Abstract
The processes of pollen grain development and germination depend on the uptake and metabolism of pollen sugars. In pepper (Capsicum annuum L.), initial sugar metabolism includes sucrose hydrolysis by invertase and subsequent phosphorylation of glucose and fructose by hexose kinases. The main objective of this study was to investigate changes in fructokinase (EC 2.7.1.4) and hexokinase (EC.2.7.1.1) activities in pepper flowers during their development, and to study the possible roles of these enzymes in determining pollen germination capacity under high temperature and under CO2 enrichment, previously shown to modify sugar concentrations in pepper pollen (Aloni et al., 2001 Physiologia Plantarum 112: 505–512). Fructokinase (FK) activity was predominant in pepper pollen, and increased during pollen maturation. Pollen hexokinase (HK) activity was low and did not change throughout pollen development. High‐temperature treatment (day/night, 32/26 °C) of pepper plants reduced the percentage of pollen that germinated compared with that under normal temperatures (26/22 °C), and concomitantly reduced the activity of FK in mature pollen. High temperature also reduced FK and HK activity in the anther. Under high ambient CO2 (800 µl l–1) pollen FK activity was enhanced. The results suggest that pollen and anther FK may play a role in the regulation of pollen germination, possibly by providing fructose‐6‐phosphate for glycolysis, or through conversion to UDP‐glucose (UDPG) to support the biosynthesis of cell wall material for pollen tube growth. High temperature stress and CO2 enrichment may influence pollen germination capacity by affecting these pathways.
Key words: (Capsicum annuum L.), pollen, fructokinase, hexokinase, high temperature
INTRODUCTION
The pepper crop is susceptible to various environmental stresses. High temperature conditions commonly cause flower abscission and seed abortion because of pollination failure (Wien, 1997; Aloni et al., 2001). Development and functioning of pollen are more susceptible to high temperatures than are development and functioning of the maternal part of the flower (Han et al., 1996; Peet et al., 1998). Pepper pollen grains accumulate sucrose during their maturation (Aloni et al., 2001), and following its hydrolysis by invertase, the glucose and fructose liberated must be rapidly metabolized at the onset of germination. Pollen acid invertase activity has been demonstrated to vary under environmental stresses leading to changes in pollen sucrose concentration (Dorion et al., 1996; Sheoran and Saini, 1996; Saini, 1997). We have recently demonstrated that high temperature reduces pollen germination in pepper, whereas high atmospheric CO2 increases it, possibly by affecting sucrose utilization in pollen grains under these conditions (Aloni et al., 2001). Following sucrose cleavage by invertase, the liberated glucose and fructose are phosphorylated before undergoing further metabolism. Nakamura et al. (1991) demonstrated the existence of fructokinase (FK) in Camellia japonica, Lillium longiflorum and L. lancifolium pollen grains, and showed that fructokinase had a much higher specific activity than hexokinase (HK). In the present research, the effect of high temperature stress and high atmospheric CO2 on FK and HK activity in pepper pollen was investigated in relation to pollen germination under these conditions.
MATERIALS AND METHODS
Plant material and growth conditions
Capsicum annuum L. ‘Mazurka’ seedlings were transplanted into 3 l pots containing peat : perlite : sponge (60 : 20 : 20, v/v/v) as the growth medium. Plants were grown in a glasshouse with natural light and minimum/maximum temperatures of 20/28 °C, respectively. Initially, fruitlets were removed immediately after formation until the plant had five internodes (approx. 45 d after transplanting). During that period, plants were drip‐irrigated with commercial nutrient solution (‘Mor’, Deshanim, Haifa, Israel) which included 100 mg l–1 N with an NO2/NH4 ratio of 9 : 1, and micronutrients. Irrigation occurred once a day with 50 % leaching. After 45 d plants were transferred to growth chambers for application of the temperature treatments. At the onset of the treatment each plant was fruitless but bore an average of ten flowers at anthesis and additional flower buds at various stages of development. The treatments imposed were either a normal temperature (26/22 °C day/night) or high temperature (32/26 °C day/night), regime. The photoperiod was 12 h and light was natural with supplementary incandescent light, bringing the maximal light intensity (at 1200 h) to 500 µmol photons m–2 s–1. Management of irrigation and nutrition was as described above except that the high temperature‐treated plants received an additional pulse of irrigation daily. Each treatment was applied to three replicates of 20 plants each.
CO2 enrichment
The CO2 enrichment treatment was applied to plants growing under normal temperature conditions. CO2 enrichment involved flowing pure CO2 into the glasshouse and began when plants had reached the stage of fruit setting in the fifth internode. Temperature and humidity set points for the CO2 enrichment treatment were 30 °C and 90 %, respectively. When the set points were reached, glasshouse ventilation was initiated for 5 min during which the CO2 flow was halted. The minimum light level for enrichment was 200 µmol photons m–2 s–1 and the maximum ambient CO2 concentration was 800 µl l–1. Temperature and humidity conditions in the control, non‐enriched glasshouse were similar to those in the CO2‐enriched glasshouse.
Pollen extraction and in vitro pollen germination assay
Flower buds at various stages of development [designated as days to anthesis, A, A–1, A–2, etc.] were collected from plants in each treatment following 12 d of treatment and were incubated with their peduncles in water for 24 h at 26 °C in light (500 µmol photons m–2 s–1), until the corollas were fully open. Anthers of each flower were harvested and weighed, and pollen grains were collected from some of them.
Five replicates of two anthers per flower were used in the pollen germination assay. Each anther was cut transversely and shaken to release the pollen grains into 200 µl of germinating solution containing 100 g l–1 sucrose, 2 mm calcium nitrate, 2 mm magnesium sulfate, 1 mm potassium nitrate and 2 mm boric acid. The released pollen grains were allowed to germinate for 24 h at 25 °C under constant fluorescent light. Drops of the germinating solution containing pollen grains were mounted on a haemocytometer slide and the grains were counted under a light microscope. The percentage of germinating pollen was determined by counting the number of germinating pollen grains in eight fields (each containing at least 50 pollen grains). Germinating pollen grains were classified into the following size groups: 1, tip‐germination (pollen grains with an extended tip only); 2, > ×10 (grains with elongated pollen tubes); and 3, non‐germinating grains. The percentage of pollen grains in each group was calculated.
Sugar assays
To assay pollen sugars, 30 flowers at A, A–1, A–2, A–4, A–6 and A–8 were collected and their anthers were separated from the corolla. Each anther was cut transversely and immersed in a tube containing 5 ml cold (4 °C) germination solution without sucrose. The tubes were shaken thoroughly to release the pollen. The suspended pollen grains were pelleted by centrifugation at 10 000 g for 10 min. Sugars were extracted by resuspending the resulting pollen pellet in 10 ml of 800 ml l–1 ethanol at 80 °C for 30 min, and the suspension was centrifuged (10 000 g for 10 min) to remove non‐extractable residue. This procedure was repeated twice. The ethanolic solutions were combined and evaporated to dryness at 40 °C with the aid of continuous ventilation. The dried sugars were dissolved in 1 ml distilled water and stored frozen (–80 °C) until determination. Sucrose, the principal soluble sugar in pepper pollen grains, was determined using the anthrone reagent method as modified for determination of non‐reducing sugars (Van Handel, 1968). The ethanol‐insoluble residue was used for determination of starch concentration in the pollen. The insoluble material was dissolved in 2 ml 0·2 m KOH, incubated at 100 °C for 30 min, cooled and then adjusted to pH 5·0 using 2 m acetic acid. Digestion of starch was carried out by incubating samples with 3 ml water containing 200 units of amyloglucosidase for 1 h at 55 °C (Dinar et al., 1983). The released glucose was determined colorimetrically using dinitrosalicylic acid (Miller, 1959).
Extraction and assay of soluble and insoluble enzymes
FK and HK were determined as described by Schaffer and Petreikov (1997a). Twelve days after the start of each treatment 30 flowers at each stage were collected from ten plants per treatment. The flowers were allowed to open as described above and their anthers were separated. Each anther was cut transversely in half and the pollen grains were released into 5 ml extraction buffer by vigorous shaking. Pollen grains were counted in 0·5 ml of the pollen suspension. The rest of the pollen mixture was pelleted by centrifugation (10 000 g for 10 min). The pollen pellet and the residual anther tissue were kept at –80 °C until assayed. The anther tissue (1 g f. wt) and the pollen pellet were each ground with a mortar and pestle in 2 ml chilled extraction solution containing 50 mm HEPES–NaOH buffer (N‐(2‐Hydroxyethylpiperazine‐N (2‐ethane‐sulfonic acid) (pH 7·5), 1 mm MgCl2, 1 mm EDTA, 10 mm KCl, 2·5 mm DTT (DL‐Dithiothreitol), 20 mg insoluble PVP (polyvinylpolypyrrolidone), 3 mm DIECA (diethyldithiocarbamic acid) and centrifuged at 10 000 g for 30 min. The supernatant was precipitated with 80 % ammonium sulfate, centrifuged at 10 000 g for 10 min, resuspended in 1 ml of extraction buffer and desalted on a Sephadex G‐25 column with washing buffer containing 50 mm HEPES–NaOH (pH 7·5), 1 mm EDTA and 1 mm DTT. The desalted extract (soluble enzymes) was used for the enzyme assays. The pellet was washed three times with extraction buffer containing 20 mm HEPES‐NaOH (pH 7·5) with all other ingredients as above. The final pellet was resuspended in 1 ml of the latter solution.
Soluble HK and FK activities were assayed according to Schaffer and Petreikov (1997b) as follows: a total volume of 1 ml of the assay solution contained 200 µl of the extracted enzyme, 50 mm HEPES‐NaOH (pH 7·5), 5 mm MgCl2, 1 mm EDTA, 15 mm KCl, 1 mm NAD, 1 mm ATP and 2 units of NAD‐dependent glucose‐6‐phosphate dehydrogenase (G6PDH) (from Leuconostoc; Sigma, St Louis, USA). In some experiments MgCl2 or CaCl2 concentrations were varied. The HK reaction was initiated with 1 mm or varying concentrations of glucose. To assess FK activity, two units of phosphoglucose isomerase (PGI) (type III; Sigma) were added and the reaction was initiated with 1 mm or varying concentrations of fructose. Reactions were carried out at 37 °C and absorbance at 340 nm was monitored continuously. Enzymatic activities were expressed on the basis of protein content (Bradford, 1976), pollen grain number or fresh weight. Insoluble enzymes were assayed as follows: 200 µl of the resuspended pellet was incubated in 1 ml of the reaction mixture excluding NAD and G6PDH for 10 min at 37 °C. The mixture containing the resulting fructose‐6‐phosphate (F6P) or glucose‐6‐phosphate (G6P) was boiled, centrifuged at 10 000 g for 10 min, and NAD and G6PDH were added to the supernatant. The reduction of NAD was recorded as above.
RESULTS
FK and HK activity
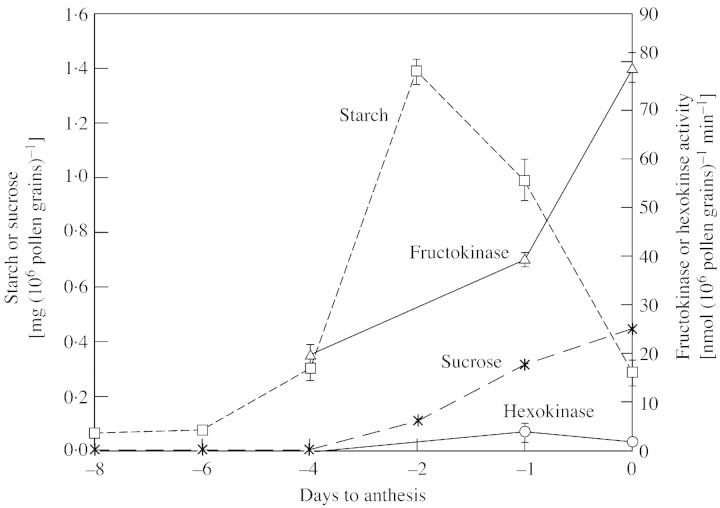
FK and HK activity was found mainly in the soluble fraction of pepper pollen, with little activity (less than 10 % of the total) in the insoluble fraction (data not shown). Therefore, further study was restricted to the soluble fraction. The activity of FK increased during pollen grain development. There was a sharp increase in enzyme activity 1 d before anthesis that lasted until anthesis. This pattern parallelled the change in pollen sucrose concentration, but the rise in FK activity occurred as pollen starch content declined (Fig. 1). In contrast, HK activity was low and did not change significantly during pollen grain development.
Fig. 1. Changes in the activity of fructokinase and hexokinase over the course of pollen development in relation to changes in pollen grain starch content. Stages of pollen development are designated in relation to days before anthesis (A, A–1, A–2, etc.). Data are means (n = 5) ± s.e. Assay mixtures contained 1–30 µg protein.
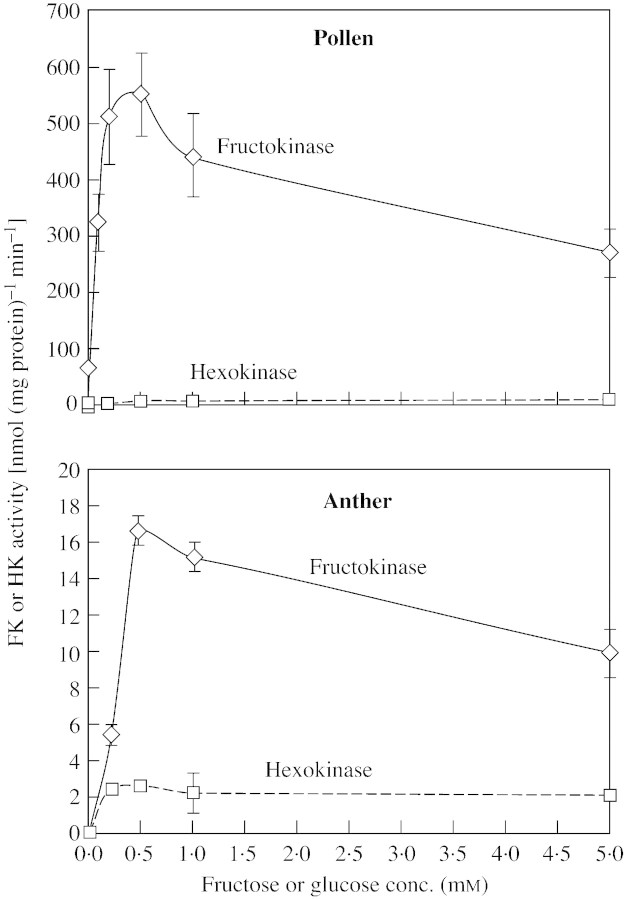
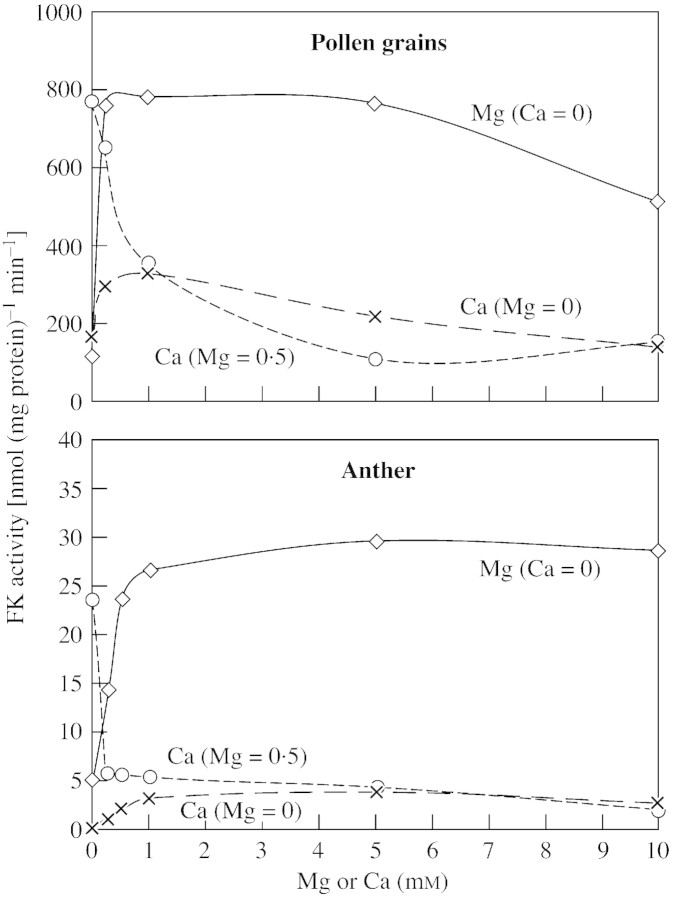
FK activity from the pollen grains and the anther tissue was reduced as the fructose concentration was increased above 1 mm. On the other hand, HK activity was not affected by high glucose concentrations (Fig. 2). Magnesium was essential for FK activity in both pollen and anther tissue, and calcium was no substitute (Fig. 3). However, there were some differences in the response of FK activity from the different organs towards magnesium: Mg2+ was slightly inhibitory above 5 mm to pollen grain FK, but FK activity from the anther tissue was not reduced by high Mg2+ concentrations. In the presence of optimal magnesium concentrations (0·5 mm), calcium was strongly inhibitory towards pollen and anther FK, even at the lowest concentration tested (100 µm).
Fig. 2. The effect of substrate concentration in the reaction mixture on the activity of fructokinase extracted from pollen grains (A) and anther tissue (B). Data are means (n = 5) ± s.e. Assay mixtures contained 20 µg protein.
Fig. 3. Effects of CaCl2 and MgCl2 concentrations in the reaction mixture on the activity of fructokinase extracted from pollen grains (A) and anther tissue (B). The dashed line with open circles represents the effect of CaCl2 in the presence of 0·5 mm MgCl2. Data are means (n = 5) ± s.e. Each assay mixture contained 20 µg protein.
Effects of high temperature and high CO2
Exposing pepper plants with flower buds to 12 d of high temperature, beginning when microspores were at meiosis and continuing until anthesis, caused a substantial reduction in the percentage of mature pollen grains germinating and also reduced the percentage of pollen grains with an elongated germination tube. On the other hand, pollen grain germination was enhanced in plants grown under normal temperature conditions with increased concentrations of CO2 (Table 1).
Table 1.
Effect of high temperature and CO2 enrichment on pollen germination and on the fraction of elongated pollen tubes
| Pollen germination | |||
| Treatment (temp. °C) | % of total | % of control | Elongated pollen tubes (% of total) |
| 26/22 | 37 ± 3 | 100 | 15 ± 2 |
| 32/26 | 12 ± 2 | 32 | 0 |
| 26/22 | 31 ± 3 | 100 | 17 ± 3 |
| 26/22+ CO2 | 48 ± 5 | 154 | 29 ± 3 |
Control treatment is considered as 26/22 °C (day/night) at normal CO2 concentration. Data are means (n = 8) ± s.e.
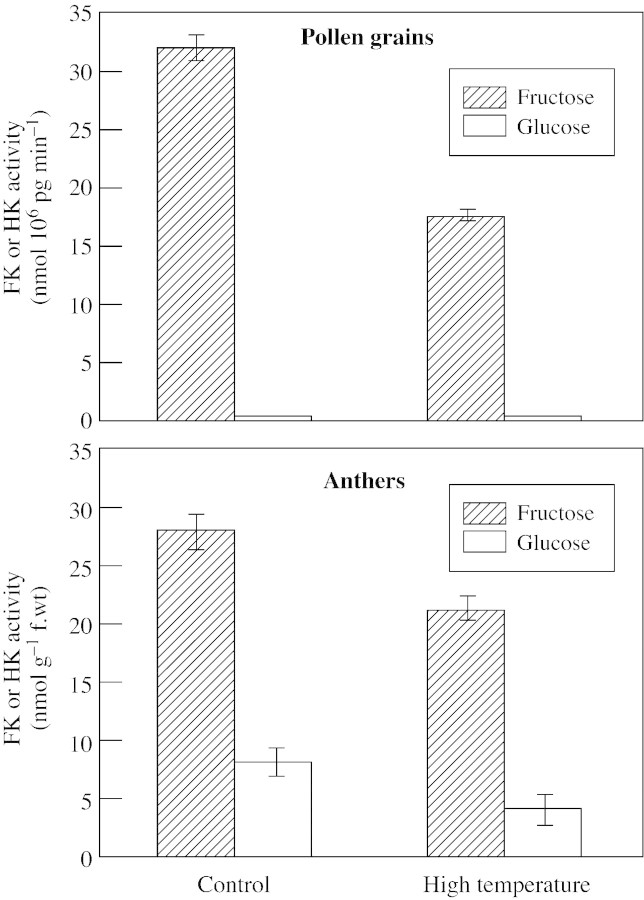
High temperature caused a reduction of approx. 50 % in the activity of FK in mature pollen grains. The activity of HK in pollen grains was relatively low and was not affected by high temperature (Fig. 4). In the anther tissue, activities of both FK and HK were also reduced by high temperature treatment. CO2 enrichment increased pollen FK activity, whereas that of HK was increased only slightly by this treatment. Anther FK and HK activities were also increased when the flower reached anthesis under CO2 enrichment (Table 2).
Fig. 4. Effect of high temperature (32/26 °C, day/night) on FK and HK activities extracted from pollen grains (A) and anther tissue (B). Data are means (n = 5) ± s.e. Each assay mixture contained 10–30 µg protein.
Table 2.
Effects of CO2 enrichment on the activities of soluble fructokinase and hexokinase from different organs of pepper flowers
| Enzymatic activity | ||||
| Fructokinase | Hexokinase | |||
| Organ | Control | High CO2 | Control | High CO2 |
| nmol (10–6 pollen grains)–1 min–1 | ||||
| Pollen | 32 ± 2 | 48 ± 3 | 3·0 ± 0·1 | 3·8 ± 0·1 |
| nmol (mg protein)–1 min–1 | ||||
| Pollen | 650 ± 100 | 1080 ± 85 | 56 ± 7 | 84 ± 8 |
| Anther | 3·6 ± 0·5 | 4·9 ± 0·2 | 1·3 ± 0·2 | 2·6 ± 0·3 |
| nmol (g f.wt used in test)–1 min–1 | ||||
| Anther | 15 ± 1·2 | 25 ± 1·8 | 2·0 ± 0·3 | 2·8 ± 0·2 |
Control treatment is 26/22 °C (day/night) and high CO2is 26/22 °C + high CO2.
Data are means (n = 5) ± s.e.
DISCUSSION
Fructokinase and hexokinase are both pivotal enzymes for the initial step of sugar utilization in sink tissues (Quick and Schaffer, 1996). Pollen grains are considered to be a sink, and during pollen development the incoming sucrose is initially cleaved by invertase to fructose and glucose and these are subsequently phosphorylated by FK and HK, respectively. Therefore, these two enzymes are expected to exhibit similar rates of activity. However, Nakamura et al. (1991) found that, in pollen, FK was predominant whereas the activity of HK was relatively low. In agreement with this finding, the present experiments showed that the activity of soluble FK is several times higher than that of soluble HK in pepper pollen, and that a sharp increase in FK activity occurred just before anthesis (Fig. 1). It has been reported that part of the HK protein is particulate, especially in mitochondrial membranes, and that this form is strongly inhibited by micromolar concentrations of ADP (Da Silva et al., 2001). Nevertheless, in the present study less than 10 % of FK and HK activities were associated with the pollen pellet (data not shown). The particulate form of HK might have been inhibited by ADP, but the ADP concentrations generated in the reaction mixture were calculated to be in the nanomolar range in the present study, which would make any such inhibition insignificant. The pattern of change in FK activity suggests that this enzyme has an important role in pollen germination. FK has been suggested to be associated with starch accumulation (Kanayama et al., 1998). However, this enzyme seems to have a different role in the pollen grain, since FK activity reached a maximum at anthesis, while starch content in the pollen grain was maximal at 4 d before anthesis, and had almost completely disappeared at anthesis (Fig. 1). These findings suggest that in pollen, FK may function either to provide fructose‐6‐phosphate for glycolysis or, through conversion to UDPglucose, support the biosynthesis of cell wall material for pollen tube growth. Since HK activity in the mature pollen grain was low, the metabolic fate of glucose liberated by pollen invertase remains unclear. A possible explanation is that the demand for glucose‐6‐phosphate for the germination process is low and the low HK activity is sufficient to meet that demand. On the other hand, fructose‐6‐phosphate may rapidly be produced and utilized in different metabolic processes with only part of it being diverted to pollen germination. In agreement with this suggestion, Nakamura et al. (1980) have shown that the concentration of glucose‐6‐phosphate is two to five‐fold higher than that of fructose‐6‐phosphate in mature pollen grains of Camellia japonica.
FK from the pollen grains and from the anther tissue was inhibited by high fructose concentrations (Fig. 2) and required Mg2+ for activity, indicating that this enzyme is similar to other FKs, designated as FK II, which are found in other plant species (Copeland et al., 1978; Copeland and Morell, 1985; Nakamura et al., 1991). Activity of HK from pollen grains and from anther tissue was not inhibited by glucose, as found previously in Lilium (Nakamura et al., 1991). It is still not clear whether the differing substrate responses of FK and HK have any physiological significance. Pollen FK activity was inhibited slightly by 10 mm Mg2+, while anther FK was not affected by this Mg2+ concentration. It has recently been demonstrated that there are at least three isoforms of FK (Pego and Smeekens, 2000; Petreikov et al., 2001), of which only FK II is inhibited by Mg2+ (Schaffer and Petreikov, 1997b). This suggests that FK II is more abundant in pepper pollen, whereas additional FK forms also operate in the anther tissue. FK activity in pollen grains and anther tissue was strongly inhibited by Ca2+ in the presence of an optimal magnesium concentration (Fig. 3). The inhibition of FK by calcium may have a physiological significance; this needs to be explored further.
High temperature and high ambient CO2 concentration were shown here to have contrasting effects on in vitro pollen germination (Table 1). It has been suggested that these two environmental factors act via their influence on photosynthesis and on sugar supply to the developing flowers (Aloni et al., 2001). Aneja et al. (1992) found that CO2 and temperature had direct effects on pollen germination in cocoa. We have previously shown that high temperature and CO2 enrichment caused a decrease and an increase, respectively, in pepper pollen acid invertase activity, but the enzyme specific activity was not changed (Aloni et al., 2001). FK activity in pepper pollen grains was reduced at high temperature (Fig. 4) and enhanced under conditions of CO2 enrichment (Table 2). In the anther, both FK and HK activities decreased under high temperature conditions (Fig. 4) and were enhanced by CO2 enrichment (Table 2), suggesting that in this tissue HK and FK are regulated in the same fashion. Fructokinase and hexokinase in the anther tissue that surrounds the pollen may also have a role in determining pollen development and germination capacity, since the anther walls control sugar nutrition in Lilium pollen grains (Clement and Audran, 1995).
Overall, it remains to be seen whether the high temperature and high CO2 effects on FK and HK activities are specific. Recently Aloni et al. (2001) observed similar responses to these environmental treatments in pollen grain acid invertase. This suggests that sugar metabolism may change in a coordinated manner to these environmental conditions. It should be noted that FK and HK activities were affected even if calculated on a protein basis (Table 2), indicating that this was not merely a result of changes in overall protein synthesis or degradation.
One possible approach to verify the role of FK and HK in pollen grains and anther tissue is to transform plants with the corresponding genes under pollen‐ or anther‐specific promoters and to assay their expression and their effects on pollen performance. Such research is currently underway in our laboratory.
ACKNOWLEDGEMENTS
Contribution from the Agricultural Research Organization, The Volcani Center, Bet‐Dagan. Israel. No. 135/2001.
Supplementary Material
Received: 11 June 2002; Returned for revision: 8 July 2002; Accepted: 24 July 2002 Published electronically: 2 October 2002
References
- AloniB, Peet MM, Pharr M, Karni L.2001. The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112: 505–512. [DOI] [PubMed] [Google Scholar]
- AnejaM, Gianfagna TNE, Badilla I.1992. Carbon dioxide and temperature influence pollen germination and fruit set in cocoa. HortScience 27: 1038–1040. [Google Scholar]
- BradfordMM.1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- ClementC, Audran JC.1995. Anther wall layers control pollen sugar nutrition in Lilium Protoplasma 187: 172–181. [Google Scholar]
- CopelandL, Morell M.1985. Hexose kinases from the plant cytosolic fraction of soybean nodules. Plant Physiology 79: 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CopelandL, Harrison DD, Turner JF.1978. Fructokinase (fraction III) of pea seeds. Plant Physiology 62: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da SilvaWS, Rezende GL, Galina A.2001. Subcellular distribution and kinetic properties of cytosolic and non‐cytosolic hexokinase in maize seedling roots: implication for hexose phosphorylation. Journal of Experimental Botany 52: 1191–1201. [PubMed] [Google Scholar]
- DinarM, Rudich J, Zamski E.1983. Effect of heat stress on carbon transport from tomato leaves. Annals of Botany 51: 97–103. [Google Scholar]
- DorionS, Lalonde S, Saini HS.1996. Induction of male sterility in wheat by meiotic‐stage under water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiology 111: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HanXB, Li RQ, Wang JB, Miao C.1996. Effect of heat stress on pollen development and pollen viability of pepper. Acta Horticultura Sinica 23: 359–364. [Google Scholar]
- KanayamaY, Granot D, Dai N, Petreikov M, Schaffer A, Powell A, Bennett AB.1998. Tomato fructokinases exhibit differential expression and substrate regulation. Plant Physiology 117: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnightMR, Campbell AK, Smith SM, Trewavas AJ.1991. Transgenic plant aequorin reports the effects of touch, cold shock and elicitors on cytoplasmic calcium. Nature 352: 524–526. [DOI] [PubMed] [Google Scholar]
- LynchJ, Polito VS, Lauchli A.1989. Salinity stress increases cytoplasmic Ca activity in maize root protoplasts. Plant Physiology 90: 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MillerGL.1959. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry 31: 426–428. [Google Scholar]
- NakamuraN, Sado M, Arai Y.1980. Sucrose metabolism during the growth of Camelia japonica pollen. Phytochemistry 19: 205–209. [Google Scholar]
- NakamuraN, Shimizu M, Suzuki H.1991. Characterization of hexose kinases from camellia and lily pollen grains. Physiologia Plantarum 81: 215–220. [Google Scholar]
- PeetMM, Sato S, Gardner RG.1998. Comparing heat stress effects on male‐fertile and male‐sterile tomatoes. Plant Cell and Environment 21: 225–231. [Google Scholar]
- PegoJV, Smeekens CM.2000. Plant fructokinases: a sweet family get‐together. Trends in Plant Science 5: 531–536. [DOI] [PubMed] [Google Scholar]
- PetreikovM, Dai N, Granot D, Schaffer AA.2001. Characterization of native and yeast‐expressed tomato fruit fructokinase enzymes. Phytochemistry 58: 841–847. [DOI] [PubMed] [Google Scholar]
- PriceAH, Tayler A, Ripley SJ, Griffiths A, Trewavas AJ.1994. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QuickWP, Schaffer AA.1996. Sucrose metabolism in sources and sinks. In: Zamski E, Schaffer AA, eds. Photoassimilate distribution in plants and crops. New York, Basel, Hong Kong: Marcel Dekker, Inc. 115–156. [Google Scholar]
- SainiHS.1997. Effects of water stress on male gametophyte development in plants. Sexual Plant Reproduction 10: 67–73. [Google Scholar]
- SchafferAA, Petreikov M.1997a Sucrose‐to‐starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiology 113: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchafferAA, Petreikov M.1997b Inhibition of fructokinase and sucrose synthase by cytosolic levels of fructose in young tomato fruit undergoing transient starch synthesis. Physiologia Plantarum 101: 800–806. [Google Scholar]
- SheoranIS, Saini HS.1996. Drought‐induced male sterility in rice: changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sexual Plant Reproduction 9: 161–169. [Google Scholar]
- SomssichIE, Hahlbrock K.1998. Pathogen defense in plants – a paradigm of biological complexity. Trends in Plant Science 3: 86–90. [Google Scholar]
- Van HandelE.1968. Direct microdetermination of sucrose. Analytical Phytochemistry 22: 280–283. [DOI] [PubMed] [Google Scholar]
- WienHC.1997. Peppers. In: Wien HC, ed. The physiology of vegetable crops. Wallingford: CAB International, 259–293. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.