Abstract
In protogynous plants, female flowers of early blooming plants are at a reproductive disadvantage because they cannot set fruit due to the lack of available pollen. To study this phenomenon, gender expression of the monoecious herb Sagittaria trifolia was investigated over the entire flowering season in two field and two cultivated populations in Hubei and Hunan Provinces, China. In racemes of S. trifolia, flowers open sequentially from bottom to top, with female flowers opening first followed by male flowers. This creates a temporal separation of sexes in the species. Under field conditions small plants are often male, with production of both male and female flowers increasing with plant size. Femaleness increased among sequential inflorescences since female flower production increased whereas male flower production did not. Seed production was greater in large inflorescences because they contain more female flowers, and the number of ovules increased in female flowers at basal positions within the raceme. A consistent pattern of high seed set was observed in flowers from both field and cultivated populations. About 1 % of unfertilized ovules resulted from no pollination and 2 % of the seeds produced were only partly developed due to resource limitation. In the first inflorescence of the six experimental populations, 6·7–40·0 % of individuals produced only male flowers, and female flowers of 1·9–6·5 % individuals were aborted. The occurrence of male flowers in early blooming inflorescences could be an adaptive strategy to conserve resources and enhance pollination of female flowers in protogynous S. trifolia.
Key words: Sagittaria trifolia, gender variation, sequential inflorescences, pollination, seed production, monoecy, dioecy, dichogamy
INTRODUCTION
Plant species may separate their sex functions spatially and temporally, as well as by physiological self‐incompatibility (Wyatt, 1983). If there is no overlap between male and female phases of a monoecious plant, an early blooming flower—whether it is in male or female phase—will be unlikely to achieve reproductive success because only one gender may exist in the population. There are many reports of the advantages of sex segregation, but few studies have considered the effects of single‐sex, early blooming flowers and the detrimental effects of sex separation in dichogamous plants. Darwin (1877) deduced that the first flowers to open sometimes aborted because they were of no use in dichogamous hermaphrodite plants. Although dichogamy is considered an important factor influencing gender expression (de Jong, 1976; Lloyd 1980b; Pellmyr, 1987; Brunet and Charlesworth, 1995), its roles in promoting the evolution of gender specialization are still poorly understood.
Plants groups displaying a gradation of gender specialization provide good opportunities to investigate the possible transitional pathways from one sexual system to another (Thomson and Brunet, 1990; Freeman et al., 1997; Sakai and Weller, 1999). Sagittaria is one such taxon enabling studies of the evolution of dioecy from monoecy (Delesalle and Muenchow, 1992; Barrett et al., 2000; Sarkissian et al., 2001). There are about 20 species in the genus, most of which are basically monoecious (Bogin, 1955). Some species display remarkable variation in gender expression in different populations (Barrett et al., 2000). For example, Sagittaria latifolia consists of various populations ranging from monoecy to complete dioecy (Wooten, 1971; Muenchow and Delesalle, 1994; Sarkissian et al., 2001). Sagittaria lancifolia subsp. lancifolia was described as subandrodioecious, a rare breeding system in plants (Muenchow, 1998). Seven species of Sagittaria occur in China (Chen, 1989), of which S. guyanensis subsp. lappula is typically andromonoecious (Huang et al., 2000). The other six species of Sagittaria are monoecious. Among these, S. trifolia L. embodies the greatest variation in morphological characters as well as gender expression. Chen (1989) previously recorded the occurrence of male plants in populations of S. trifolia. Several subspecies of this species have been described (Chen, 1989); this study concentrated on S. trifolia subspecies trifolia.
Temporal variation of gender was investigated during the entire flowering season of Sagittaria trifolia. This was undertaken to assess whether temporal separation in gender (dichogamy) influences gender specialization in this monoecious species. Of particular interest was gender variation of early blooming inflorescences in this protogynous species. Three specific questions were addressed: (1) is S. trifolia a strictly monoecious species or does androdioecy also occur? Experiments were carried out to determine whether male morphs occurring in the field were simply small individuals that were capable of female function if they were larger, or whether gender expression was stable and they were strictly male. (2) Do sequential inflorescences produced by a plant display differences in gender, and what is the reproductive fate of early blooming female flowers? (3) Is reproductive success of the species pollen‐limited and/or resource‐limited? In particular, the number of female and male flowers varies daily in populations of S. trifolia, changing the mating environments of flowers during the flowering sequence (Huang et al., 1999). If dichogamy affects floral sex allocation in sequential inflorescences of monoecious species, theory predicts that protogyny would be expected to select for male‐biased allocation in early inflorescences compared with late inflorescences (Brunet and Charlesworth, 1995). In the mating environment of a male‐biased sex allocation, we predict that fruit and seed set are less likely to be pollen limited.
MATERIALS AND METHODS
Study species and populations
Sagittaria trifolia (Alismataceae) is a widely distributed perennial, emergent, aquatic plant in Asia (Chen, 1989). It occurs in the shallow waters of marshes, ponds, stream margins and rice fields. Plants reproduce both sexually and clonally by producing shoot tubers. Unisexual flowers with three white petals produce numerous stamens or carpels and both present floral nectar. Each carpel has a terminal stigma and a basal ovary with a single ovule. Approximately 3 weeks after pollination, one carpel will mature into one achene. Flowers are generally arranged in whorls of three and open sequentially from bottom to top within an inflorescence. Flowers open for a single day only. In an inflorescence, female flowers in the lower whorls bloom for 1–2 d followed by male flowers in the upper whorls which bloom over a period of 4–10 d. Less than 3 % of male and female flowers of individuals were found to open in a day (Huang et al., 1999). Sagittaria trifolia is self‐compatible and is pollinated by a variety of insects including flies, bees and syrphids (Huang et al., 1999).
Two field populations of S. trifolia approx. 420 km apart were studied from July to September in 1999 and 2000. The ‘Hao’ population occupies the edge of a 16 ha cultivated lotus pond, located in Haogou Cun, Wuchang, Hubei Province (30°40′N, 114°22′E), China, and the ‘Cha’ population is sparsely distributed over a 300 ha wetland located in Chaling, Huli Wetland, a natural reserve of Hunan Province (26°50′N, 113°42′E), China. Ninety‐one plantlets at the two‐ or three‐leaf stage were transplanted from the Hao population in May 2000 to the Garden of Wuhan University, 8 km away.
To examine whether sex expression varies with plant resource status, these plantlets were randomly divided into two treatments: 45 plantlets were cultivated in loam soil mixed with 50 % sand (garden 1) and 46 plantlets were cultivated in loam soil without sand (garden 2). The two treatments were devised to assess the influence of plant size on female and male flower production. Floral visitors to S. trifolia were observed in the two experimental populations; the visitors were similar to those observed in field populations.
Measurement of gender variation
Gender variation was measured in sequential inflorescences following the method of Lloyd (1980a) and Thomson and Barrett (1981). All inflorescences produced by a plant were recorded once a week in the Hao population and twice a week in the Cha population. Sex expression of every tagged individual was recorded by counting male and female flowers in each inflorescence. During the flowering period from July to September, the two experimental populations were visited daily. Male and female flowers produced in each inflorescence were counted in all six populations (garden 1, garden 2, Hao 1999, Hao 2000, Cha 1999 and Cha 2000).
To investigate whether size‐dependent patterns of female and male flower production occur in S. trifolia [for evidence of this in S. latifolia see Sarkissian et al. (2001)], 45 plants were collected at random from garden 1 and garden 2 at the end of September. Plant height, mid‐vein length of the largest leaf, circumference of the largest peduncle (measured at the level of the lowest whorl of flowers) and biomass of each plant were recorded. The dry mass of leaves with roots and inflorescences was also measured. Some fruits released by the early inflorescences were also collected and were included in the mass of all inflorescences.
Measurement of Lloyd’s phenotypic gender (Gi) (Lloyd, 1980a) of S. trifolia plants followed the methods outlined for S. latifolia (Sarkissian et al., 2001).
Seed production
To determine whether seed production of S. trifolia is pollen‐limited and/or resource‐limited, the status of ovules in female flowers was observed. In a previous study it was noted that stigmatic pollen loads of this species are very high. All of the stigmas of plants in the Hao population had pollen deposited on them (Huang et al., 1999). Carpels and ovules did not enlarge when flowers were bagged to prevent pollination, indicating that pollen deposition is required for seed set. Some carpels and ovules were only slightly enlarged despite the flowers being cross‐pollinated by hand. Three types of achenes were recognized according to size: (1) fully developed achenes; (2) partly developed achenes that were smaller than fully developed achenes and had a slightly developed ovary that received pollen but whose development was constrained; and (3) undeveloped achenes that were the same size as unfertilized carpels which did not receive pollen. This finding permits us to analysis pollen or resource limitation based on the development of ovules in S. trifolia. Inflorescences from both field and cultivated populations were collected at random in August and September 2000 before achenes were dispersed. Achenes were classified as described above in each aggregate fruit and the location of this fruit in the inflorescence was recorded.
Statistical analyses
All statistical tests were performed using the SAS program (SAS Institute, 1998). Correlation analysis and tests of significance were used to determine relationships between male and female flower production and plant size. Differences in flower production or floral sex ratio between populations or between individuals within populations as well as between inflorescences produced sequentially on a plant were tested by ANOVA and a posteriori Games–Howell tests. Intra‐ and inter‐population variation in ovule and seed production was also tested by ANOVA.
RESULTS
Plant size and phenotypic gender
Biomass allocation to inflorescences increased significantly with vegetative biomass in the two cultivated populations (R2 = 0·887, F1,44 = 346·852, P < 0·0001). Total flower production, male and female flower production, and floral sex ratio were positively correlated with the four measurements of plant size (Table 1). Large individuals produced more inflorescences and more flowers. Production of both female and male flowers increased with plant size, but the correlation was stronger for female flower production than male flower production, with coefficients consistently higher for all four measurements of plant size. Floral sex ratio was also related to plant size in the cultivated populations but its coefficient was lower than that for male and female flower production.
Table 1.
Relationship between plant size and flower production in Sagittaria trifolia
| Total number of flowers | Number of male flowers | Number of female flowers | F/M ratio | |||||
| F | R 2 | F | R 2 | F | R 2 | F | R 2 | |
| Leaf weight | 30·671*** | 0·588 | 23·638*** | 0·524 | 38·114*** | 0·639 | 10·764** | 0·334 |
| Leaf length | 22·902*** | 0·516 | 16·880*** | 0·440 | 35·080*** | 0·620 | 10·905*** | 0·337 |
| Plant height | 19·854*** | 0·480 | 17·032*** | 0·442 | 19·758*** | 0·479 | 5·909* | 0·216 |
| Peduncle circumference | 38·708*** | 0·643 | 30·933*** | 0·590 | 45·005*** | 0·677 | 9·725** | 0·311 |
Dry mass of all leaves produced, length of the longest leaf, circumference of the largest peduncle (measured at the level of the lowest whorl of flowers) and height of each plant were measured for 45 plants from two cultivated populations.
*P < 0·01; **P < 0·001; ***P < 0·0001.
Gender variation in populations
Total flower production, male and female flower production, and floral sex ratio varied significantly among the six experimental populations (Table 2). The plants of garden 2 growing in a high‐nutrient soil produced larger inflorescences and more female flowers than those of garden 1. Comparing average flower production of garden 2 with garden 1 plants (Table 1), the proportion of female flowers increased more (40·5/16·2 = 2·5) than that of males (164·9/123·1 = 1·3). In the Hao 2000 population, plants had larger inflorescences than in 1999. In Cha 2000, female flower production was especially low, probably because most plants were small. Small plant stature was related to late flowering in August because heavy rains meant that the water level in the wetland was high that year.
Table 2.
Mean number of flowers produced (± s.d.), floral sex ratio, significance tests and relationships between male and female flower production (F value and R2) in six experimental populations of Sagittaria trifolia
| Population | Number of individuals | Total number of flowers | Number of male flowers | Number of female flowers | F‐value | R 2 | F/M ratio |
| Garden 1 | 45 | 139·4 ± 82·8 | 123·1 ± 72·5 | 16·2 ± 12·2 | 91·771*** | 0·681 | 0·136 |
| Garden 2 | 46 | 205·3 ± 107·0 | 164·9 ± 80·6 | 40·5 ± 29·8 | 109·57*** | 0·713 | 0·233 |
| Hao 1999 | 71 | 110·3 ± 74·1 | 93·2 ± 62·0 | 17·1 ± 13·3 | 252·955*** | 0·781 | 0·184 |
| Hao 2000 | 83 | 165·0 ± 91·7 | 138·3 ± 71·6 | 26·7 ± 24·0 | 135·055*** | 0·625 | 0·176 |
| Cha 1999 | 105 | 102·8 ± 56·3 | 83·9 ± 44·9 | 18·8 ± 14·0 | 145·362*** | 0·585 | 0·225 |
| Cha 2000 | 105 | 48·9 ± 22·9 | 41·5 ± 19·0 | 7·3 ± 5·8 | 54·975*** | 0·35 | 0·179 |
| ANOVA results F5,450 | 42·512*** | 44·332*** | 28·161*** | 7·119*** |
Inter‐population comparison of male and female flower production and floral sex ratio is also shown.
*** P < 0·0001.
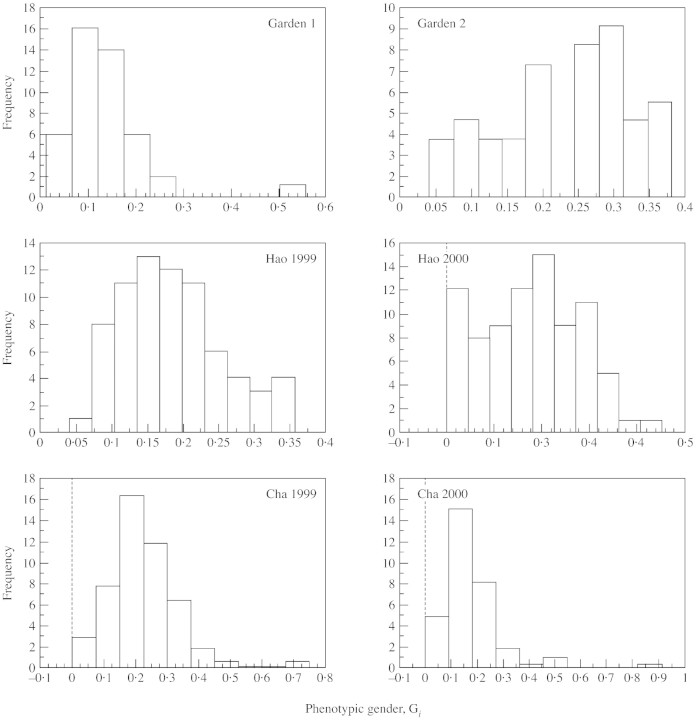
No plants that only produced male flowers occurred in either of the cultivated populations. Some plants in field populations of Hao 2000, Cha 1999 and Cha 2000 were male only and did not produce female flowers. These male plants were small and produced only one inflorescence. No plant with more than one inflorescence was male only. In general, gender variation among the six populations was male‐biased (Fig. 1). Analysis of variance indicated a significant difference in total flower production, male and female flower production, and floral sex ratio among the six populations (Table 2). Games–Howell tests showed significant differences in these four parameters between the two samples from garden, Hao and Cha, except male flower production between garden 1 and 2 and floral sex ratio in both years from Hao and Cha. Male and female flower production was positively correlated in all populations (Table 2).
Fig. 1. Variation of phenotypic gender in six experimental populations of Sagittaria trifolia. The observed frequency distribution of standardized phenotypic femaleness (Gi) is shown for plants of each sample (Gi = 0 for pure male; Gi = 1 for pure female).
Temporal trends in female and male flower production
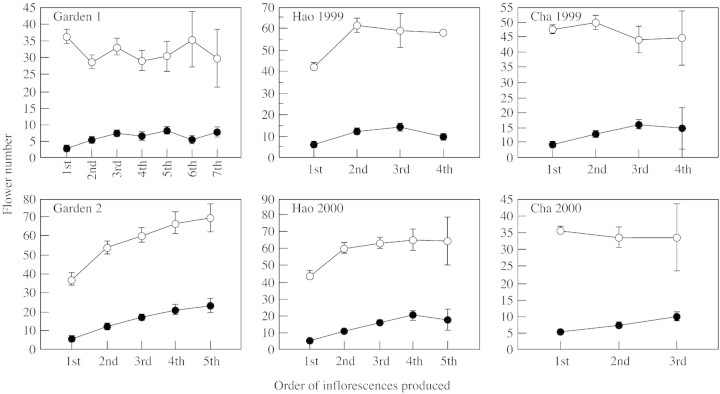
In the six experimental populations there was no consistent relationship between the number of both male and female flowers produced by sequential inflorescences on a plant, but the trend for female flower production was more distinct than that for males (Fig. 2). Average female flower production of the last inflorescences decreased in Hao 1999, Hao 2000 and Cha 1999, perhaps due, in part, to the limited number of individuals with four or five inflorescences in these samples (Table 3). In garden 2 plants, which received sufficient resources, the numbers of both male and female flowers increased gradually in sequential inflorescences.
Fig. 2. Production of male (open circles) and female flowers (closed circles) in sequential inflorescences of Sagittaria trifolia in six experimental populations. Bars represent ± 1 s.d.
Table 3.
Percentage of inflorescences with pure male flowers and aborted female flowers (which occurred in the first inflorescence only) and the total number of inflorescences in the blooming sequence in six experimental populations of Sagittaria trifolia
| First inflorescence | Second inflorescence | Third inflorescence | Fourth inflorescence | Fifth inflorescence | Sixth inflorescence | Seventh inflorescence | |||||||||
| Population | Total | % Pure male | % Aborted female | Total | % Pure male | Total | % Pure male | Total | % Pure male | Total | % Pure male | Total | % Pure male | Total | % Pure male |
| Garden 1 | 45 | 40·0 | 4·4 | 44 | 15·9 | 37 | 8·1 | 24 | 0 | 14 | 0 | 7 | 0 | 4 | 0 |
| Garden 2 | 46 | 28·3 | 6·5 | 43 | 0 | 31 | 3·2 | 17 | 5·8 | 8 | 12·5 | ||||
| Hao 1999 | 71 | 14·1 | 4·2 | 46 | 0 | 15 | 0 | 1 | 0 | ||||||
| Hao 2000 | 83 | 33·7 | 2·4 | 69 | 8·7 | 39 | 7·7 | 14 | 7·1 | 4 | 25 | ||||
| Cha 1999 | 105 | 6·7 | 1·9 | 64 | 4·7 | 11 | 0 | 2 | 0 | ||||||
| Cha 2000 | 105 | 10·5 | 2·9 | 16 | 6·2 | 4 | 0 | ||||||||
Variation in male flower production between sequential inflorescences was non‐significant in garden 1, Cha 1999 and Cha 2000, but was significant in the other three populations (Table 4). All populations showed significant differences among inflorescences for female flower production. Games–Howell tests showed that the difference existed between the first inflorescences and the other sequential inflorescences, with the exception of the second with the third and fourth inflorescences in garden 2, and the second and third inflorescences in Hao 2000 (Table 4). The ratio of female to male flower production (F/M) was significantly different among inflorescences in all populations except Cha 1999. Games–Howell tests showed that this difference in floral sex ratio existed only between the first inflorescence and the remainder (Table 4). If the first inflorescence is excluded from this analysis, no significant difference was found for floral sex ratio or male flower production between inflorescences in any population. However, there were still significant differences for female flower production in garden 2 and Hao 2000 (Table 4).
Table 4.
ANOVA results (F‐values) for male and female flower production and ratios of female to male flowers (F/M) in sequential inflorescences of S. trifolia in six experimental populations
| Comparison of sequential inflorescences | Excluding first inflorescence | |||||
| Sample | Male | Female | F/M | Male | Female | F/M |
| Garden 1 | 1·555 | 7·378*** | 6·846*** | 0·588 | 1·830 | 1·262 |
| 1 and 2–5 | 1 and 2–5 | |||||
| Garden 2 | 10·232*** | 27·228*** | 4·019* | 2·199 | 8·190*** | 0·415 |
| 1 and 2–5 | 1 and 2–5, 2 and 3–4 | 1 and 3 | 2 and 3–4 | |||
| Hao 1999 | 9·478*** | 18·484*** | 7·061** | 0·948 | 0·753 | 2·273 |
| 1 and 2 | 1 and 2–3 | 1 and 2–3 | ||||
| Hao 2000 | 8·495*** | 27·811*** | 7·673*** | 0·370 | 7·474*** | 1·938 |
| 1 and 2–4 | 1 and 2–4, 2 and 3 | 1 and 2–4 | 2 and 3 | |||
| Cha 1999 | 0·716 | 8·914*** | 0·095 | 0·857 | 2·607 | 0·130 |
| 1 and 2–3 | ||||||
| Cha 2000 | 1·095 | 6·103** | 16·063*** | 0·000 | 2·516 | 3·181 |
| 1 and 2 | 1 and 2 | |||||
The same analysis excluding the first inflorescence in each sample is also shown.
For each sample, the second row summarizes results of the Games–Howell tests but only significant differences between inflorescences are presented.
The number of inflorescences (sample size) used in this analysis is shown in Table 2.
*P < 0·01; **P < 0·001; ***P < 0·0001.
Inflorescences with male flowers only were found in various sequential inflorescences in the six populations. The majority occurred in the first inflorescence although a few plants produced male flowers in other inflorescences in the sequence (Table 3).
Early in the flowering period, all female flowers were aborted and no fruit was produced in the first inflorescences of 1·9–6·5 % of individuals (Table 3). These inflorescences had less than seven female flowers [5 ± 1·5 (mean ± s.e.), n = 15].
Ovule production and patterns of seed set
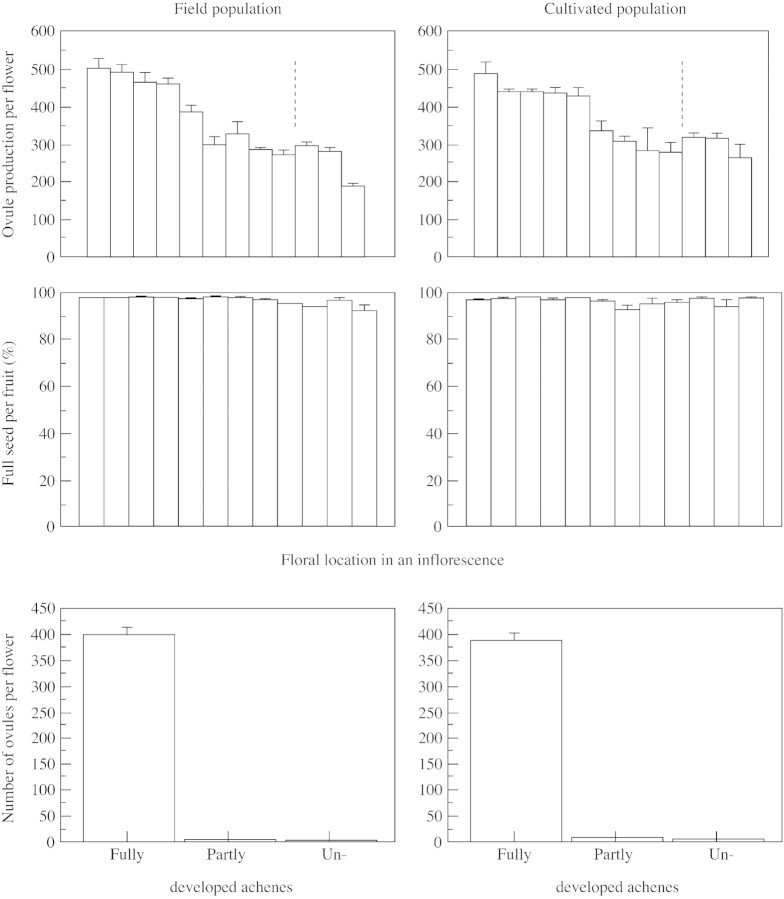
Ovule number per flower varied considerably within inflorescences of S. trifolia. For example, in an inflorescence with 20 female flowers, the first flower at the bottom had 553 ovules but flower number 15 in the top whorl of the main branch had 300 ovules (Fig. 3). Ovule production in female flowers on the lateral branch, which usually flowered at the same time as several upper whorls of flowers of the main branch, was also reduced. Flower number 20 in this inflorescence had 258 ovules. There was a significant linear decrease in ovule production between whorls from the bottom to the top of one inflorescence in both field and cultivated populations (Fig. 4). The mean ovule number per flower was not significantly different among inflorescences of differing size (6–30 flowers) (F1,8 = 0·276, P = 0·61, n = 158 female flowers on ten inflorescences). Large inflorescences with more female flowers had significantly more ovules than smaller inflorescences (F1,8 = 52·9, R2 = 0·87, P < 0·0001). Ovule number per flower was significantly different within cultivated and field populations (Table 5).
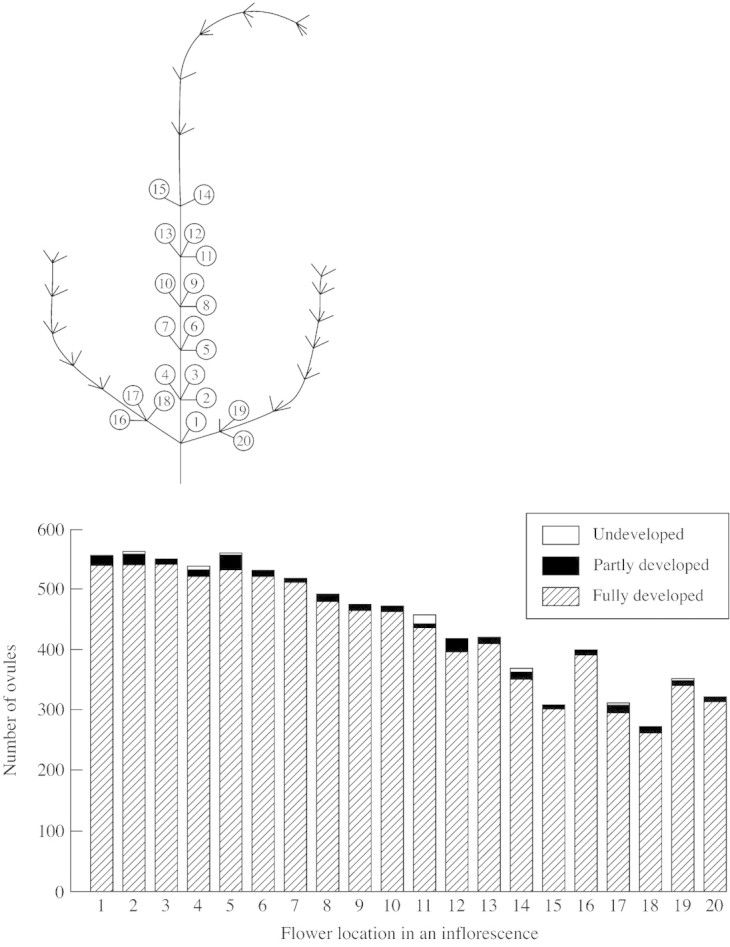
Fig. 3. Ovule production and flower location in an inflorescence of Sagittaria trifolia with 20 flowers. The proportion of fully developed, partly developed and undeveloped ovules is shown for each flower. Five flowers (numbers 16–20) are on the lateral branch of the inflorescence. The location of the 20 female flowers is indicated on the illustration. Male flowers are located on the upper whorls of each branch.
Fig. 4. Ovule production and seed set in field and cultivated populations of Sagittaria trifolia. Ovule production and the number of full seeds differ significantly between floral locations within each population but not between the two populations (see Table 5). No significant difference (P > 0·05) was observed within or between populations in the percentage of full seed set. Flowers to the right of the dotted lines are on lateral branches of inflorescences. Bars represent s.e.
Table 5.
ANOVA results (F‐values) for intra‐ and inter‐population variance of ovule and seed production in field (Hao 2000) and cultivated populations (gardens 1 and 2)
| Sample (d.f.) | Ovule number per flower | Fully developed seeds per fruit | Partly developed seeds per fruit | Undeveloped seeds per fruit |
| Field population (1,58) | 68·115*** | 67·254*** | 0·276 | 0·994 |
| Cultivated population (1,70) | 153·291*** | 157·817*** | 0·743 | 1·770 |
| Inter‐population comparison (1,136) | 0·985 | 1·050 | 0·902 | 0·298 |
*** P < 0·0001.
The pattern of seed set was the same in fruits collected from the Hao population and from the two cultivated populations, garden 1 and 2 (Fig. 4). Three types of seeds were found as about 1 % of ovules were undeveloped, 2 % were partly developed and over 96 % developed fully to mature seeds (Fig. 4). No difference was found between field and cultivated populations in the number of fully developed, partly developed or undeveloped seeds per fruit (Table 5). Among fruits on different whorls, the production of fully developed seed differed significantly for field and cultivated populations, but that of partly developed and undeveloped seed did not. All fruits sampled from the field and cultivated populations exhibited high seed set (Fig. 4), except the aborted female flowers in the first inflorescence, which were not included in this analysis.
DISCUSSION
Plant size and gender variation
Size‐dependent variation in gender has been observed in some monoecious species (Fox, 1993; Sarkissian et al., 2001). Femaleness usually increases with plant size; for example, in Arisaema dracontium, female flower production increased and male flower production decreased with plant size (Clay, 1993). In the monoecious species Arum italium, production of both male and female flowers increased with plant and inflorescence size (Méndez, 1998), as occurred in Sagittaria trifolia in the present study. In monoecious populations of S. latifolia female flower production generally increased with plant size although male flower production did not decrease (Sarkissian et al., 2001). These data generally support the theoretical prediction that smaller plants should be functionally male and that relative femaleness increases with plant size (Charnov, 1982). Femaleness generally increased in late inflorescences in S. trifolia due to an increase in plant size. The male plants observed in field populations were small individuals that only produced one inflorescence. It seems that their growth may have been limited due to lack of resources. All cultivated plants produced functional female flowers. Thus, S. trifolia is typical monoecious not androdioecious.
Gender variation in sequential inflorescences
Gender variation in S. trifolia occurs both among individuals and populations. Furthermore, within a plant gender, modification is displayed among sequential inflorescences. The gender of the first inflorescence was largely male in its expression. Of the early blooming inflorescences, a proportion was purely male, and some female flowers of the first inflorescence were aborted. Female gender function generally increased in late inflorescences as flower number and ovule and seed production increased. No significant difference was observed in male flower production between inflorescences excluding the first inflorescence. However, in two populations, garden 2 and Hao 2000, plants with a good resource status increased their femaleness by producing more female flowers in late inflorescences. This observation supports the prediction for protogynous species (Brunet and Charlesworth, 1995) that resource allocation to female gender increases in late‐blooming flowers.
Dichogamy and gender variation in Sagittaria
Temporal changes of sex allocation and pollination dynamics are considered to play a role in the evolution of plant sexual systems (Bawa and Beach, 1981; Wyatt, 1983; Brunet and Charlesworth, 1995; Freeman et al., 1997). With the exception of some early aborted female flowers, S. trifolia achieved high reproductive success in late inflorescences in this study. The six populations were all male‐biased, producing many more male flowers than female flowers, and suggesting that seed production was unlikely to be pollen limited. Only 1 % of undeveloped achenes was observed due to pollen limitation. A previous study indicated that daily ratios of female to male flowers were positively related to the mean distance that pollen was dispersed (Huang et al., 1999). If many male flowers were available in a day, pollen mainly reached neighbouring inflorescences. When female flowers were relatively abundant, pollen could be dispersed over long distances (Huang et al., 1999), which is likely to promote cross pollination because nearby individuals (ramets) potentially come from the same genet. Consequently, late inflorescences producing more female flowers could yield high femaleness without a decline in out‐crossing.
Dichogamous monoecious species derive benefits through the promotion of out‐crossing and reducing pollen–stigma interference and geitonogamy (Wyatt, 1983; Lloyd and Webb, 1986; Bertin, 1993; Harder et al., 2000). However, the early flowering sex phenotype is at a disadvantage because there are no mates available. As in S. trifolia, there is a trend towards maleness in early blooming flowers. Pure male inflorescences were found in S. trifolia that behaved as protandrous individuals in this protogynous species and aborted females in early blooming flowers. A decline in female investment could reflect an adaptive strategy to conserve resources (Brunet, 1996).
The present study has demonstrated a size‐dependent gender variation and a significant variation in gender among sequential inflorescences in the monoecious, perennial herb S. trifolia. More insights into such temporal gender variation, though largely unexplored, may permit determination of the influence of temporal factors on the shift or maintenance of plant sexual systems (Thomson and Barrett, 1981; Brunet and Charlesworth, 1995). Sagittaria is likely to be an example to support Darwin’s prediction that a tendency to produce either male or female flowers will spread to an inflorescence and even to a whole plant when the first flowers to open are sometimes aborted in dichogamous plants (Darwin, 1877). The discovery of size‐dependent gender variation was considered an important clue to understanding the possible pathways from monoecy to dioecy in this genus (Sarkissian et al., 2001). Our finding that femaleness increases with plant size in S. trifolia is consistent with the finding in monoecious populations of S. latifolia. Yet the possibility of geitonogamy will increase greatly in self‐compatible species if male and female flowers of different ramets in the same genet bloom on the same day. Avoidance of inbreeding depression and geitonogamous pollination in large clones may provide a necessary condition favouring the evolution of unisexuality (Barrett et al., 2001), as it has recently been demonstrated that selfing rates in monoecious populations are significantly higher than those in dioecious populations in S. latifolia (Dorken et al., 2002).
ACKNOWLEDGEMENTS
We thank Spencer Barrett for critical comments and helpful suggestions that greatly improved the first draft of the manuscript; S.‐P. Song, H. Dong, J. Wu, L. Wang and G.‐H. Liu for their help in the field; and the first author’s parents for their management of the cultivated plants. This work was supported by a grant from the National Science Foundation of China and a JSPS fellowship from Japan to S.‐Q.H.
Supplementary Material
Received: 21 January 2002; Returned for revision: 17 July 2002; Accepted: 28 July 2002 Published electronically: 2 October 2002
References
- BarrettSCH, Baker AM, Jesson LK.2000. Mating strategies in monocotyledons. In: Wilson KL, Morrison DA, eds. Systematics and evolution of monocots Sydney: CSIRO Publishing, 256–267. [Google Scholar]
- BarrettSCH, Dorken ME, Case AL.2001. A geographical context for the evolution of plant reproductive systems. In: Silvertown J, Antonovics J, eds. Integrating ecology and evolution in a spatial context Oxford: Blackwell, 341–364. [Google Scholar]
- BawaKS, Beach JH.1981. Evolution of sexual systems in flowering plants. Annals of the Missouri Botanical Garden 62: 254–274. [Google Scholar]
- BertinRI.1993. Incidence of monoecy and dichogamy in relation to self‐fertilization in angiosperms. American Journal of Botany 80: 557–560. [DOI] [PubMed] [Google Scholar]
- BoginC.1955. Revision of the genus Sagittaria (Alismataceae). Memoirs of the New York Botanic Garden 9: 179–233. [Google Scholar]
- BrunetJ.1996. Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology 77: 2458–2471. [Google Scholar]
- BrunetJ, Charlesworth D.1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- CharnovEL.1982. The theory of sex allocation. Princeton: Princeton University Press. [PubMed] [Google Scholar]
- ChenJK.1989. Systematic and evolutionary studies on Sagittaria from China. Wuhan, China: Wuhan University Press. [Google Scholar]
- ClayK.1993. Size‐dependent gender change in green dragon (Arisaema dracontium; Araceae). American Journal of Botany 80: 769–777. [Google Scholar]
- DarwinC.1877. The different forms of flowers on plants of the same species. London: John Murray. [Google Scholar]
- de JongPC.1976. Flowering and sex expression in Acer L. A biosystematic study. Mededelingen Landbouwhogeschool Wageningen 76–2: 1–201. [Google Scholar]
- DelesalleVA, Muenchow GE.1992. Opportunities for selfing and inbreeding depression in Sagittaria congeners (Alismataceae) with contrasting sexual systems. Evolutionary Trends in Plants 6: 81–91. [Google Scholar]
- DorkenME, Friedman J, Barrett SCH.2002. The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 56: 31–41. [DOI] [PubMed] [Google Scholar]
- FreemanDC, Doust JL, El‐Keblawy A, Miglia KJ, McArthur ED.1997. Sexual specialization and inbreeding avoidance in the evolution of dioecy. Botanical Review 63: 65–92. [Google Scholar]
- FoxJF.1993. Size and sex allocation in monoecious woody plants. Oecologia 94: 110–113. [DOI] [PubMed] [Google Scholar]
- HarderLD, Barrett SCH, Cole WW.2000. The mating consequences of sexual segregation within inflorescences of flowering plants. Proceedings of the Royal Society, Series B 267: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HuangSQ, Jin BF, Wang QF, Guo YH.1999. Floral display and pollen flow in a natural population of Sagittaria trifolia.Acta Botanica Sinica 41: 726–730. [Google Scholar]
- HuangSQ, Song N, Wang Q, Tang LL, Wang XF.2000. Sex expression and the evolutionary advantages of male flowers in an andromonoecious species, Sagittaria guyanensis subsp. lappula (Alismataceae). Acta Botanica Sinica 42: 1108–1114. [Google Scholar]
- LloydDG.1980a A quantitative method for describing the gender of plants. Sexual strategies in plants. New Zealand Journal of Botany 18: 103–108. [Google Scholar]
- LloydDG.1980b The distribution of gender in four angiosperm species illustrating two evolutionary pathways to dioecy. Evolution 34: 123–134. [DOI] [PubMed] [Google Scholar]
- LloydDG, Webb CJ.1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- MéndezM.1998. Modification of phenotypic and functional gender in the monoecious Arum italicum (Araceae). American Journal of Botany 85: 225–234. [PubMed] [Google Scholar]
- MuenchowGE.1998. Subandrodioecy and male fitness in Sagittaria lancifolia subsp. lancifolia (Alismataceae). American Journal of Botany 85: 513–520. [PubMed] [Google Scholar]
- MuenchowGE, Delesalle VA.1994. Pollinators response to male floral display size in two Sagittaria (Alismataceae). American Journal of Botany 81: 568–573. [Google Scholar]
- PellmyrO.1987. Multiple sex expression in Cimicifuga simplex: dichogamy destabilizes hermaphroditism. Biological Journal of the Linnean Society 31: 161–174. [Google Scholar]
- SakaiAK, Weller SG.1999. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in flowering plants New York: Springer‐Verlag, 1–31. [Google Scholar]
- SarkissianTS, Barrett SCH, Harder LD.2001. Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology 82: 360–373. [Google Scholar]
- SAS.1998. SAS/STAT user’s guide. Cary, NC: SAS Institute. [Google Scholar]
- ThomsonJD, Barrett SCH.1981. Temporal variation of gender in Aralia hispida (Araliaceae). Evolution 35: 1094–1107. [DOI] [PubMed] [Google Scholar]
- ThomsonJD, Brunet J.1990. Hypotheses for the evolution of dioecy in seed plants. Trends in Ecology and Evolution 5: 11–16. [DOI] [PubMed] [Google Scholar]
- WootenJW.1971. The monoecious and dioecious conditions in Sagittaria latifolia L. (Alismataceae). Evolution 25: 549–553. [DOI] [PubMed] [Google Scholar]
- WyattR.1983. Pollinator‐plant interactions and the evolution of breeding systems. In: Real L, ed. Pollination biology London: Academic Press, 51–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.