mice (in most strains), nearly 100% complete the task; this avoids the problem of selection bias that is a common problem in appetitive tasks where there can be significant dropout rates.
Third, in the MWM, 100% of control rats and nearly 100% of mice (in most strains) master the task; this aspect of the task is important because flat or shallow learning curves may not only prevent one from detecting group differences, but they also raise concerns about the validity of the test. Every standard rat strain tested in the MWM shows good learning, as do many mouse strains, but in mice, there are strains that show poor learning and may not be good experimental choices (Clapcote et al. 2005; Crabbe et al. 1999; Lipp and Wolfer 1998; Upchurch and Wehner 1988; Wahlsten et al. 2005).
Interestingly, when MWM data were analyzed in a large dataset of 1500 mice by factor analysis, the principle factors affecting MWM performance in mice were noncognitive (Lipp and Wolfer 1998). Factor 1 showed that measures related to thigmotaxis, percentage of time in goal quadrant, escape time, and time floating accounted for most of the variance. Factor 2 included swim speed and time floating. Factor 3 included percentage of time in goal quadrant and probe trial performance. Factor 1 accounted for 48% of the variance, factor 2 accounted for 20%, and factor 3 accounted for 13%, indicating that in this large mouse dataset most of the variance was accounted for by nonspatial influences (factors 1 and 2 together being 68% of the variance). It is important to note that this is not the case in rats, but the fact that performance factors are salient in mice provides an important cautionary note when interpreting mouse MWM data.
This and other factors have led to other indices being developed to better differentiate learning from other factors in the MWM. One is a proximity index typically called cumulative distance from the platform, defined as path length divided by latency. Another is an adjustment to cumulative distance called search error (Gallagher et al. 1993) and later named corrected integrated path length (CIPL) (Barnes et al. 1997). CIPL scores behavior in terms of deviation in distance traveled from the start to the goal relative to an efficient, straight line path, adjusted for average swim speed. The CIPL adjusts path for average swim speed and corrects for the fact that start positions are different distances from the platform. Unfortunately, CIPL as implemented in some software, may not be valid because it generates positive and negative values. If corrections to path-speed calculations result in positive and negative values, net CIPL can be small or zero despite large deviations. Moreover, if the animal does not reach the platform during a trial, the value for CIPL will be much larger than that for animals that do reach the platform. If the CIPL is used, it is probably best reserved for late trials when trail failures are no longer occurring. This brings up an important issue when testing mice: some investigators indicate that if mice float they prod or push them. Nonsystematic experimenter interventions such as this may have unintended consequences. We avoid this as follows: If a mouse floats, it is left alone. If it floats the entire trial, it is removed and is given a second trial later. If it still floats on the second trial, it is given up to two trials the next day. If it never searches, it is eliminated. In our experience, dropouts using this procedure are rare.
A fourth advantage is the minimal training required for the MWM and water mazes in general. Because rodents are natural swimmers, only a few trials are needed for animals to learn that active searching leads to escape and, once found, leads to removal from the pool. The simplest and most effective way to introduce the task is to give cued trials first with a visible platform. On the first few trials, the animal learns that the only prominent cue within the pool is the platform and the platform is the escape. Mice require a few additional trials sometimes to learn to remain on the platform rather than climbing on and jumping off again. Mice show improved performance on the hidden platform version if the cued version is given first to eliminate these off-task behaviors.
A fifth advantage is that the MWM and most swimming mazes are efficient compared with other learning tests (i.e., within a few days, with relatively few trials per day, and relatively short times per trial, rats and mice show good learning curves). This is different from appetitive mazes. For example, a typical MWM procedure may involve four trials per day for 4 to 6 days with a time limit per trial of 90 or 120 seconds to obtain good learning. Appetitive mazes may have similar numbers of trials per day but require more days of testing. They also require more training days and longer times per trial. This is because in appetitive mazes animals exhibit off-task behaviors such as sniffing, grooming, looking, rearing, urinating, defecating, and so on that interrupt searching for the goal; these behaviors are not seen in water mazes.
A sixth advantage is that water is as motivating on the last trial as it is on the first, whereas appetitive tasks are subject to satiation effects. The more trials given during a test session, the more rewards the animal consumes, which reduces hunger and motivation. Experimenters typically try to minimize this by making the rewards highly palatable but quantitatively small in proportion to the fasted animal's hunger. This, combined with limits on the number of rewards available per test session, reduces the influence of declining appetite as testing proceeds. This is effective at keeping most animals working for the rewards but requires a high level of hunger to maintain motivation and places boundaries on daily session length.
Disadvantages
One disadvantage already mentioned is caused by species-specific response characteristics in some strains that are not conducive to the task requirements. This is most often seen in inbred strains of mice that float or exhibit persistent thigmotaxis rather than active searching for the goal throughout the area of the pool, but this also applies to shock and appetitive tasks. For example, some strains of rats do well in shock avoidance tasks because foot shock elicits running, whereas in other strains it elicits freezing; freezing interferes with learning to escape or avoid shock because the task requires the animal to move to the opposite compartment to experience the reinforcement, which leads to learning the task contingencies (Barrett et al. 1973; Barrett et al. 1974; Caul and Barrett 1973; Ray and Barrett 1975).
Another disadvantage is that of working memory. Working memory versions of the MWM have been described by using new platform positions each day and looking for savings between the first sample trial and performance on subsequent trials given thereafter (matching to sample). However, these methods have not proven to be as sensitive as other tests and are not widely reported (but see the radial-arm water maze as a way around this limitation of the standard MWM).
The most often stated criticism of the MWM (and all swimming mazes) is that they are unduly stressful. The implication is that stress is always negative and is to be avoided at all costs. Food restriction has also been shown to activate stress responses in animals, as has exposure to novel environments (Armario and Jolin 1986; Coover et al. 1984; Garcia-Belenguer et al. 1993; Heiderstadt et al. 2000; Honma et al. 1986; Johansson et al. 2008; Marinkovic et al. 2007; Pesic et al. 2010). However, stress is not always negative. Stress in relation to performance is an inverted U-shaped function. Too little as well as too much is counterproductive to performance, but in the midrange there are levels of stress that optimize performance. What critics of water mazes omit is that assessing learning and memory requires an appropriate level of motivation (or stress) to incentivize performance. How are optimal levels of stress-related motivation determined? Most learning tests are validated empirically. Using this criterion, because most rodents show high levels of learning in the MWM and high levels of retention of platform location, it is difficult to suggest that the task is overly stressful.
What is the evidence that the MWM is stressful? One study compared singly housed with group-housed rats on MWM learning (Wade and Maier 1986). Three weeks of isolation housing caused rats to learn the MWM more slowly than group-housed rats, but this does not show that swimming is counterproductive, only that preexisting stress slows learning. In a second experiment, group-housed rats were compared with rats singly housed for 1, 2, or 3 weeks or 3 weeks followed by a loud noise. The 3-week isolation group learned the MWM more slowly than group-housed rats, whereas results did not differ for the 1-week isolation group. The 2-week isolation group was intermediate, but curiously the 3-week plus noise group performed as well as group-housed controls. The fact that compound stressors reversed the effect of 3-week single housing shows that simplistic explanations of how stress affects MWM learning are not straightforward. Current National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) housing requirements ensure that animals are not isolation housed (except when there are specific experimental reasons to do so), and AAALAC guidelines require within-cage enrichment, thereby reducing stress.
In another study, nonhandled versus handled rats were compared for MWM learning (Holscher 1999). Handled rats learned more rapidly than nonhandled rats. A further experiment showed that rats tested in an RAM improved subsequent MWM performance. Hence, inducing stress before testing affects MWM learning, but pretesting stress affects all types of learning, not only MWM learning. One factor about stress that is important is whether a stressor is escapable. Inescapable stressors, such as inescapable shock, lead to learned helplessness (Anisman and Merali 2001), which is used to model depression (Yan et al. 2010). Similarly, extended periods of inescapable water confinement, as in the Porsolt forced swim test of swimming despair, also induce a form of immobility resembling helplessness and are similarly used to model depression-related effects (Porsolt, Bertin et al. 1977; Porsolt et al. 1978; Porsolt, LePichon et al. 1977). However, in the latter case, the test environment is designed to maximize frustration by making the swimming chamber small, the walls high, and the confinement long to induce defeat, very much in contrast with the MWM, in which escape is reinforced. Also, in the MWM trial lengths are short compared with the Porsolt test. In addition, if the animal fails to find the platform, it is not allowed to remain in the water and become fatigued or discouraged but is guided or lifted to the platform to prevent the onset of defeat behaviors from developing.
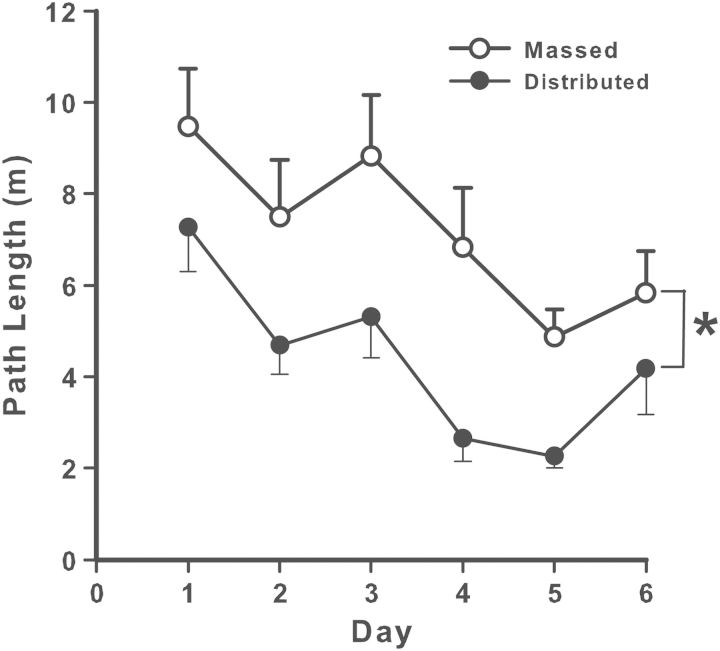
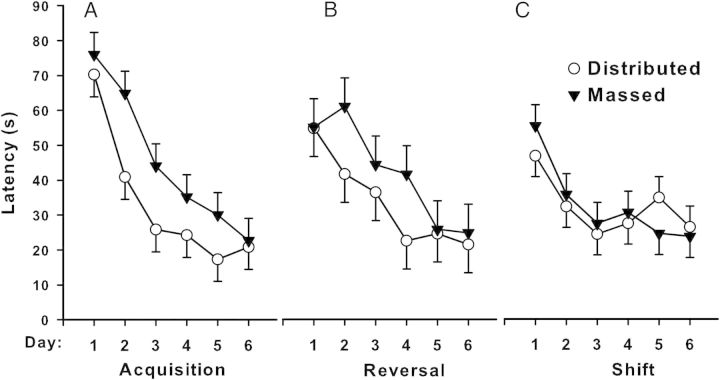
We compared rats tested in the MWM to a group of untested rats. Separate groups of rats had blood samples collected 0, 30, 60, or 90 minutes after removal from the maze. Those tested showed increased plasma corticosterone compared with those not tested. The elevation was approximately double in the group assayed immediately after the last trial. At 30 minutes, corticosterone levels were 30% above control rats, with no differences by 60 and 90 minutes (Skelton et al. 2007). In another experiment, three groups of male C57BL/6J mice were matched for dominance and assigned to groups either not tested or tested in a MWM or Barnes maze for six trials per day for 5 days. Thirty minutes after the last trial, mice were anesthetized with isoflurane, and blood was collected. Relative to untested mice, maze-tested groups had increased plasma corticosterone. The Barnes maze group showed a 4-fold and the MWM a 5-fold corticosterone increase after testing. Errors in the Barnes maze did not correlate with corticosterone levels, whereas search error, latency, and path length in the MWM were significantly correlated with corticosterone levels. It is important to bear in mind that this experiment used a massed practice method (i.e., trials were given back-to-back) (Harrison et al. 2009), which is known to cause reduced body temperature in mice and interfere with learning. Mice exhibit significant core body temperature reductions using closely spaced trials, and this likely accounts for the correlations found for the MWM in this experiment. Because it is known that both distributed practice with longer intertrial intervals and preventing hypothermic conditions improve learning, today's common practice of using spaced trials in the MWM obviates this concern.
Flexibility
The MWM is adaptable to many experimental conditions in that a number of variations of the basic visible and hidden platform versions may be used. Morris introduced working memory and discrimination versions of the maze (Morris 1984; Stewart and Morris 1993). Another method was developed to distinguish hippocampally mediated from striatally mediated learning in the MWM. In this case, dual targets were used; two balls of the same size and large enough for the animal to stand on were painted with different striped patterns and positioned above the water. In the spatial version, the correct ball was always in a fixed location and provided a firm escape platform, whereas the incorrect ball was placed in different quadrants on every trial and sank if climbed. In addition, the pattern on the balls was changed so that pattern did not provide differential cues. Start positions were randomized. Rats with hippocampal lesions could not learn the task but control rats did. In a second version, the correct ball always had the same pattern on it whereas the incorrect ball had a changing pattern and both balls were moved; the correct ball was moved every fourth trial and the incorrect ball every trial. Rats with striatal lesions could not learn this version but hippocampally lesioned rats could. This represents a useful way that the MWM can be changed to get at different types of learning (Packard and McGaugh 1992). Another approach is to place an insert into the pool to create a structured configuration. One of these is the four-arm plus water maze. When comparing inbred strains of mice, it was found that the plus configuration produced faster learning than the standard open version (Wahlsten et al. 2005). Although the plus water maze may have advantages in poorly performing inbred strains of mice, there is no evidence that it has value in rats or most strains of mice, and it introduces proximal cues that are an issue in the RAM that the MWM was designed to eliminate.
Procedures for the MWM
There are multiple methods for running the MWM. It is worthwhile to review the original descriptions of Morris before deciding on apparatus design and testing procedures. The three most salient papers in this regard are Morris's foundational paper (Morris 1981) and his two methods papers (Morris 1984; Stewart and Morris 1993). Subsequent MWM protocol papers that provide more detailed guidance and some methodological improvements have been published (Vorhees and Williams 2006; Wenk 2004).
Pool and Platform Size
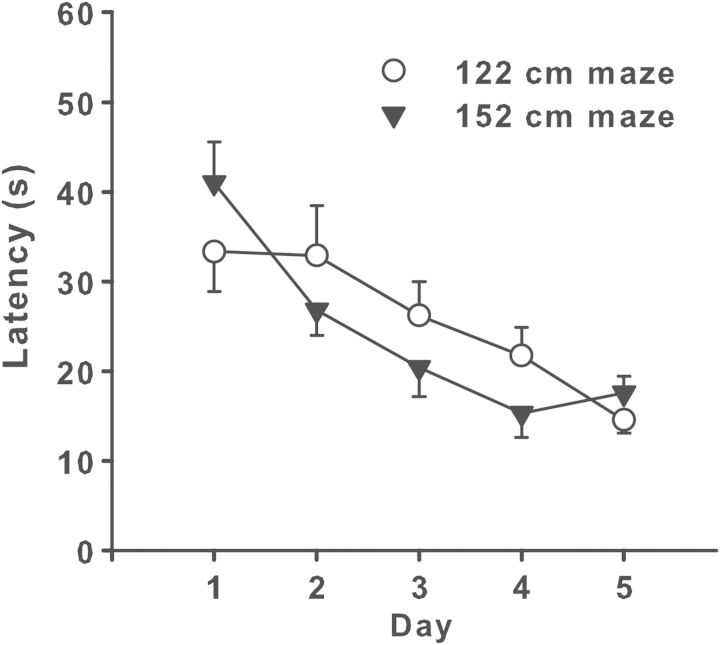
There are no exact standards for what size the pool or goal platform should be. It is noteworthy, however, that Morris's first maze was 132 cm in diameter but by the time he published his first methodological paper, he increased it to 214 cm in diameter in subsequent experiments. There has been very little systematic study of pool size as a factor in allocentric navigation. We have shown that rats learn faster in a 122-cm pool than in a 210-cm pool using identical conditions (platform position and start positions) (Vorhees and Williams 2006). Although this is not surprising, the steepness of the learning curve in the 122-cm pool clearly showed that when the pool is small the task is very easy, and this may preclude seeing deficits in the rate of learning where control animals reach asymptotic performance by day 2. There may be other risks if the pool is too small as well. There is a report indicating that if the pool is too small in relation to the goal size, rats solve the maze without using spatial cues (Mactutus and Booze 1994). At the other extreme, the pool can be too large. We recently constructed a 244-cm diameter maze and have established that rats can learn in this large environment without difficulty (Williams et al. 2014). Therefore, the upper boundary on pool size has not yet been established for rats. There are MWM pools smaller than 122 cm [e.g., 117 cm in diameter (Clapcote et al. 2005); 109 cm in diameter (Stackman et al. 2012)]; however, 122 cm is the most commonly used size for adult mice (Maei et al. 2009; Nguyen et al. 2000; Schaefer et al. 2009; Schaefer et al. 2011; Silva et al.1992; Skelton et al. 2011; Upchurch and Wehner 1988; Vorhees and Williams 2006). There is evidence that mice cannot learn when placed in a 210-cm diameter pool (Schaefer et al. 2011). We compared a 122-cm diameter pool to a 152-cm diameter pool in wild-type C57BL/6J mice. The mice received cued trials first for 6 days (1 day with the start and platform in fixed positions for six trials and 5 days with random start and platform positions for two trials per day). The result of the hidden platform trials are shown in Figure 4 when the mice were given four trials per day for 5 days. As can be seen, the mice in the smaller maze found the platform slightly faster on day 1, but after that both groups showed similar rates of improvement through day 5. Similar results in C57BL and DBA mice in a 150-cm diameter pool have also been reported (Sung et al. 2008).
Figure 4.
Morris water maze (MWM) performance in mice in different size tanks. C57BL/6J adult male mice were randomly divided into two groups of equal numbers and tested in either a 122-cm or 152-cm diameter tank. The maze conditions were identical for both groups. The smaller diameter was achieved by placing an inner ring within the larger diameter tank constructed of the same polypropylene material as the outer tank. Both groups first received cued training trials first for 6 days. Training consisted of 1 day with the start and platform in fixed positions for six trials and 5 days with random start and platform positions for two trials per day. After this, mice received 5 days of acquisition to find a fixed hidden platform for four trials per day from randomized start positions balanced such that they received one trial from each of four start positions each day. The platform was in the SW quadrant and start positions were arranged such that two were from cardinal positions (N and E) and two were from distal ordinal positions (NW and SE) to eliminate very close start positions (W and S). Average latency to reach the platform is shown (mean ± SEM). Because both groups were untreated, latency, path length, and cumulative distance indices all gave the same results. No group differences occurred on training trials. The effect of the pool was not significant. Group sizes were 12 per group.
Search Area to Target Ratio
Absolute pool diameter, although important, may be only part of what determines task difficulty. The other factor is the size of the goal. Although 10-cm diameter platforms are the most common, there are many that are larger or smaller. One way to conceptualize the difficulty of the task is the relative size of the pool in relation to the area of the platform because pool size reflects the area over which the animal must search to find the platform. Clearly, the larger the pool and smaller the platform, the more difficult the task becomes; therefore, considering the ratio of pool surface area to platform surface area permits a systematic examination of this factor. Using a 10-cm platform and a 122-cm diameter tank, the ratio of pool area to platform area is 149:1 (Table 1). Many mouse strains learn well at this ratio.
Table 1.
Morris water maze pool-to-platform area ratio
| Pool diameter (cm) | Platform diameter (cm) | Pool-to-platform ratio |
|---|---|---|
| 122 | 10 | 149:1 |
| 7 | 304:1 | |
| 5 | 596:1 | |
| 156 | 10 | 243:1 |
| 7 | 496:1 | |
| 5 | 975:1 | |
| 183 | 10 | 335:1 |
| 7 | 741:1 | |
| 5 | 1342:1 | |
| 210 | 10 | 454:1 |
| 7 | 925:1 | |
| 5 | 1818:1 | |
| 244 | 10 | 596:1 |
| 7 | 1214:1 | |
| 5 | 2386:1 |
To illustrate this, mice on a mixed Swiss Black × 129/Sv background were trained in either a 122-cm pool or a 210-cm pool with 10-cm platforms. The larger pool has a pool-to-platform ratio of 441:1. Mice learned well in the 122-cm pool but showed almost no learning in the 210-cm pool (Schaefer et al. 2011). This experiment showed a further effect of task difficulty. The mice that learned well in the 122-cm pool with a 10-cm platform were then tested on reversal with the platform placed in the opposite quadrant and the platform reduced to 7 cm in diameter, making the surface-to-platform ratio 304:1. The mice were slow at first but gradually learned to locate the platform, even though they never reached the same level of performance they showed with the 10-cm platform. The mice were then tested with the platform moved to an adjacent quadrant and the platform reduced to 5 cm, creating a surface-to-platform ratio of 596:1, a ratio even higher than that for mice tested initially in the 210-cm pool. With this high 596:1 ratio, mice showed modest improvement (Schaefer et al. 2011). Therefore, mice cannot learn at high ratios (444:1) if started at this level of difficulty but can learn at ratios higher than 149:1, such as 304:1, if given an easier version first. These data suggest that pool-to-platform ratio can provide useful information on task difficulty and indicate that some ratios are counterproductive to spatial navigation in mice. Another experiment compared three inbred strains of mice, C57BL, BALBc, and 129/SvEvBrd (Van Dam et al. 2006), in 75-, 120-, or 150-cm diameter pools with platforms of 9.6, 15, or 15 cm in diameter, respectively, and hence ratios of 61:1, 64:1, and 100:1, respectively. C57BL mice learned in all diameter pools. In the 75-cm pool this strain learned so rapidly their learning curve was nearly flat. BALBc mice also learned in all three pool sizes, but not as well as C57BL mice except in the 75-cm pool where their learning curve was also flat. The 129/SvEvBrd mice showed almost no learning in the 150-cm pool, some learning in the 120-cm pool, and rapid learning in the 75-cm pool. These data show how sensitive some strains of mice are to task difficulty. If the task is too easy or too difficult, no useful data are obtained. The data suggest that for most mouse strains, pools of 120 to 122 cm in diameter are in the optimal range.
Findings in rats are different. Rats have been tested in all the pool sizes shown in Table 1, and even in the 244-cm diameter pool with 10-cm platforms, they learn well. What happens when the ratio is changed in rats? We previously showed that rats become proficient in a 210-cm diameter MWM with a 10-cm platform and show clear deficits after neonatal exposure to methamphetamine (Williams et al. 2003b). In a follow-up experiment where we wanted to test lower doses of methamphetamine, we sought to make the task more challenging, and hence, we presumed, more sensitive. Therefore, we used the same 210-cm pool but used a smaller 5-cm platform from the beginning (Williams et al. 2004). Although the rats showed learning in this configuration, when we compared saline controls across experiments, there was a dramatic difference in levels of performance. Control rats with the 10-cm platform had average latencies to reach the platform of just under 40 seconds on day 1 to approxiately 10 seconds on day 5 (4 trials/day) whereas those tested with the 5-cm platform had latencies of approximately 95 seconds on day 1 to approximately 50 seconds on day 5 (Williams et al. 2004). The situation is quite different, however, if one starts with a 10-cm platform during acquisition and reduces it to a 5-cm platform in steps. In this experiment, again on developmental methamphetamine, we tested the rats as adults in the 210-cm diameter pool with a 10-cm platform during acquisition, with a 7-cm platform during reversal, and with a 5-cm platform during shift (platform in a quadrant adjacent to the reversal quadrant position) (Vorhees et al. 2008). Groups showed good learning curves during all three phases, including in the final phase with the 5-cm platform, which, in the previous experiment, rats could not become proficient at when they had the 5-cm platform from the outset (acquisition). Whereas rats improved to just less than 50 seconds on day 5 when they began with the 5-cm platform, after having experience in previous phases with 10- and 7-cm platforms, they improved to approximately 20 seconds by day 5 with the 5-cm platform when used last (both experiments used 4 trials/day with identical start and goal positions). Thus, although pool-to-platform ratio is informative, it is not the whole story. Prior experience has a major effect on how difficult a task is, even if all the test parameters are identical.
Cues
The distal cues used to assess allocentric learning and memory in the MWM also affect the rate and ultimately the level of proficiency achieved. One experiment determined the minimally sufficient distal cues for rats. For this, the maze was covered with a white plastic dome so the experimenters could place distinct distal cues around the pool to precisely control how many and where cues were located. At minimum, rats needed two distinct distal cues by which to navigate. Moreover, the two cues had to have a minimum angular separation (distance between prominent cues located around the pool) to provide adequate navigational information, but in addition to this rats always used distance to the edge of the pool as a cue (Maurer and Derivaz 2000). As Morris and others have noted, even rats with hippocampal lesions that can no longer spatially navigate to a hidden platform nonetheless find the platform by adopting a strategy of swimming at a distance from the wall (Morris et al. 1990). In another study in which the pool was physically moved within the room but the position of the hidden platform was in the same spot, rats swam in the correct direction but not to the exact spot of the platform (Hamilton et al. 2007), demonstrating that rats do not navigate by absolute position but by relative position in association with directional cues. Hence, rats use distal and directional cues together, a concept similar to the idea that animals use the cues of the boundaries of the maze as an important guide to finding a specific locale.
Cued versus Hidden Versions: Which Comes First?
The advantage of providing the cued version of the MWM first is, as previously noted, it has the effect of reducing or eliminating problems that animals have with sensorimotor function and, for learning subordinate skills such as staying on the platform and searching away from the wall, effects similar to those learning by using nonspatial pretraining methods (Hoh et al. 1999). Providing cued trials before hidden trials is especially valuable in mice because mice are more prone to thigmotaxis and to floating and other behaviors that reduce the rate of learning (i.e., not recognizing the platform as the escape by swimming over it, pushing off from it, or climbing on and jumping off). Cued trials eliminate most of these nonspatial behaviors and serve as a control procedure to determine that proximal cue learning is intact. Giving cued trials first also causes some positive transfer of learning that flattens the acquisition learning curve, but the effect is modest. For this reason, we experimented in rats with giving the cued trials first or after hidden platform trials and found better separation of experimental groups when cued trials are given second. Although one might be concerned with this approach because of the nonspecific behaviors that can sometimes falsely appear as a spatial deficit on acquisition, we have shown that if one assesses rats in multiple phases (acquisition, reversal, and shift), the issues associated with nonspecific factors are eliminated. For mice, we always do cued training before hidden platform trials because mice benefit from this procedure, but we have modified this slightly. We find that for mice, a single training day with six trials to a fixed visible platform from a fixed start is sufficient to acclimate mice to swimming, to teach them the basic task requirements, and for eliminating problems of thigmotaxis, floating, and jumping off the platform once having found it. We then proceed to hidden platform acquisition and reversal and then conduct cued trials with random start and random platform positions to assess proximal cue learning.
Hidden Platform Acquisition
The most basic version of the MWM is the initial or acquisition phase of the test. The number of trials given per day and the number of days vary from one laboratory to another, but by far the most common number is four trials per day. If one divides the pool into four equal quadrants by perpendicular lines intersecting in the center, one has four cardinal positions: north (N), south (S), east (E), and west (W). If the platform is positioned in the middle of one of these zones, it is designated as being in the SW, NW, NE, or SE quadrant. Morris started rats in a randomized order from each of the cardinal positions, thereby creating four different start locations. To balance across each of these start positions within a test session, it logically follows to have four trials in each block. From this, it followed that most labs conduct either one or two four-trial blocks per day to ensure that animals get all possible starts per session. But there is nothing special about using the four cardinal positions as the only possible start positions. Some have gone beyond using start positions oriented every 90o and have divided the perimeter into eight equal segments at 45o angles, creating four cardinal and four ordinal positions (Silva et al. 1992). One difficulty using cardinal start positions is that two positions are farther from the goal than the remaining two. If the platform is in the SW quadrant, then the N and E starts are significantly farther from the goal than the W and S start positions. This creates a saw-tooth pattern of results because animals find the platform faster when started near compared when started far from the platform (Morris 1981; Vorhees et al. 1995).
We smoothed the learning curves by reducing the difference in distances from the different start positions using what we call distal start positions (Vorhees and Williams 2006). If the platform is in the SW quadrant, we use two cardinal and the two ordinal positions farthest from the goal—in this example, NW, N, SE, and E. Although the two ordinal starts are still closer to the SW quadrant than to N or E, they are farther away than W and S.
Hidden Platform Reversal and Other Phases
One of the most useful ways of getting more information from MWM testing is to add a reversal phase. This is done by moving the platform to the opposite quadrant. There are many examples of treatments that produce small or even no differences during acquisition but significant deficits during reversal. Rats with hippocampal or hippocampal input pathway lesions or pharmacological blockade of key receptors often show striking reversal deficits. One of the reasons for this is that lesioned animals sometimes show perseveration for the acquisition platform position and have difficulty giving up looking in the previous location and adapting to the changed contingencies (Whishaw and Tomie 1997). There is some debate about the interpretation of perseverative interference effects and whether they represent a spatial navigation deficit or a dysfunction secondary to spatial learning based on inflexibility rather than cognitive remapping. In the case of NMDA receptor inhibitors, acquisition deficits are eliminated by prespatial navigation testing using either nonspatial pretraining or cued trials first (Hoh et al. 1999), but importantly reversal deficits remain. For example, we used nonspatial pretraining to show that developmental methamphetamine's effects on MWM acquisition were eliminated by nonspatial pretraining but reversal deficits persisted (Williams et al. 2002). We showed that the reversal deficits were unlikely to be perseverative because multiple shift phases (which should gradually diminish perseverative behavior) did not diminish the drug effects. Furthermore, probe trials showed that methamphetamine-exposed offspring could not localize the platform's vicinity even after they had learned where it was no matter how many prior test phases they had learned to a high level of proficiency (Williams et al. 2003b).
Using the MWM repeatedly can also be done on the same animals to assess time-dependent effects such as in neurodegenerative and aging models. We have shown that if one uses a platform in the SE quadrant for acquisition, one can use the NW quadrant weeks later. One can continue this with the same animals by moving the platform to other quadrants; for example if one started in SE and then moved to NW, one can then move to NE, then to SW, then back to SE, back to NW, and so on. Doing this, we saw that acquisition latencies were the longest and reversal latencies were the second longest, whereas shift latencies (moving to an adjacent quadrant) were shorter than reversal latencies, then shift-reversal latencies were longer again, reacquisition latencies were shorter, and re-reversal latencies (this time we used a smaller platform) were much longer (Williams et al. 2003b), but in each case the rats exposed early in development to methamphetamine were impaired. Using a similar approach but with only three phases, we showed that early exposures to 3,4-methylenedioxymethamphetamine induced MWM deficits across multiple MWM phases (Vorhees, Schaefer, et al. 2009) as did developmental exposure to (+)-fenfluramine (Morford et al. 2002). In these cases, the deficits are difficult to explain as perseverative in that they were not greatest on the first trial or two of each day of a new phase, as a perseveration effect would predict, but were as large or larger on the last trial of each day of each phase.
Memory/Probe Trials
The probe or memory trial is a standard feature of MWM in which the platform is removed. Where the animal spends its time is a measure of spatial bias. If the animal has learned the platform's location, it should show a preference for the quadrant in which the platform was located and even for the site where the platform was within the target quadrant. If a probe trial is given shortly after the last platform trial, the question arises as to whether performance on this trial reflects reference memory or a mixture of reference and working memory because the animal could be using its short-term recall of the last trial. One study tested this by giving multiple probe trials, one each day before and one each day after the platform trials, and found that only probe trials given 24 hours after a session of platform trials reflected consolidated reference memory (Baldi et al. 2005). For this reason, many investigators give the probe trial 24 hours after the last platform trial, thereby assuring that working memory is not contributing to retention. In addition, some investigators give a second probe trial 72 hours after the last platform trial to test for forgetting (e.g., Daily et al. 2011). Probe trials also have been given interspersed with platform trials as a means of assessing the incremental increase in bias as learning progresses (e.g., Baldi et al. 2005; Williams et al. 2003a).
Another important issue concerning probe trials is how long they should be because the longer the trial lasts the more likely it is that extinction will begin. A common probe trial length is 60 seconds, but there are data showing that 30-second trials show greater spatial bias to the platform's location than longer probe trial lengths. In one experiment, this was tested by giving a 120-second probe trial and analyzing the data in 30-second intervals (Blokland et al. 2004). In the second, third, and fourth 30-second intervals, time in the target zone progressively declined. It is possible to prevent extinction at intervals longer than 30 seconds if need be. This may be done by using variable-length probe trials and having a platform that can be raised from under the water so that at the end of each trial the animal can find the platform and be reinforced for escaping (Markowska et al. 1993). Although the variable-interval elevating platform effectively maintains probe trial performance, it is not a procedure everyone can engineer into their maze set-up; therefore probe trials of 30 to 45 seconds are optimal under most experimental conditions. If longer probe trials are used, it is prudent to fractionate the time and analyze the trial in intervals, making sure to pay particular attention to the first 30 seconds.
There are many indices of probe trial performance, but an interesting study done on 1600 mice spanning many experiments in which different probe trial measures were compared shows that some measures are better than others. The results showed that proximity to the platform site was consistently a better measure of spatial reference memory than quadrant preference, zone (time in the platform area), and crossings (number of platform site crossovers) (Maei et al. 2009). The proximity index they used was average distance to the platform site. Although no comparable study has been done in rats, it is a reasonable inference that proximity measures are likely to be the optimal way of assessing spatial memory in rats as well.
Water Temperature
Water temperature affects performance, and in the early years after the MWM became widely used, there was a preference to use warm water because it was suggested that room temperature water (typically 19–22oC in most vivaria) was too cold. Cooling or heating rats to change their core body temperature by cooling them so that body temperature decreased 28–30oC or warming them and raising body temperature to 40oC impaired MWM learning (Rauch et al. 1989a; Rauch et al. 1989b). Such extreme core body temperatures are not induced by typical room temperature water with two exceptions: even ambient temperature water can induce hypothermia and impair spatial learning in old rats (Lindner and Ribkoff 1991) and in mice. The solution in old rats and mice is to warm them between trials and/or provide longer intertrial intervals. In nonaged rats, there is no evidence that warming is needed. Thus, under typical conditions, water temperature of 20–22oC works well for both rats and mice.
Intertrial Interval
It has long been known that massed practice reduces learning and memory performance compared with distributed or spaced practice in almost all settings. Therefore, it is not surprising that experiments testing whether massed or spaced trials facilitate MWM learning find that, in general, spaced trials enhance learning and memory (Commins et al. 2003). We have also evaluated this. In male C57BL/6J mice, we tested two groups in a 122-cm diameter MWM with a 10-cm diameter platform. Mice first received cued trials for 6 days; this consisted of six trials on day 1 to a fixed platform from a fixed start, and on days 2 to 6, two trials per day with random start and random platform positions. For these trials, the platform was marked with an orange ball mounted on a pole positioned 8 cm above the center of the submerged platform; curtains were closed around the pool to obscure distal cues. For this phase, trials were back-to-back. The trial limit was 90 seconds; the intertrial interval was 5 seconds on the platform plus 20 seconds in a holding cage. Mice then entered hidden platform testing with either massed or distributed practice of four trials per day for 6 days. For the massed group, the intertrial interval was 10 seconds on the platform, and for the distributed group, it was 10 seconds on the platform plus 10 minutes in a holding cage. The difference between groups is illustrated in Figure 5. Analysis of variance showed a significant effect of group (F(1,21) = 13.11; p < 0.002). As can be seen, the distributed group performed significantly better than the massed group, and this was confirmed by analyses of path length and cumulative distance to the platform, whereas swim speeds were not different between groups. The study in rats was done slightly differently. In this case, adult male Sprague-Dawley CD IGS rats (Charles River strain 001) were acclimated to swimming by testing them for four back-to-back trials in a 15 × 244 cm straight water-filled channel to find a hidden platform at one end. After this, they were tested in a 244-cm diameter pool in three phases, each consisting of four trials per day for 6 days with a probe trial on day 7. The phases were acquisition, reversal, and shift, and the platforms for each successive phase were 10, 7, and 5 cm in diameter. The effect of massed versus distributed practice showed a trend toward better performance in the distributed group on acquisition(F(1,14) = 4.33; p < 0.06). On reversal and shift phases, the influence of trial spacing was not close to being significant (for reversal: F(1,14) = 1.26, p < 0.30; for shift: F(1,14) = 0.02, p > 0.90. There were no group × day interactions on any phase. The results are illustrated in Figure 6. Although the acquisition data suggest that spaced trials improve performance in rats, the effect is much less pronounced than in mice and insignificant on reversal or shift.
Figure 5.
Morris water maze (MWM) performance in mice given massed or distributed trials. C57BL/6J adult male mice were randomly divided into two groups of equal numbers and tested in a 122-cm diameter tank with either massed (back-to-back) or distributed trials (spaced 10 minutes apart) during hidden platform acquisition. Both groups first received cued training trials for 6 days. Training consisted of 1 day with the start and platform in fixed positions for six back-to-back trials and 5 days with random start and platform positions for two back-to-back trials per day. After this, mice received 6 days of acquisition to find a fixed hidden platform for four trials per day from randomized start positions balanced such that they received one trial from each of four start positions each day. The platform was in the SW quadrant, and start positions were arranged such that two were from cardinal positions (N and E) and two were from distal ordinal positions (NW and SE) to eliminate very close start positions (W and S). Average path length to reach the platform is shown (mean ± SEM). Because both groups were untreated, latency, path length, and cumulative distance indices all gave the same results. There were no significant differences on training trials. There was a significant main effect of trial spacing during hidden platform acquisition: analysis of variance showed a significant effect of group (F(1,21) = 13.11; p < 0.002). The distributed group performed significantly better than the massed group on each day of testing. Group sizes were 12 per group. *p < 0.05 averaged across days.
Figure 6.
Morris water maze (MWM) performance in rats given massed or distributed trials. Sprague-Dawley CD IGS (Charles River strain 001) adult male rats were randomly divided into two groups of equal numbers and tested in a 244-cm diameter tank with either massed (back-to-back) or distributed trials (spaced 10 minutes apart) during hidden platform acquisition, reversal, and shift phases with the platform in the SW, NE, and NW quadrants, respectively, and platform sizes of 10, 7, and 5 cm, respectively. Both groups first received acclimation trials in a 244 × 10 cm straight swimming channel (4 trials given back-to-back) on a single day. After this, rats received 6 days of learning to a fixed hidden platform for four trials per day from randomized start positions, balanced such that they received one trial from each of four start positions each day. The platform was in the SW quadrant, and start positions were arranged such that two were from cardinal positions (N and E) and two were from distal ordinal positions (NW and SE) to eliminate very close start positions (W and S). Average latency to reach the platform is shown (mean ± SEM). Because both groups were untreated, latency, path length, and cumulative distance indices gave the same results. There were no significant differences on straight-channel training trials. There was a marginally significant main effect of trial spacing during hidden platform acquisition: analysis of variance showed a nearly significant effect of group (F(1,14) = 4.33; p < 0.06); there were no significant differences on reversal or shift trials. There were no significant group × day interactions. The trial spacing trend on acquisition suggested that the distributed group learned the task faster than the massed group, except on day 6 when the groups converged. Group sizes were eight per group.
Sex Differences
In most tests of allocentric navigation in rodents, male animals show an advantage compared with female animals. Examples in rats from our laboratory and others consistently show that male rats learn the MWM faster during all phases of the test (acquisition, reversal, shift) and perform better on probe trials than female rats (Hamilton et al. 2007; Vorhees et al. 2008). Interestingly, in an egocentric navigation task (the Cincinnati Water Maze; Figure 3) just the opposite was seen; female rats consistently performed better than male rats (Vorhees et al. 2008). A meta-analysis across studies of RAM and MWM showed that in both tasks male rats performed better than female rats (Jonasson 2005); this is especially true in the RAM on the reference memory component compared with the working memory when the combined working/reference memory procedure is used. Interestingly, in mice the meta-analysis showed a slight female advantage in the MWM and slight male advantage in the RAM.
Ontogeny of Allocentric Learning
Not surprisingly, age has significant effects on MWM performance. Using a small 87-cm diameter pool, one study tested rats for 5 days starting on P21, P28, P34, P43, or P64 (Schenk 1985). Rats were divided into two groups—those trained in the cued and those trained in the hidden platform conditions; both groups were given a single 60-second probe trial at the end. The P21 and P28 groups were slower to learn the hidden version and never became proficient, and the P21 group never became proficient in the cued condition, but overall the cued groups performed better than the hidden version groups, a finding that shows that the cued version is easier to learn than the hidden version. When the hidden group was switched to cued, only the P21 group continued to have difficulty; the older groups quickly reached asymptotic performance. When the cued condition was switched to hidden, both the P21 and P28 groups had difficulty in locating the platform. On the probe trial given at the end of the first phase of testing before the switch in conditions, the hidden group showed target quadrant preference at all ages, except the P21 group, which had fewer site crossovers (i.e., tested at P25). All cued groups showed significant target quadrant preference but not significant site crossovers, except at the oldest age. Hence, spatial learning is not developed at P21 but cued learning is, and spatial learning in an 87-cm pool emerges on or about P28.
Another study showed that P17 to P19 rats could not learn the hidden version of the MWM in a 120-cm diameter pool with an 11.5-cm diameter platform (Rudy et al. 1987). The same authors tested preweaning rats in the cued version and measured their spatial bias on a probe trial at the end (Rudy and Paylor 1988). Young rats had difficulty spatially navigating, but they learned the cued task, indicating that egocentric navigation was developed before weaning. Another study of the ontogeny of spatial navigation included control of litter effects by testing rats of different ages against littermates (Tonkiss et al. 1992). The pool was 150 cm in diameter, and the platform was 12 cm in diameter. Male rats were tested over 2 days, and two strains were compared: Sprague-Dawley and Long-Evans. The ages were P20 to P21, P22 to P23, P24 to P25, P26 to P27, and P90 to P91. The young rats were tested in 27oC water and the adults in 25oC water. All groups and both strains showed progressively shorter latencies across the 24 test trials across the 2 days of testing, but the Long-Evans rats had shorter latencies than the Sprague-Dawley rats. Moreover, the strain effect remained until the last trial in the P20 to P21, P22 to P23, and P24 to P25 groups, but in the P26 to P27 and P90 to P91 groups, Sprague-Dawley rats reached the same level of performance as the Long-Evans rats. On the probe trial, target preference was greater in the P24 to P25, P26 to P27, and P90 to P91 groups than in the younger two age groups. Hence, before P23, spatial learning in rats is reduced.
In a study of younger rats, a 40-cm diameter pool was used with a 2.5 × 2.5 cm platform. Rats were given eight trials per day on P17 and P18 and four trials on P19, followed by a single probe trial (Carman and Mactutus, 2002). Distal cues were arranged in three of four corners of the room, with one corner having twice as many cues as the other two and one having none. One set of rats was tested facing the corner with no cues (null-cue) and the other group facing the corner with twice as many (double-cue). These groups were divided into those given cued versus hidden trials. Acquisition was slow and erratic on day 1, better on day 2, and by day 3 the two cued groups (double-cue and null-cue) reached the platform in 5 to 10 seconds, whereas the hidden null-cue group showed almost no learning, and the hidden double-cue group was intermediate. On the probe trial, the cued groups showed a small preference for the target quadrant, whereas the hidden null-cue group showed no preference. The hidden double-cue group showed the best quadrant preference (approximately 22 seconds out of 30 seconds). Hence, very young rats can learn the MWM but only under special conditions of extra cues and a very small pool.
Odor Trails
There is one report in a swimming T maze that rats can follow odor trails in a win-stay paradigm (Means et al. 1992). We have tested the idea of odor trails in the CWM in rats using several procedures. In one, we used a pathfinder (i.e., a rat that had learned the maze to errorless performance put through the maze before each trial of a naive rat) versus controls with no pathfinder run first; we found no pathfinder/odor trial effect (unpublished data). In another study in the CWM the water was stirred and walls scrubbed between trials versus controls tested without such procedures, and again we found no evidence of odor trial effects (unpublished data). To the best of our knowledge, there are no data of odor trails in the MWM or CWM, or any other water maze for that matter; to the contrary, the data continue to support the view that one of the advantages of water mazes is the absence of odor trails, something dry mazes cannot assure.
Rearing/Housing Conditions
As noted herein, singly housed and nonhandled rats perform worse in the MWM than group-housed and handled rats. Because group housing is standard practice in modern vivaria, the adverse effects of isolation housing is no longer an issue. What about enhanced housing? These vary widely but fit within the rubric of environmental enrichment. This literature is full of complex environmental enrichment types, but the general phenomenon is that highly enriched environments during development enhance later learning. These methods reliably improve learning in the MWM as well (Tees et al. 1990). Such effects can be demonstrated in the MWM without elaborate enrichments. We demonstrated that in rats reared in pairs in box cages with woodchip bedding with arched stainless steel semicircular enclosures in each cage versus rats raised the same way but without the enclosures, the enriched group showed facilitated MWM learning as adults compared with the standard cage group (Vorhees et al. 2008).
Experimental Design
There has been much discussion recently that many animal studies have not proven to be replicable (Couzin-Frankel 2013). A variety of sources of this problem have been identified and include inadequate sample sizes, lack of randomization of animals to groups, nonblinding of experimenters, and inadequate control of litter effects. Although these apply to all experiments, they are worth considering here because experiments using the MWM and other learning and memory tests often suffer from these same problems.
Sample Size
Statisticians have long warned about the problem of type I errors when sample sizes are small (declaring a difference significant by chance; i.e., a false positive). Investigators persist in the belief that for any result, once a significant difference is obtained it is real even if only three to five animals per group are used. Many MWM experiments are reported with small group sizes. In our experience with the MWM and other water mazes, group sizes less than 10 can be unreliable and we use 15 to 20 animals per group, especially for mice, whose performance in learning and memory tests tends to be more variable than for rats. It is noteworthy that regulatory authorities require that safety studies have 20 or 25 animals per group. This number is for each of at least four groups (control and three dose levels) (Food and Drug Administration 2007; Gad 2009; Tyl and Marr 2012). Such group sizes are used by the US Environmental Protection Agency, the US Food and Drug Administration, the Organization for Economic Cooperation and Development, and Japanese and European Union regulatory agencies. Although the 3 Rs (reduce, refine, and replace) are worthwhile goals in the use of animals in research, it is not a justification to underpower experiments and run the risk of false positives, which, in the long run, cost more time, more animals, and more money to prove or disprove.
An excellent example of this occurred in research using a mouse model of amyotrophic lateral sclerosis (ALS). A mouse model of familial ALS with a mutation of the SOD1 gene became a common model to test new treatments. The ALS Therapy Research Institute undertook studies to replicate 70 drugs reported in the literature to extend the life of SOD1 mutant mice. They tested 18,000 mice. They found that not a single drug reported as extending the survival of SOD1 mutant mice could be replicated. They then used their dataset of control mice to randomly select samples of different group sizes and tested for whether they were significantly different or not. Because the controls should show no survival differences, they tested how often randomly drawn samples produce false positives (i.e., statistically significant differences by chance). For repeatedly drawn sample sizes of four, significant differences occurred 30% of the time. If sample size was increased to 10 per group, only 10% false-positive differences were obtained, and with sample sizes of 16 per group, this dropped to less than 5% (Scott et al. 2008). In trying to identify the sources of the errors in the original experiments, they found that small sample sizes, litter effects, and several other effects were salient.
Litter Effects
The basic issue in multiparous species such as rats and mice is that littermates have overlapping genetic, epigenetic, and experimental influences that make littermates more similar to one another than to offspring from another litter. This is comparable with fraternal twins. Fraternal twins are dizygotic but have overlapping genetics, the same intrauterine environment, and the same rearing environment and hence are more similar than are children born to other parents. Littermate variance clustering has long been recognized by statisticians as a factor that needs to be accounted for in data analyses. It can be handled in statistical models by considering littermates as a matching factor and treated as a within-subject factor (i.e., a repeated measure factor). Because in prenatal studies, it is the pregnant female animal that is randomly assigned to experimental groups, the offspring are yoked to the maternal treatment and hence within a litter the pups are not orthogonal to one another. If treated as if they are orthogonal, this violates one of the basic assumptions of inferential statistics that subjects be independent. The problem of oversampling multiple offspring per litter and treating them as if independent has been well described elsewhere, as have solutions (Holson and Pearce 1992; Lazic and Essioux 2013). Solutions include sampling only one pup per litter (or only one male and one female pup) or, if breeding heterozygotic mice for a genetic mutation, sampling only one pup of any given genotype per litter or per sex per litter. There are also more complex hierarchical analysis of variance models that nest pup within litter, or there are randomized block designs. Alternatively, one can test multiple offspring per litter and average their data together to generate one value per litter for each outcome. Whatever the approach, failure to account for litter was a major cause of error in the ALS replication project (Scott et al. 2008).
Randomization
Although most experimenters are taught that animals should be randomly assigned to groups, it is not always done and even less often reported. Many experimenters have come to accept the practice of arbitrary selection as an approximation to random assignment, but the lack of reproducibility of animal experiments suggests that this assumption is flawed. Our approach is to take a group of animals before an experiment is begun and assign each animal an arbitrary temporary number, then use a random number chart or computerized random number generator to determine which temporary number is assigned to which treatment group. Whatever the method, it should be described in the methods in the paper.
Blinding
Several reviews have noted that blinding experimenters to treatment group assignment is another often omitted or not mentioned procedure. This is an important safeguard no matter what the experiment, including tests of learning and memory. Inadvertent bias can affect handling and outcome of tests without the person even recognizing it. Routinely blinding (and stating as much in the methods) is essential.
Conclusions
In weighing the pros and cons of different methods of assessing allocentric navigation in rodents, the MWM has been demonstrated to possess many advantages and few disadvantages compared with other methods. Criticisms have been few but the one recurrent issue is that placing animals in water causes undue stress. However, food deprivation that reduces an animal's body weight to 80–85% or more of its free-feeding weight is also stressful (Armario and Jolin 1986; Coover et al. 1984; Garcia-Belenguer et al. 1993; Heiderstadt et al. 2000; Honma et al. 1986; Johansson et al. 2008; Marinkovic et al. 2007; Pesic et al. 2010). Even tests such as novel object recognition and spontaneous alternation that rely on curiosity and not experimenter-imposed sources of motivation induce some stress, but it does not follow from this that the stress of any of these procedures is ipso facto aversive. Although positive reinforcement is effective at changing behavior, in rodents positive reinforcement is accompanied by food and/or water restriction, which is stress inducing. A task that may be intermediate in stress between food restriction and tasks such as novel object recognition and spontaneous alternation is the Barnes maze. In this test, the animal is placed on an open elevated platform with multiple holes around the perimeter. The animal is placed in the center and allowed to search for an escape box beneath one of the holes. To induce searching, the maze has bright lights shining down from above, or a loud tone, or uses blowing air from a fan mounted above the maze. Once the animal enters the escape box, the stimulus is disabled, but how stressful these motivators are has not been tested or compared with water mazes in rats. By far the most stressful methods are those based on escaping or avoiding electrical foot shock. Swimming is not as aversive as foot shock, but likely more aversive than novel object recognition or spontaneous alternation. Swimming mazes may be more aversive than the Barnes maze, but even here this depends on what motivator is used in the Barnes maze. Whether the stress generated in water mazes is more or about the same as food restriction is unknown because direct comparisons are lacking. However, the remarkable ability of swimming mazes to motivate learning and memory with little training to high levels of proficiency has made water mazes, including the MWM, highly useful. The other characteristic that makes the MWM stand out is that by testing allocentric navigation it assesses a conserved form of learning and memory essential for survival and is convergent with semantic and episodic memory systems in humans, functions often adversely affected by disease and injury and which are the targets of research to develop treatments. As such, the assessment of MWM spatial navigation is likely to remain a central methodology in the study of mechanisms underlying how the brain captures, consolidates, and retrieves allocentric information to navigate through the world.
In sum, the entire field of learning and memory assessment would benefit if laboratories would increasingly standardize apparatus and test methods to the fullest extent possible, attending to the design and procedural evidence reviewed herein above without limiting creativity or customizing procedures for testing specific hypotheses. Within regulatory settings, improved standardization would be especially beneficial. Only by improving methods and procedures will data from different labs or across experiments within labs become sufficiently comparable to allow experimental findings to become better synthesized. Standardization is challenging for tests of learning and memory, more so than for tests of locomotor activity and acoustic startle, but nonetheless feasible if the evidence of best practices reviewed above are implemented to maximum advantage.
Acknowledgments
This review was made possible by the authors' research, which was supported in part by past and present grants (past: NIH DA006733, DA021394, and ES015689; current: NIH MH101609 and ES023319 and DOD grant W81XWH-13-1-0306).
References
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Rodent models of depression: learned helplessness induced in mice. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8) doi: 10.1002/0471142301.ns0810cs14. 10C. [DOI] [PubMed] [Google Scholar]
- Armario A, Jolin T. Effects of water restriction on circadian rhythms of corticosterone, growth hormone and thyroid stimulating hormone in adult male rats. Physiol Behav. 1986;38:327–330. doi: 10.1016/0031-9384(86)90102-2. [DOI] [PubMed] [Google Scholar]
- Baldi E, Efoudebe M, Lorenzini CA, Bucherelli C. Spatial navigation in the Morris water maze: working and long lasting reference memories. Neurosci Lett. 2005;378:176–180. doi: 10.1016/j.neulet.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RGM. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Leith NJ, Ray OS. A behavioral and pharmacological analysis or variables mediating active avoidance behavior in rats. J Comp Physiol Psychol. 1973;82:489–500. doi: 10.1037/h0034111. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Leith NJ, Ray OS. An analysis of the facilitation of avoidance acquisition produced by d-amphetamine and scopolamine. Behav Biol. 1974;11:189–203. doi: 10.1016/s0091-6773(74)90353-8. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A, Geraerts E, Been M. A detailed analysis of rats' spatial memory in a probe trial of a Morris task. Behav Brain Res. 2004;154:71–75. doi: 10.1016/j.bbr.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Brun VH, Solstad T, Kjelstrup KB, Fyhn M, Witter MP, Moser EI, Moser MB. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus. 2008;18:1200–1212. doi: 10.1002/hipo.20504. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DP. LTP, NMDA, genes and learning. Curr Opin Neurobiol. 1997;7:235–242. doi: 10.1016/s0959-4388(97)80012-8. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D. The neuroscience of spatial navigation: Focus on behavior yields advances. Rev Neurosci. 1996;7:215–231. doi: 10.1515/revneuro.1996.7.3.215. [DOI] [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behav Neurosci. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Carman HM, Mactutus CF. Proximal versus distal cue utilization in spatial navigation: the role of visual acuity? Neurobiol Learn Mem. 2002;78:332–346. doi: 10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- Caul WF, Barrett RJ. Shuttle-box versus Y-maze avoidance: value of multiple response measures in interpreting active-avoidance performance of rats. J Comp Physiol Psychol. 1973;84:572–578. doi: 10.1037/h0034876. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lazar NL, Bechard AR, Wood GA, Roder JC. NIH Swiss and Black Swiss mice have retinal degeneration and performance deficits in cognitive tests. Comp Med. 2005;55:310–316. [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. The hippocampus and spatial memory: Findings with a novel modification of the water maze. J Neurosci. 2007;27:6647–6654. doi: 10.1523/JNEUROSCI.0913-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins S, Cunningham L, Harvey D, Walsh D. Massed but not spaced training impairs spatial memory. Behav Brain Res. 2003;139:215–223. doi: 10.1016/s0166-4328(02)00270-x. [DOI] [PubMed] [Google Scholar]
- Coover GD, Murison R, Sundberg H, Jellestad F, Ursin H. Plasma corticosterone and meal expectancy in rats: effects of low probability cues. Physiol Behav. 1984;33:179–184. doi: 10.1016/0031-9384(84)90097-0. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. When mice mislead. Science. 2013;342:922–925. doi: 10.1126/science.342.6161.922. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Cravens RW. Effects of maternal undernutrition on offspring behavior: Incentive value of a food reward and ability to escape from water. Dev Psychobiol. 1974;7:61–69. doi: 10.1002/dev.420070110. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Daily JL, Nash K, Jinwal U, Golde T, Rogers J, Peters MM, Burdine RD, Dickey C, Banko JL, Weeber EJ. Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027221. e27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalm S, Grootendorst J, De Kloet ER, Oitzl MS. Quantification of swim patterns in the Morris water maze. Behav Res Methods Instrum Comput. 2000;32:134–139. doi: 10.3758/bf03200795. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: Relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Dubreuil D, Tixier C, Dutrieux G, Edeline JM. Does the radial arm maze necessarily test spatial memory? Neurobiol Learn Mem. 2003;79:109–117. doi: 10.1016/s1074-7427(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Etienne AS. Navigation of a small mammal by dead reckoning and local cues. Curr Dir Psychol Sci. 1992;1:48–52. [Google Scholar]
- Food and Drug Administration. College Park MD: US Department of Health and Human Services; 2007. Guidance for Industry and Other Stakeholders Toxicological Principles for the Safety Assessment of Food Ingredients Redbook 2000. [Google Scholar]
- Fouquet C, Babayan BM, Watilliaux A, Bontempi B, Tobin C, Rondi-Reig L. Complementary roles of the hippocampus and the dorsomedial striatum during spatial and sequence-based navigation behavior. PLoS One 8:e67232. 2013 doi: 10.1371/journal.pone.0067232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad SC. Hoboken NJ: John Wiley & Sons; 2009. Drug safety evaluation. [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Garcia-Belenguer S, Oliver C, Mormede P. Facilitation and feedback in the hypothalamo-pituitary-adrenal axis during food restriction in rats. J Neuroendocrinol. 1993;5:663–668. doi: 10.1111/j.1365-2826.1993.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, Sutherland RJ. How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J Exp Psychol Anim Behav Process. 2007;33:100–114. doi: 10.1037/0097-7403.33.2.100. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiderstadt KM, McLaughlin RM, Wright DC, Walker SE, Gomez-Sanchez CE. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab Anim. 2000;34:20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Heys JG, MacLeod KM, Moss CF, Hasselmo ME. Bat and rat neurons differ in theta-frequency resonance despite similar coding of space. Science. 2013;340:363–367. doi: 10.1126/science.1233831. [DOI] [PubMed] [Google Scholar]
- Hodges H. Maze procedures: The radial-arm and water maze compared. Cog Brain Res. 1996;3:167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Hoh T, Beiko J, Boon F, Weiss S, Cain DP. Complex behavioral strategy and reversal learning in the water maze without NMDA receptor-dependent long-term potentiation. J Neurosci 19:RC2. 1999 doi: 10.1523/JNEUROSCI.19-10-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh TE, Cain DP. Fractionating the nonspatial pretraining effect in the water maze task. Behav Neurosci. 1997;111:1285–1291. doi: 10.1037//0735-7044.111.6.1285. [DOI] [PubMed] [Google Scholar]
- Hollup SA, Molden S, Donnett JG, Moser M-B, Moser EI. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci. 2001;21:1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C. Stress impairs performance in spatial watermaze learning tasks. Behav Brain Res. 1999;100:225–235. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hirai T, Katsuno Y, Hiroshige T. Food ingestion is more important to plasma corticosterone dynamics than water intake in rats under restricted daily feeding. Physiol Behav. 1986;37:791–795. doi: 10.1016/0031-9384(86)90186-1. [DOI] [PubMed] [Google Scholar]
- Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides. 2008;29:1588–1595. doi: 10.1016/j.peptides.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci 14:37. 2013 doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005a;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol. 2005b;15:738–746. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Ribkoff VK. Relationship between performance in the Morris water task, visual acuity, and thermoregulatory function in aged F-344 rats. Behav Brain Res. 1991;45:45–55. doi: 10.1016/s0166-4328(05)80179-2. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Wolfer DP. Genetically modified mice and cognition. Curr Opin Neurobiol. 1998;8:272–280. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res. 1999;99:143–152. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Booze RM. Accuracy of spatial navigation: The role of platform and tank size. Soc Neurosci Abst 20:1014. 1994 [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the most sensitive measure of water maze probe test performance? Front Integr Neurosci 3:4. 2009 doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—A decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Marinkovic P, Pesic V, Loncarevic N, Smiljanic K, Kanazir S, Ruzdijic S. Behavioral and biochemical effects of various food-restriction regimens in the rats. Physiol Behav. 2007;92:492–499. doi: 10.1016/j.physbeh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Long JM, Johnson CT, Olton DS. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behav Neurosci. 1993;107:627–632. doi: 10.1037//0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- Maurer R, Derivaz V. Rats in a transparent morris water maze use elemental and configural geometry of landmarks as well as distance to the pool wall. Spatial Cogn Comput. 2000;2:135–156. [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Means LW, Alexander SR, O'Neal MF. Those cheating rats: Male and female rats use odor trails in a water-escape "working memory" task. Behav Neural Biol. 1992;58:144–151. doi: 10.1016/0163-1047(92)90387-j. [DOI] [PubMed] [Google Scholar]
- Menzel R, Geiger K, Joerges J, Muller U, Chittka L. Bees travel novel homeward routes by integrating separately acquired vector memories. Anim Behav. 1998;55:139–152. doi: 10.1006/anbe.1997.0574. [DOI] [PubMed] [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, Hefft S, Merkow M, Polyn SM, Jacobs J, Kahana MJ, Schulze-Bonhage A. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342:1111–1114. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with d-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Morris RGM. An attempt to dissociate "spatial-mapping" and "working-memory" theories of hippocampal function. In: Seifert W, editor. Neurobiology of the Hippocampus. New York: Academic Press. p 405–432; 1993. [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate antagonist, AP5. Nature. 1986;329:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Hagan JJ, Rawlins JNP. Allocentric spatial learning by hippocampectomized rats: A further test of the "spatial mapping" and "working memory" theories of hippocampal function. Quart J Exp Psychol 38B:365-395. 1986 [PubMed] [Google Scholar]
- Morris RGM, Schenk F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: Dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2:1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser M-B, Morris RGM. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadal L. Oxford: Oxford University Press; 1978. The Hippocampus as a Cognitive Map. [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. J Exp Psychol Anim Behav Processes. 1976;2:97–116. [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Paquette C, Franzen E, Jones GM, Horak FB. Walking in circles: navigation deficits from Parkinson's disease but not from cerebellar ataxia. Neuroscience. 2011;190:177–183. doi: 10.1016/j.neuroscience.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KE, Telugu BP. Role of stem cells in large animal genetic engineering in the TALENs-CRISPR era. Reprod Fertil Dev. 2013;26:65–73. doi: 10.1071/RD13258. [DOI] [PubMed] [Google Scholar]
- Penner MR, Mizumori SJ. Neural systems analysis of decision making during goal-directed navigation. Prog Neurobiol. 2012;96:96–135. doi: 10.1016/j.pneurobio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Pesic V, Marinkovic P, Janac B, Ignjatovic S, Popic J, Kanazir S, Ruzdijic S. Changes of behavioral parameters during long-term food restriction in middle-aged Wistar rats. Physiol Behav. 2010;101:672–678. doi: 10.1016/j.physbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn. 1977;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, LePichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;226:730–731. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Post AM, Wultsch T, Popp S, Painsipp E, Wetzstein H, Kittel-Schneider S, Sontag TA, Lesch KP, Reif A. The COGITAT holeboard system as a valuable tool to assess learning, memory and activity in mice. Behav Brain Res. 2011;220:152–158. doi: 10.1016/j.bbr.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Rauch TM, Welch DI, Gallego L. Hyperthermia impairs retrieval of an overtrained spatial task in the Morris water maze. Behav Neural Biol. 1989a;52:321–330. doi: 10.1016/s0163-1047(89)90442-1. [DOI] [PubMed] [Google Scholar]
- Rauch TM, Welch DI, Gallego L. Hypothermia impairs performance in the Morris water maze. Physiol Behav. 1989b;45:315–320. doi: 10.1016/0031-9384(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Ray OS, Barrett RJ. Behavioral, pharmacological, and biochemical analysis of genetic differences in rats. Behav Biol. 1975;15:391–417. doi: 10.1016/s0091-6773(75)92184-7. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci. 2006;26:4071–4081. doi: 10.1523/JNEUROSCI.3408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Paylor R. Reducing the temporal demands of the Morris place-learning task fails to ameliorate the place-learning impairment of preweanling rats. Psychobiology. 1988;16:152–156. [Google Scholar]
- Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: Dissociation of "proximal" and "distal"-cue-based behaviors. Behav Neurosci. 1987;101:62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Weiss DS. Exploiting CRISPR/Cas systems for biotechnology. Bioessays. 2014;36:34–38. doi: 10.1002/bies.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier D, Hargreaves EL, Boon F, Vanderwolf CH, Cain DP. Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: Nonspatial pretraining eliminates spatial learning deficits. Behav Neurosci. 1996;110:103–116. [PubMed] [Google Scholar]
- Schaefer TL, Lingrel JB, Moseley AE, Vorhees CV, Williams MT. Targeted mutations in the Na,K-ATPase alpha 2 isoform confer ouabain resistance and result in abnormal behavior in mice. Synapse. 2011;65:520–531. doi: 10.1002/syn.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Vorhees CV, Williams MT. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F. Development of place navigation in rats from weaning to puberty. Behav Neural Biol. 1985;43:69–85. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, Al-Nakhala BM, Vieira FG, Ramasubbu J, Heywood JA. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87:521–536. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J Neurosci. 2013;33:19304–19313. doi: 10.1523/JNEUROSCI.1825-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Neural basis of the cognitive map: path integration does not require hippocampus or entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:12034–12038. doi: 10.1073/pnas.0805414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in a-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Schaefer TL, Graham DL, deGrauw TJ, Clark JF, Williams MT, Vorhees CV. Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One 6:e16187. 2011 doi: 10.1371/journal.pone.0016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Jr, Lora JC, Williams SB. Directional responding of C57BL/6J mice in the Morris water maze is influenced by visual and vestibular cues and is dependent on the anterior thalamic nuclei. J Neurosci. 2012;32:10211–10225. doi: 10.1523/JNEUROSCI.4868-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Morris RGM. The watermaze. In: Sahgal A, editor. Behavioural Neuroscience, Volume I, A Practical Approach. Oxford: IRL Press at Oxford University Press; 1993. pp. 107–122. [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Sung JY, Goo JS, Lee DE, Jin DQ, Bizon JL, Gallagher M, Han JS. Learning strategy selection in the water maze and hippocampal CREB phosphorylation differ in two inbred strains of mice. Learn Mem. 2008;15:183–188. doi: 10.1101/lm.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tees RC, Buhrmann K, Hanley J. The effect of early experience on water maze spatial learning and memory in rats. Dev Psychobiol. 1990;23:427–439. doi: 10.1002/dev.420230505. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Shultz P, Galler JR. Long-Evans and Sprague-Dawley rats differ in their spatial navigation performance during ontogeny and at maturity. Dev Psychobiol. 1992;25:567–579. doi: 10.1002/dev.420250804. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Marr MC. Developmental toxicity testing—Methodology. In: Hood RD, editor. Developmental and Reproductive Toxicology: A Practical Approach. New York: Informa Healthcare; 2012. pp. 129–183. [Google Scholar]
- Upchurch M, Wehner JM. Differences between inbred strains of mice in Morris water maze performance. Behav Genet. 1988;18:55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- Van Dam D., Lenders G, De Deyn PP. Effect of Morris water maze diameter on visual-spatial learning in different mouse strains. Neurobiol Learn Mem. 2006;85:164–172. doi: 10.1016/j.nlm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Acuff-Smith KD, Schilling MA, Cappon GD, Fisher JE, Pu C. Long-term learning deficits and changes in unlearned behaviors following in utero exposure to multiple daily doses of cocaine during different exposure periods and maternal plasma cocaine concentrations. Neurotoxicol Teratol. 1995;17:253–264. doi: 10.1016/0892-0362(94)00061-h. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Skelton MR, Grace CE, Herring NR, Williams MT. (+/-)3,4-Methylenedioxymethamphetamine (MDMA) dose-dependently impairs spatial learning in the morris water maze after exposure of rats to different five-day intervals from birth to postnatal day twenty. Dev Neurosci. 2009;31:107–120. doi: 10.1159/000207499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci 27:289-298. 2009 doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SE, Maier SF. Effects of individual housing and stressor exposure upon the acquisition of watermaze escape. Learn Motiv. 1986;17:287–310. [Google Scholar]
- Wahlsten D, Cooper SF, Crabbe JC. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav Brain Res. 2005;165:36–51. doi: 10.1016/j.bbr.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Assessment of spatial memory using the radial arm and Morris water maze. In: Crawley JN, Gerhardt GA, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Current Protocols in Neuroscience. New York: Wiley Interscience. Unit 8.5A.; 2004. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J-A. Perseveration on place reversals in spatial swimming pool tasks: Further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Williams MT, Braun AA, Amos-Kroohs R, McAllister JPI, Lindquist DM, Mangao FT, Vorhees CV, Yuan W. Kaolin-induced ventriculomegaly at weaning produces long-term learnng, memory, and motor deficits in rats. Int J Dev Neurosci. 2014;35:7–15. doi: 10.1016/j.ijdevneu.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Behavioral and growth effects induced by low dose methamphetamine administration during the neonatal period in rats. Int J Dev Neurosci. 2004;22:273–283. doi: 10.1016/j.ijdevneu.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects, or injection stress. Brain Res. 2003a;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003b;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal days 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Wittlinger M, Wehner R, Wolf H. The ant odometer: Stepping on stilts and stumps. Science. 2006;312:1965–1967. doi: 10.1126/science.1126912. [DOI] [PubMed] [Google Scholar]
- Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull. 2010;26:327–337. doi: 10.1007/s12264-010-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]