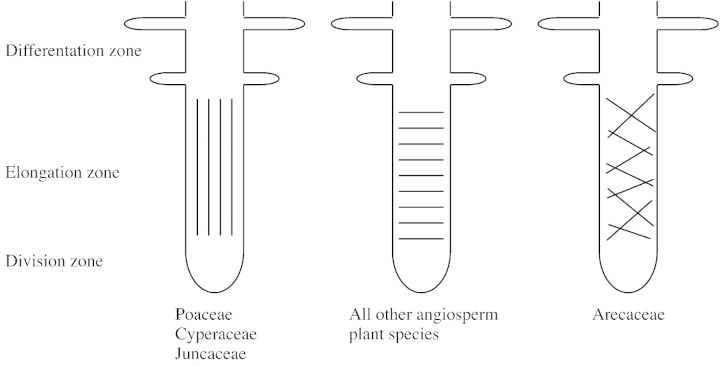
Abstract
The net orientation of cellulose fibrils in the outer epidermal wall of the root elongation zone of 57 angiosperm species belonging to 29 families was determined by means of Congo Red fluorescence and polarization confocal microscopy. The angiosperms can be divided in three groups. In all but four plant families, the net orientation of the cellulose fibrils is transverse to the root axis. Three families, the Poaceae, Juncaceae and Cyperaceae, have a totally different organization. In the root elongation zone of these plants, the net orientation of cellulose fibrils in the outer epidermal wall is parallel with the root axis. In roots of one family, the Arecaceae, an elongation zone in the literal sense of the word is absent and cellulose fibrils are randomly oriented.
Key words: Cell wall, cellulose fibrils, Congo Red, polarized confocal microscopy, commelinoid monocotyledons, Poales, Poaceae, Cyperaceae, Juncaceae
INTRODUCTION
The structural organization of the primary cell wall is similar for all angiosperms. All plants have (1→4)‐β‐d‐linked glucose polymers condensed to form semi‐crystalline cellulose microfibrils (Taiz and Zeiger, 1998). These cellulose fibrils are interlaced and cross‐linked with hydrogen bonds by complex glucans to form a strong framework. This framework is said to be the load‐bearing domain of the plant cell wall (Pauly et al., 1999). This load‐bearing network is embedded in a second domain that consists of a less stiff gel matrix of pectic polysaccharides (Carpita and Gibeaut, 1993). This organization gives the cell wall features of a complex composite material in which the orientation of the cellulose fibrils determines anisotropic mechanical characteristics (Kerstens et al., 2001). Although the basic organization is thus the same for all angiosperms, the chemical composition of primary walls varies among plant families. This feature has been used in biosystematics, particularly of monocotyledons (Harris, 2000). Monocotyledons can be divided into two groups depending on whether their primary walls show UV‐fluorescence or not (Harris and Hartley, 1980). The first group shows fluorescence due to the presence of phenolic acids, whereas in the second group these phenolic constituents are not present in the primary cell wall. The first group is identical to the commelinoid group of monocotyledons identified as a monophyletic clade in cladograms using the rbcL gene (Chase et al., 1993; Duvall et al., 1993). It contains the orders of the Poales and of the non‐graminaceous Zingiberales, Commelinales and Arecales (APG, 1998; Chase et al., 2000). The distinction between the commelinoid group and the other monocotyledons and, by extension, the dicotyledons, coincides with the now classic denomination of type I and type II cell walls (Carpita and Gibeaut, 1993; Carpita, 1996). Commelinoids have a type II cell wall. The other monocotyledons and the dicotyledons have a type I wall. In the latter, the polymers that interconnect the cellulose fibrils are xyloglucans, linear chains of (1→4)‐β‐d‐glucan, with several xylosyl units added at the O‐6 position of the glucosyl. In type II cell walls of the commelinoids, the crosslinking polymers are mainly glucuronoarabinoxylans (GAXs), linear chains of (1→4)‐β‐d‐xylose with single arabinose units at the O‐3 end and, less frequently, single glucosyluronic acid units at the O‐2 end of the xylosyl units. The phenolic compounds that are responsible for the typical UV‐fluorescence of these cell walls are ester‐linked to these GAXs (Harris, 2000). The pectin content in type II cell walls appears to be very low compared with that in type I cell walls (Jarvis et al., 1988). The structural glycoprotein extensin is also present in high amounts only in type I cell walls (Cassab, 1998). Additionally, the graminaceous monocotyledons (families of the Poales) differentiate themselves from other commelinoid monocotyledons by the presence of a so‐called mixed linked (1→3,1→4)‐β‐d‐glucan (Smith and Harris, 1999).
Besides these chemical differences in primary wall structure, structural differences have also been observed. In an earlier report (Verbelen and Kerstens, 2000), the remarkable difference in the mean or net orientation of cellulose fibrils in the outer epidermal wall of roots of arabidopsis and maize was highlighted. In arabidopsis roots, the orientation of cellulose fibrils in the elongation zone was transverse to the growth direction. However, in maize roots the orientation of cellulose fibrils in the elongation zone was clearly parallel to the direction of extension. The aim of this study was to investigate whether this longitudinal net orientation of cellulose fibrils in the outer epidermal wall of the root could be assigned to a specific taxonomic group of angiosperm plants.
MATERIALS AND METHODS
Seeds were obtained commercially or from the National Botanical Garden of Belgium. They were sown on vermiculite or potting compost and grown under normal cultivation conditions. A few days after germination, seedlings were harvested. Some plants were harvested in the wild. The species used are listed in Table 1A1B. Classification is according to the Angiosperm Phylogeny Group with recent minor corrections for some families of monocotyledons (APG, 1998; Chase et al., 2000; see also http://www.biologie.uni‐hamburg.de/b‐online/APG.html).
Table 1. List of species studied

Table 1 Continued
The mean or net orientation of cellulose fibrils in the outer epidermal wall was revealed using Congo Red and polarization confocal microscopy (Verbelen and Stickens, 1995; Verbelen and Kerstens, 2000). After harvesting, roots were washed and incubated in a 1 % aqueous solution of Congo Red (Merck, CI22120, Darmstadt, Germany) for approx. 30–60 min. Samples were then rinsed in water and studied using a confocal microscope, consisting of a Biorad MRC600 mounted on a Zeiss Axioskop upright microscope. Congo Red is a fluorescent dichroic dye that binds in a highly ordered fashion to cellulose fibrils in such a way that the degree of fluorescence depends on the orientation of the electrical vector of the polarized laser light from the confocal microscope. In the case of a cell wall with a preferential orientation, the fluorescence of the cell wall will be greatest when the electrical vector of the laser light is parallel with the net orientation of the cellulose fibrils. Fluorescence will be at a minimum when the vector of the laser light is transversely oriented with respect to the net orientation of cellulose fibrils. If there is a net random orientation of cellulose fibrils in the cell wall, there will be no difference in fluorescence intensity with changing orientations of the electrical vector of the laser light. The pinhole of the confocal microscope was adjusted adequately to avoid ‘out of focus’ fluorescence from underlying cell walls and layers.
RESULTS
In the figures, fluorescence intensity is illustrated by colour coding. High, intermediate and low fluorescence intensities are coded by red, green and blue, respectively (see bar in Fig. 1A).
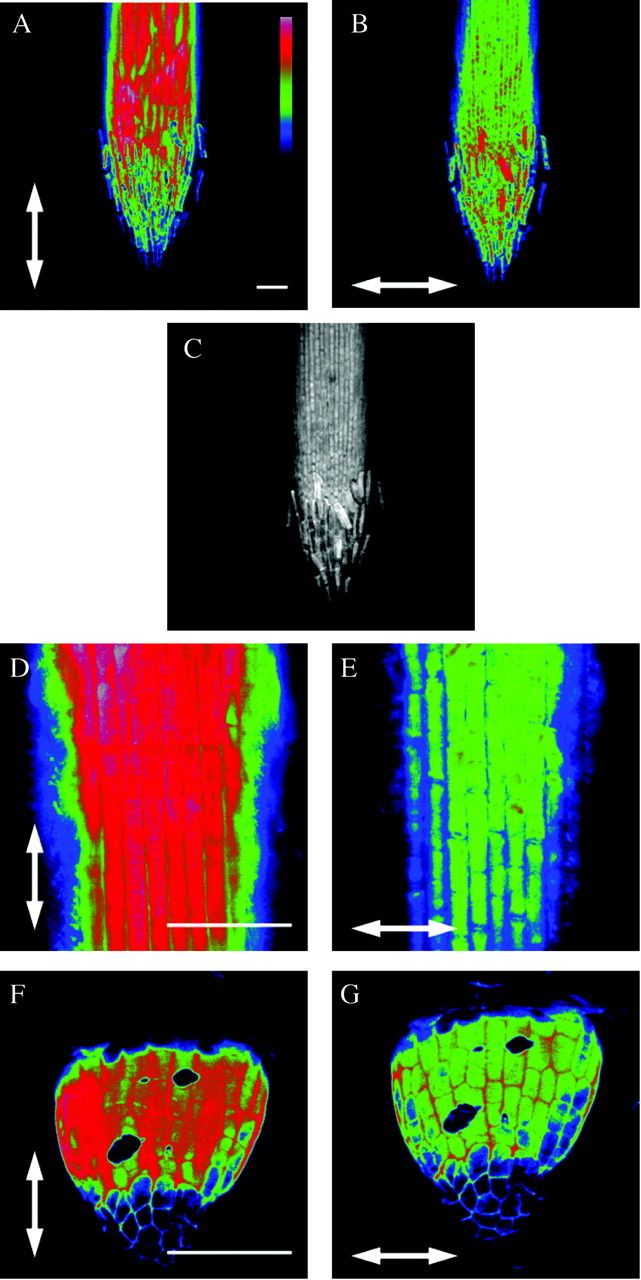
Fig. 1. Figures 1–3 are polarization confocal micrographs of roots of different species after staining with Congo Red. Arrows indicate the vector of polarization of the laser. On all micrographs, the microscope was focused on the outer epidermal wall and fluorescence is colour coded from low (blue) to high (red), except in C where colour coding was omitted. The bar in A represents the scale bar for colour coding. Scale bars = 100 µm. In all Poaceae a longitudinal orientation of cellulose fibrils with respect to the root axis was detected, indicated by the greatest fluorescence intensity in A and D (Triticum dicoccon Schrank), and was present from the very beginning of root growth (F, exact species unknown). The position of the root cap is clearly visible in C.
All Poaceae tested exhibited a net longitudinal orientation of cellulose fibrils (parallel with the root axis) in the outer epidermal wall of the elongation zone (Figs 1 and 2). These results are illustrated for Triticum dicoccon Schrank (Fig. 1). In Fig. 1B, where the vector of the laser light is parallel with the root axis, the fluorescence is higher (red in Fig. 1B) than when it is transverse to the root axis (green in Fig. 1A). In the elongation zone visualized here, cells increase in length from 35 ± 2 µm behind the root cap to 65 ± 7 µm (towards the top of the figure). A micrograph without colour coding of fluorescence clearly shows the position of the root cap (Fig. 1C). At higher magnification the size of elongating cells is clearly visible (Fig. 1D and E). Thus, in this species as in all other Poaceae, the net orientation of cellulose fibrils in the epidermis of the root tip is parallel with the growth direction of the root cells. This orientation has been found in all roots of the root system, and is present from the very beginning of root growth. This is illustrated in Fig. 1F and G, showing net cellulose orientation in an emergent adventitious root in a commercially obtained unidentified grass species.
The same conclusions can be drawn for all species of the Juncaceae (Fig. 2A and B, Juncus effusus L.) and Cyperaceae (Fig. 2C and D, Cyperus congestus Vahl). In these families the net orientation of cellulose fibrils in the root tip is also parallel with the growth direction.
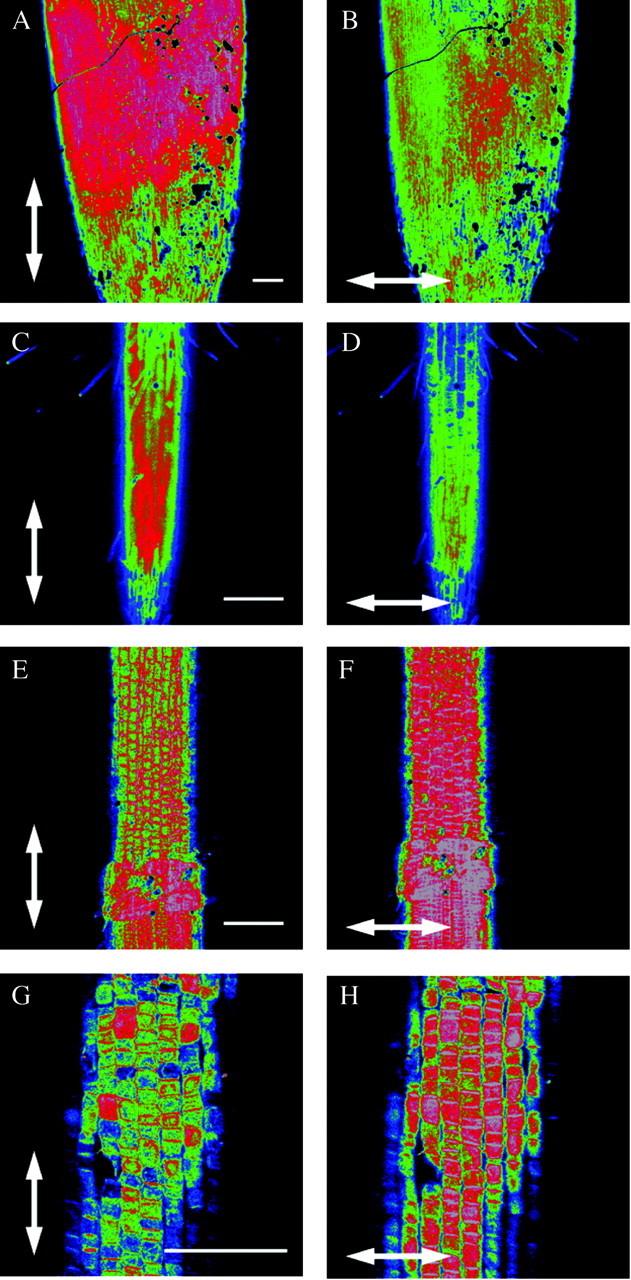
Fig. 2. See Fig. 1 legend for details. A–D, A longitudinal orientation of the cellulose fibrils in relation to the root axis was also seen in the Juncaceae (A and B, Juncus effusus L.) and the Cyperaceae (C and D, Cyperus congestus Vahl.). E–H, In all other species of the Poales not belonging to the families Poaceae, Cyperaceae or Juncaceae, the orientation of cellulose fibrils in the outer epidermal wall of the root elongation zone was transverse with respect to the growth axis of the root. This is indicated by greater fluorescence in F (Sparganium erectum L.: Sparganiaceae) and H (Typha angustifolia L.: Typhaceae). Scale bars = 100 µm.
The situation is different for all other families belonging to the order Poales. The net orientation of cellulose fibrils in the epidermis of the elongation zone is transverse to the growth direction of the root. Results are shown for a representative of the Sparganiaceae (Fig. 2E and F, Sparganium erectum L.) and of the Typhaceae (Fig. 2G and H, Typha angustifolia L.). The greatest fluorescence in these cell walls is indeed seen when the electrical vector of the laser light is transverse to the growth direction of the root (Fig. 2F and H).
The transverse orientation is found in all other orders of the commelinoid group of monocotyledons, and is shown here for a species of the Commelinaceae (Fig. 3A and B, Tradescantia zebrina Hort. Ex Heynh.). The surface of this root is less smooth than that of most other species investigated as the plant was grown in sand. This also results in a less homogeneously stained root, as is the case here.
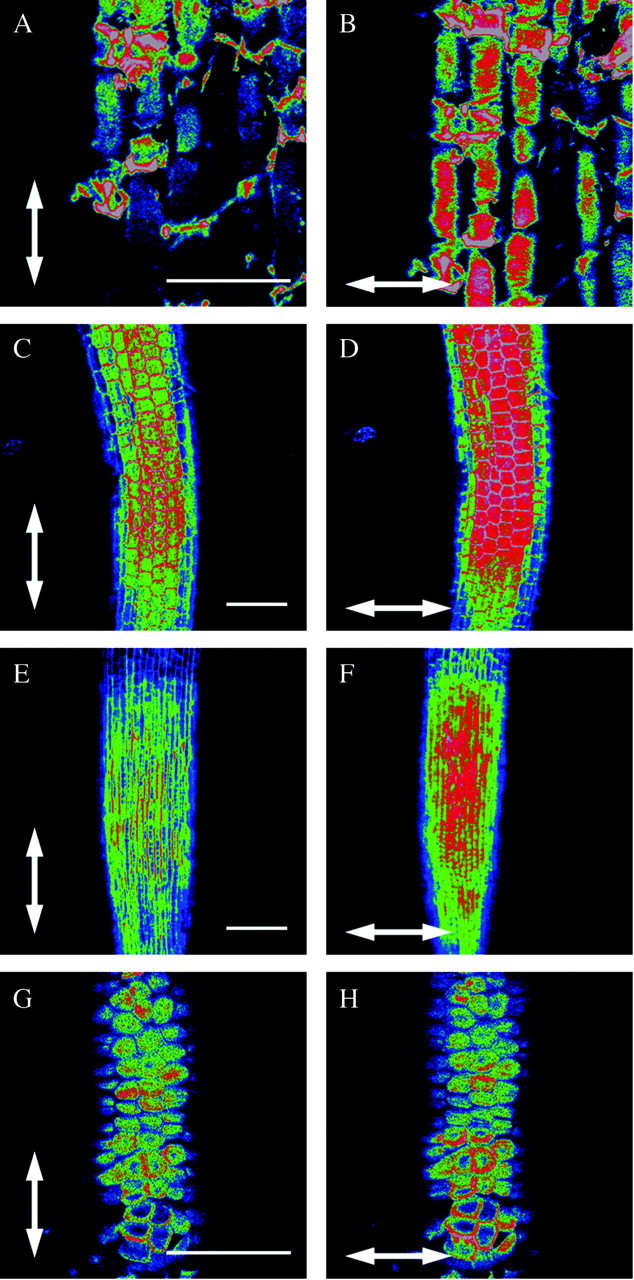
Fig. 3. See Fig. 1 legend for details. In all other species tested belonging either to the commelinoid monocotyledons, the non‐commelinoid monocotyledons or the dicotyledons, the orientation of cellulose fibrils in the outer epidermal wall of the expanding zone of the root is also transverse with respect to the root growth axis (A and B, Transdescantia zebrina Hort. Ex Heynh.: Commelinaceae; C and D, Lemna minor L.: Araceae; E and F, Elsholtzia ciliata (Thunb.) Hyl: Lamiaceae). G and H, The Arecaceae (palms) occupy a special position as their roots show no clear elongation zone and the epidermal cell wall has no preferential orientation of cellulose fibrils. The species shown is Phoenix laureirii Kunth. Scale bars = 100 µm.
The non‐commelinoid monocotyledons and all dicotyledons exhibit a net transverse orientation of cellulose fibrils in the root epidermis. Figure 3C and D shows the root tip of Lemna minor L. (a non‐commelinoid monocotyledon), the root cap of which was removed. Elsholtzia ciliata (Thunb.) Hyl from the order Lamiales is a typical example of all dicotyledons tested (Fig. 3E and F). There, too, the epidermis of the elongation zone has a net transverse orientation of cellulose fibrils with respect to the direction of growth. The results for arabidopsis roots have already been shown in previous papers (Verbelen and Kerstens, 2000; Verbelen et al., 2001).
A third type of structural organization was found in a clearly defined group. Species belonging to the Arecales, or palms (commelinoid monocotyledons), do not have a clearly defined elongation zone, unlike all other families and orders investigated to date (Fig. 3G and H, Phoenix laureirii Kunth). The epidermal cells have a scaly appearance and they hardly increase in length when they leave the meristematic zone. In the outer cell wall there is no clear preferential orientation of cellulose fibrils (compare G and H in Fig. 3) and hence their orientation can be considered random.
Thus, all species of the Poaceae, the Cyperaceae and the Juncaceae (PCJ) clearly had a net longitudinal orientation of cellulose fibrils in the outer epidermal wall of the elongation zone. The five species of Arecaceae did not have a clear elongation zone and showed a random orientation of cellulose fibrils in the epidermal cells adjacent to the meristematic zone. All other species examined, including commelinoid monocotyledons, non‐commelinoid monocotyledons and all dicotyledons had a net transverse orientation (Fig. 4).
Fig. 4. Schematic representation of the net orientation of cellulose fibrils in the outer epidermal wall of the elongation zone of angiosperm roots. A longitudinal orientation is only seen in species belonging to the families Poaceae, Cyperaceae and Juncaceae. A random orientation is unique to the Areceaceae. All other angiosperm roots have a transverse orientation of cellulose fibrils.
DISCUSSION
The orientation of cellulose fibrils in the primary wall has frequently been linked to plant growth and development (Green, 1980; Niklas, 1992; Verbelen and Kerstens, 2000; Verbelen et al., 2001). In this paper the net orientation of cellulose fibrils in the epidermis of the root elongation zone of different angiosperms is reported. It is clear that the data shown here also have a taxonomic relevance. In total 57 species were studied, belonging to 29 families. Considering the data, the angiosperms can be divided into three categories. The first category comprises species with a net orientation of cellulose fibrils that is parallel to the direction of growth. To this category belong the families of the Poaceae, the Cyperaceae and the Juncaceae. The second category is limited to the Arecaceae, or palms, represented by five species. They showed a net random orientation of cellulose in the root tip, while an elongation zone in the literal sense of the word was absent. To date, these features have not been observed within any other plant, as we are not aware of any literature related to this topic. The third category comprises species with a net orientation of cellulose fibrils transverse to the direction of growth. This category includes all dicotyledons, all the non‐commelinoid monocotyledons and the families of the commelinoid monocotyledons not included in the first or second categories.
As yet, it is difficult to determine exactly the relationship between the longitudinal, transverse or random orientation of cellulose and the organization and biochemical structure of the root cell wall. However, our data could be used as an additional tool in biosystematics. The placement of many families in cladograms is still a point of discussion and is constantly evolving (Lee and Fairbrothers, 1972; Dahlgren et al., 1985; Bergner and Jensen, 1989; Harris, 2000). This longitudinal orientation of cellulose fibrils in root tips is unique to PCJs. Indeed, this feature was only detected in three families of the Poales. The other families of this order lack this longitudinal orientation of cellulose fibrils in the root. It is interesting to note that the three other families of the Poales tested (Typhaceae, Sparganiaceae and Bromeliaceae) were only recently placed in this order (APG, 1998).
The data presented here relate to the structure and organization of the outer epidermal wall. Further investigation is required to determine the exact nature of the observed differences and their bearing on plant systematics.
ACKNOWLEDGEMENTS
S.K. is a recipient of a PhD grant from the Flemish Institute for the Promotion of Scientific and Technological Research in Industry (IWT).

Supplementary Material
Received: 29 April 2002; Returned for revision: 3 July 2002; Accepted: 28 July 2002 Published electronically: 2 October 2002
References
- APG.1998. Annals of the Missouri Botanical Garden 85: 531–553. [Google Scholar]
- BergnerI, Jensen U.1989. Phytoserological contribution to the systematic placement of the Typhales. Nordic Journal of Botany 8: 447–456. [Google Scholar]
- CarpitaNC.1996. Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology 47: 445–476. [DOI] [PubMed] [Google Scholar]
- CarpitaNC, Gibeaut DM.1993. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal 3: 1–30. [DOI] [PubMed] [Google Scholar]
- CassabGI.1998. Plant cell wall proteins. Annual Review of Plant Physiology and Plant Molecular Biology 49: 281–309. [DOI] [PubMed] [Google Scholar]
- ChaseMWet al.1993. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden 80: 528–580. [Google Scholar]
- ChaseMWet al.2000. Higher level systematics of the monocotyledons: an assessment of current knowledge and a new classification. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution. Melbourne: CSIRO. [Google Scholar]
- DahlgrenRMT, Clifford HT, Yes PF.1985. The Families of the Monocotyledons: structure, evolution and taxonomy. Berlin: Springer‐Verlag. [Google Scholar]
- DuvallRMet al.1993. Phylogenetic hypothesis for the monocotyledons constructed from rbcL sequence data. Annals of the Missouri Botanical Garden 80: 607–619. [Google Scholar]
- GreenPB.1980. Organogenesis – a biophysical view. Annual Review of Plant Physiology 31: 51–82. [Google Scholar]
- HarrisPJ.2000. Compositions of monocotyledon cell walls: implications for biosystematics. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution. Melbourne: CSIRO. [Google Scholar]
- HarrisPJ, Hartley RD.1980. Phenolic constituents of the cell walls of monocotyledons. Biochemical Systematics and Ecology 8: 153–160. [Google Scholar]
- JarvisMC, Forsyth W, Duncan HJ.1988. A survey of the pectic content of nonlignified monocot cell walls. Plant Physiology 88: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KerstensS, Decraemer WF, Verbelen J‐P.2001. Cell walls at the plant surface behave mechanically like fiber‐reinforced composite materials. Plant Physiology 127: 381–385. [PMC free article] [PubMed] [Google Scholar]
- LeeDM, Fairbrothers DE.1972. Taxonomic placement of the Typhales within the monocotyledons: preliminary serological investigation. Taxon 21: 39–44. [Google Scholar]
- NiklasKJ.1992. Plant biomechanics. An engineering appoach to plant form and function. Chicago: The University of Chicago Press. [Google Scholar]
- PaulyM, Albersheim P, Darvill A, York WS.1999. Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. The Plant Journal 20: 629–639. [DOI] [PubMed] [Google Scholar]
- PritchardJ.1994. The control of cell expansion in roots. New Phytologist 127: 3–26. [DOI] [PubMed] [Google Scholar]
- SmithBG, Harris PJ.1999. The polysaccharide composition of Poales cell walls: Poaceae cell walls are not unique. Biochemical Systematics and Ecology 27: 33–53. [Google Scholar]
- TaizL, Zeiger E.1998. Plant physiology. 2nd edn. Sunderland: Sinauer Associates. [Google Scholar]
- VerbelenJ‐P, Kerstens S.2000. Polarization confocal microscopy and Congo Red fluorescence: a simple and rapid method to determine the mean cellulose fibril orientation in plants. Journal of Microscopy 198: 101–107. [DOI] [PubMed] [Google Scholar]
- VerbelenJ‐P, Stickens D.1995. In vivo determination of fibril orientation in plant cell walls with polarization CLSM. Journal of Microscopy 177: 1–6. [Google Scholar]
- VerbelenJ‐P, Vissenberg K, Kerstens S, Le J.2001. Cell expansion in the epidermis: microtubules, cellulose orientation and wall loosening enzymes. Journal of Plant Physiology 158: 537–543. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.