Abstract
To further our understanding of the greater susceptibility of apical kernels in maize inflorescences to water stress, abscisic acid (ABA) catabolism activity was evaluated in developing kernels with chirally separated (+)‐[3H]ABA. The predominant pathway of ABA catabolism was via 8′‐hydroxylase to form phaseic acid, while conjugation to glucose was minor. In response to water deficit imposed on whole plants during kernel development, ABA accumulated to higher concentrations in apical than basal kernels, while both returned to control levels after rewatering. ABA catabolism activity per gram fresh weight increased about three‐fold in response to water stress, but was about the same in apical and basal kernels on a fresh weight basis. ABA catabolism activity was three to four‐fold higher in placenta than endosperm, and activity was higher in apical than basal kernels. In vitro incubation tests indicated that glucose did not affect ABA catabolism. We conclude that placenta tissue plays an important role in ABA catabolism, and together with ABA influx and compartmentation, determine the rate of ABA transport into endosperms.
Key words: Zea mays, L., corn, maize, ABA catabolism rate, endosperm, placenta, grain, water deficit
INTRODUCTION
The phytohormone abscisic acid (ABA) regulates many important physiological and developmental processes in plants, as well as adaptive responses to imposed environmental stress (Zeevaart and Creelman, 1988). During seed development, ABA plays several important roles, including induction of storage protein and lipid synthesis, desiccation tolerance and prevention of germination (Guerrero and Mullet, 1986; Schmitz et al., 2000; Suzuki et al., 2000; Tian and Brown, 2000). The effects exerted by ABA are a function of tissue sensitivity as well as the extent to which ABA accumulates in the target tissue (Corbineau et al., 2000). In developing seeds, ABA is synthesized by embryo tissues, but there is evidence that ABA is also transferred from maternal tissues to the seed when a plant is subjected to water deficit (Ober and Setter, 1992; Jeschke et al., 1997). ABA concentrations in plant tissues are maintained dynamically by opposing forces of synthesis, transport and catabolism to inactive products. Although considerable research has focused on ABA synthesis, much less has been directed at comparisons of ABA transport and rate of catabolism in various tissues and in response to environmental conditions.
Inactivation of ABA in plant tissues can occur via two major pathways: oxidation and conjugation. Oxidation of the 8′‐methyl group of ABA, catalysed by the P450 enzyme ABA‐8′‐hydroxylase, yields 8′‐OH‐ABA (Balsevich et al., 1994; Cutler et al., 1997; Krochko et al., 1998; Cutler and Krochko, 1999). This product is unstable and rearranges to phaseic acid (PA), which can be further reduced to 4′‐dihydrophaseic acid (DPA). Conjugation primarily involves formation of ABA glucose ester (ABA‐GE) (Zeevaart and Creelmann, 1988). Different ABA inactivation pathways occur in different species, or even at different developmental stages in the same species. ABA‐GE is a major metabolite in larch somatic embryo (Label and Lelu, 2000), Xanthium plants (Zeevaart, 1999), xylem sap of sunflower (Hansen and Dörffling, 1999), leaves and root tissues in castor bean (Jeschke et al., 1997), and maize root (Sauter and Hartung, 2000). However, in suspension cultures of maize cells (Cutler et al., 1997) and somatic embryos of white spruce (Dunstan et al., 1992), ABA was quantitatively converted to PA. In addition, minor amounts of several other metabolites, (+)‐7′‐hydroxy‐ABA, trans‐ABA and the cis‐ and trans‐1′,4′‐diols of ABA, have been detected in some plant species (Balsevich et al., 1994; Walton and Li, 1995; Zeevaart, 1999).
Studies involving water deficit treatment, exogenous ABA application, and viviparous mutants in which ABA biosynthesis is compromised indicated that during early kernel development ABA inhibits endosperm cell division and kernel growth (Ober and Setter, 1990, 1992; Mambelli and Setter, 1998). If endosperm cell division is halted at an early stage, kernels abort and fail to set seed. If they continue their development but with drastically decreased rates of cell division, they form small shrivelled mature kernels that do not contribute to yield (Artlip et al., 1995; Setter et al., 2001). Water deficit primarily affects developmentally younger reproductive structures, in apical positions along the ear, whereas basal and middle kernels are relatively little affected. The physiological basis for the differential response to water deficit at apical vs. basal/middle ear positions in maize is not known. Kernels in apical regions of ears accumulate more ABA than those in basal regions in response to water stress at the early stage of kernel formation (Setter et al., 2001). This ABA accumulation correlates with endosperm cell division, indicating that the higher accumulation of ABA might contribute to increased likelihood of kernel abortion (Myers et al., 1990; Ober and Setter, 1990, 1992; Ober et al., 1991; Artlip et al., 1995; Mambelli and Setter, 1998). In maize kernels, elevated ABA levels in apical kernels could be due to a higher rate of ABA biosynthesis and import from maternal organs, or a lower rate of ABA catabolism. In this study, focus is on ABA catabolism activity in maize kernels, using detached kernel explants which eliminate the possibility of import, and permit introduction of [3H]ABA tracer. This system was used to determine the major pathway of ABA catabolism in maize kernels and to quantify its role in placenta and endosperm tissues in differential accumulation of ABA between apical and basal kernels.
MATERIALS AND METHODS
(+)‐[ 3H]ABA preparation
(±)‐2‐cis, 4‐trans‐[G‐3H]ABA (7·4 × 106 Bq ml–1, 1·11 × 1012 Bq mmol–1; Amersham Life Science, Piscataway, NJ, USA) was methylated with excess, freshly made diazomethane, and then quenched with glacial acetic acid. (±)‐[3H]MeABA was dried with flowing N2, dissolved in isopropanol‐hexane (1 : 4 v/v; 20 mg Me‐ABA ml–1) and enantiomers were resolved by repeated 0·1 ml injections onto an analytical chiral column (Chiralcel OD, 0·46 × 25 cm; Daicel Chemical Industries Ltd, Exton, PA, USA). The mobile phase employed was isopropanol‐hexane (1 : 9 v/v) at a nominal flow rate of 1 ml min–1. The eluates were collected each 0·5 min, and 1 µl was analysed for radioactivity in each fraction with liquid scintillation counting. The (+) enantiomer eluted at 10 min and the (–) enantiomer at 15 min. Each ester was converted to the corresponding acid [85 % yield for (–)‐ABA; 92 % for (+)‐ABA] by saponification with 12 m NH4OH in the dark at 40 °C for 12 h, followed by acetic acid acidification. Both (+)‐[3H]ABA and (–)‐[3H]ABA were purified by reverse‐phase C18 HPLC, dried in vacuo and stored at –20 °C.
Plant material
Maize (Zea mays, L. ‘Pioneer Brand 39K72’; Pioneer Hi‐Bred International, Des Moines, IA, USA) was sown in 12‐l pots containing a mix of vermiculite/perlite/peat (1 : 1 : 1). Each pot was supplemented with 6 g pulverized limestone, 42 g powdered FeSO4, 35 g CaSO4, 1 g fritted trace elements (Peters FTE 555; Scotts Co., Marysville, OH, USA) and 3 g granular wetting agent (AquaGro G; Aquatrols, Pennsauken, NJ, USA). Plants were grown in a glasshouse under ambient light and temperature conditions supplemented for 12 h d–1 with 1000 W metal halide lights when solar photon flux density (PFD) was less than 250 µmol m–2 s–1 of photosynthetically active radiation (PAR) (400–700 nm wavelengths). Pots were thinned to four plants per pot 3 weeks after planting, and watered with an automatic system that contained 0·6 g l–1 of Peters 15–16–17 fertilizer (Scotts Co.) at 2 h intervals during the day. Watering was sufficient to leach excess nutrient salts. Pollination was performed manually at 4 d after silk emergence. Beginning at 1 and 6 days after pollination (DAP), pots assigned to the water deficit treatment were weighed, then removed from watering system, and maintained at about 50 % of the initial wet weight of the pots and plants. After 5 d of water deficit, plants were fully rewatered and returned to the watering system.
Samples
One to 6 d after the treatment (2–7 DAP), ears of both water deficient and well‐watered (control) plants were harvested, and kernels from apical and basal ear regions were dissected to monitor endogenous ABA levels and for the explant incubation study. The number of rings of kernels from the base to the tip of the ear was counted and the apical ear region was defined as the fifth ± two nodal rings of fertilized florets, counting basipetally from the apex. The basal ear region was the 25th ± two nodal rings of fertilized florets from the apex.
Incubation
Maize kernels were dissected directly from the ear. After glumes had been removed, kernels were cut off at the top of pedicels, leaving the placental region intact with the endosperm, pericarp and nucellus, and immediately incubated in conical tubes with placenta immersed in 10 µl incubation medium (10 mm acetic acid–NaOH buffer, pH 4·8, containing 300 Bq of (+)‐[3H]ABA with or without 200 µmol l–1 of (±)‐ABA). Preliminary tests performed by varying the incubation time from 1 to 24 h showed that ABA was taken up and metabolized linearly with time (data not shown). ABA catabolism rate per gram fresh weight was calculated as:
R [nmol h–1 g–1 f. wt] = Cr S–1 g–1 f. wt h–1,
where R is ABA catabolism rate, Cr is the sum of radioactivity in PA, DPA and other ABA catabolites identified in the studies described below (Results), S is the specific radioactivity of (+)‐ABA in the tissue [Bq ABA/mol ABA], and h is length of incubation (in hours) as indicated in figure legends. After incubation, kernels were rinsed three times in 1 ml distilled water. One batch of kernels was dissected into placenta, and endosperm and these isolated tissues were incubated in 200 µl incubation medium (10 mm acetic acid–NaOH buffer, pH 4·8), containing 300 Bq of (+)‐[3H]ABA with 200 µmol l–1 of (±)‐ABA, to study the catabolism rate of different tissues in the kernel. In each experiment, treatments were at least duplicated, and experiments were repeated at least twice to confirm the results. Specific conditions for individual experiments are described in the appropriate figures.
Metabolite extraction and analysis
After incubation, kernels were placed directly into ice‐chilled extraction medium [80 % methanol, 1 % glacial acetic acid (v/v); 10 mg l–1 butylated hydroxytoluene] with a ratio of sample (g f. wt): extraction solution (ml) of 1 : 10. Samples were homogenized in a 1·7 ml conical pestle and mortar, and extracted for 12 h with shaking at room temperature. After centrifugation at 5000 g for 10 min, the supernatant was removed and the pellet was re‐extracted twice more, and supernatants were pooled, dried in vacuo and dissolved in 100 µl 1 % (v/v) acetic acid before chromatography by reversed phase HPLC. The column was 0·46 × 25 cm C18 (RSIL, 10 µm particle size; Alltech Associates, Deerfield, IL, USA). Solvent A was 1 % (v/v) glacial acetic acid in water and solvent B was acetonitrile. A two‐step linear solvent gradient delivered at 1 ml min–1 was used to separate the metabolites: injection with 12 % B then a linear gradient to 20 % B in 18 min, and a linear gradient to 88 % B in 3·5 min. Using this gradient, baseline resolution of DPA, PA, abscisic acid glucose ester (ABA‐GE) and ABA was achieved. Metabolite identification was accomplished by co‐chromatography with authentic standards (ABA from Sigma Chemical Co., St Louis, MO, USA; ABA‐GE from Apex Organic, Oxford, UK; DPA and PA from M. Brenner, University of Minnesota, MN, USA).
ABA flash chromatography
For endogenous ABA study, 100 µl aliquots of each extract were dried in vacuo at <24 °C following addition of [3H]ABA as a tracer (17 Bq for each) of yield in chromatography. For the [3H]ABA incubation study, no radioactivity was added during this step. Aliquots were redissolved in 100 µl of Solvent I [20 % (v/v) methanol, 1% (v/v) glacial acetic acid]. ABA was separated with reverse‐phase chromatography on columns packed with 15 mg of 40 µm diameter C18‐silica material (J.T. Baker Chemicals, Phillipsburg, NJ, USA). Solvents were eluted by vacuum (<35 kPa) and the flow rate was limited to about 20 µl min–1. Columns were pre‐equilibrated with Solvent I prior to samples being loaded in 100 µl; contaminants were eluted with 400 µl Solvent I. Non‐ABA fractions were analysed for losses in ABA yield by liquid scintillation counting; yield averaged >90 % and reported data are corrected for losses. ABA was eluted with 200 µl of Solvent II [55 % (v/v) methanol, 1% (v/v) glacial acetic acid]. ABA fractions were dried in vacuo at <24 °C.
ABA enzyme‐linked immunosorbant assay (ELISA)
Samples from C18 flash chromatography were dried in vacuo and suspended in 100 µl Tris‐buffered saline [50 mm Tris–HCl, 10 mm NaCl, 1 mm MgCl2, 15 mm NaN3 (pH 7·5)]. ABA was analysed by competitive‐binding ELISA employing commercially available monoclonal antibodies specific for (+)‐ABA (Idetek, San Bruno, CA, USA), essentially as described by Setter et al. (2001). Preliminary study with a series of internal standards added to extracts yielded parallel lines (Ober et al., 1991), indicating an absence of interfering compounds.
RESULTS
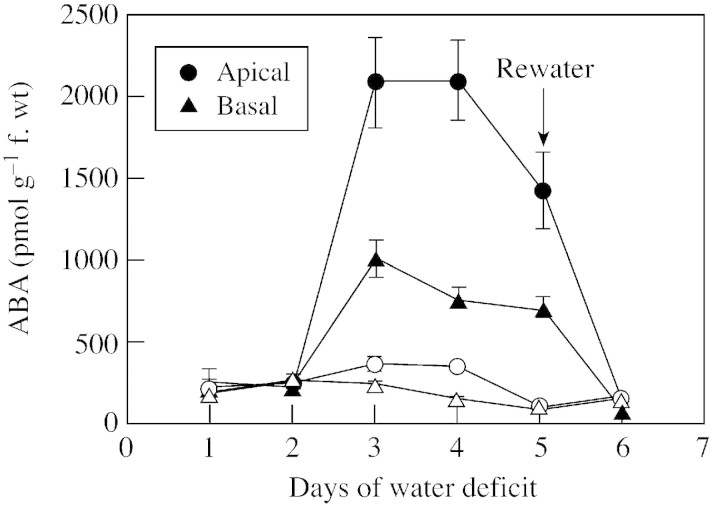
To assess the time course of ABA accumulation during stress, water was withheld at 1 d after pollination, and kernels were sampled from apical and basal regions of ear inflorescences at various times after the imposition of water deficit (Fig. 1). Although both apical and basal kernels rapidly accumulated ABA between 2 and 3 d after withholding water, the extent of ABA accumulation was about twice as high in apical than basal kernels. Maximal ABA concentrations were reached at 3 d after withholding water, corresponding to the time‐frame when soil water was depleted to the extent that leaves first exhibited incipient wilt symptoms (leaf rolling and glaucous appearance of leaf‐blade surface). ABA levels declined as the stress was continued from days 3 to 5, and when pots were rewatered ABA quickly returned to the baseline within 1 d. Other studies, in which water stress was extended an additional 2–4 d, indicated that ABA did not return to control levels, but remained elevated throughout a prolonged stress period (data not shown).
Fig. 1. Time course of ABA accumulation in apical and basal kernels on plants subjected to water deficit stress (closed symbols) and control (open symbols) treatments. Water deficit treatments started at 1 day after pollination (DAP). Pots were rewatered after 5 d water deficit treatment (6 DAP). Means ± s.e.m. for three replicates are shown.
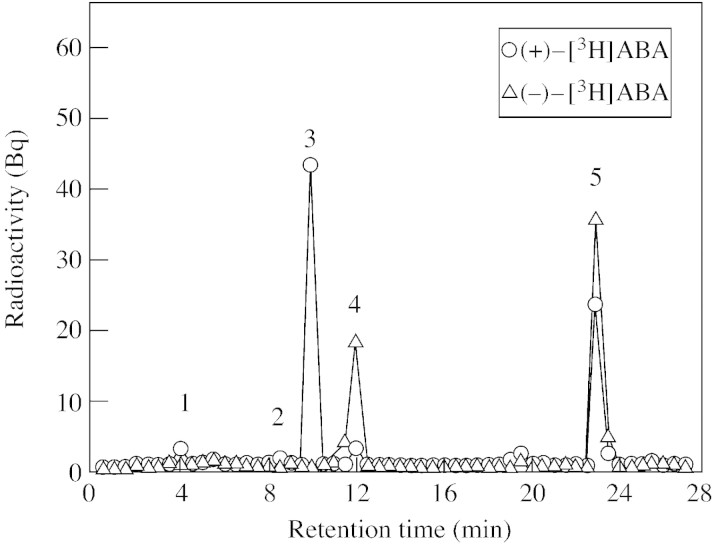
To determine the involvement of ABA catabolism in establishing steady‐state ABA concentrations in these tissues, kernels were sampled at various stages of stress and recovery, and radiotracer analysis was conducted with chirally separated (+)‐ and (–)‐[3H]ABA. After incubation of control kernels for 6 h, exogenously fed (+)‐[3H]ABA was metabolized to a variety of products (Figs 2 and 3). The metabolites identified included DPA, which eluted at 4 min, ABA‐GE (at 8·5 min), PA (at 10 min), 7′‐OH‐ABA (at 12 min) and a small peak of unidentified metabolite which eluted at 19 min (Fig. 2). Unmetabolized ABA eluted at 23·5 min. In tissues fed the natural isomer, (+)‐ABA, radioactivity that co‐eluted with ABA‐GE and 7′‐OH‐ABA never exceeded 5 % of the recovered metabolites. In contrast, the unnatural isomer (–)‐[3H]ABA was mainly metabolized to 7′‐OH‐ABA, and no PA and DPA peaks were detected among the recovered radiolabelled products. After 6 h incubation, about 58 % of (+)‐[3H]ABA fed to kernels was converted to PA, whereas 30 % of (–)‐[3H]ABA was converted to 7′‐OH‐ABA.
Fig. 2. Elution profile of ABA metabolites separated by C18 HPLC from water‐stressed maize kernels. Basal kernels were harvested after 5 d of water‐deficit treatment (6 d after pollination) and incubated for 9 h in medium containing (+)‐[3H]ABA or (–)‐[3H]ABA. 1, DPA; 2, ABA‐GE; 3, PA; 4, 7′‐hydroxy‐ABA; 5, ABA.
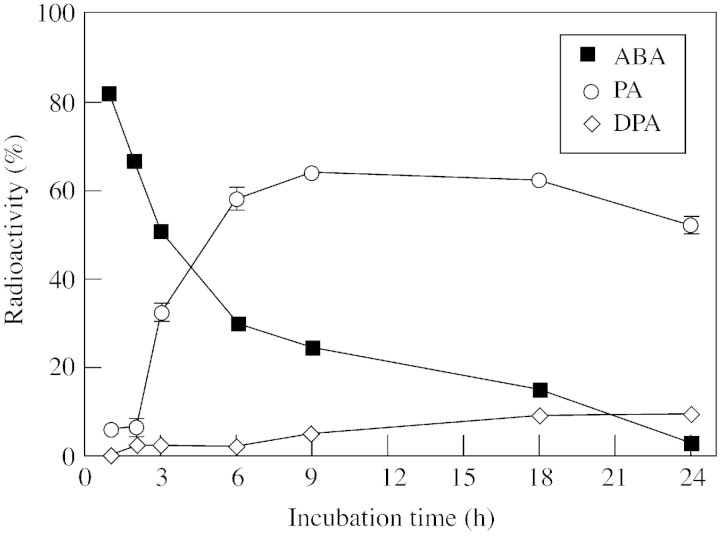
Consistent with the catabolism pathway ABA→PA→ DPA, (+)‐[3H]ABA fed to kernels decreased with incubation time, whereas PA increased sharply after 2 h and peaked at 9 h of incubation. DPA increased slightly after 9 h and reached only 9 % of the recovered radioactivity after 24 h of incubation (Fig. 3). The radioactivity in ABA‐GE, however, was a minor proportion of the metabolites recovered during 24 h of incubation, indicating that conjugation to ABA‐GE was not a major pathway for natural (+)‐ABA catabolism in maize kernels.
Fig. 3. Time course of (+)‐[3H]ABA catabolism to PA and DPA. Basal kernels were excised from water‐stressed plants at 9 days after pollination and incubated on medium containing (+)‐[3H]ABA for the indicated times. Bars represent s.e.m., and are shown if they exceed symbol size. n = 3–5.
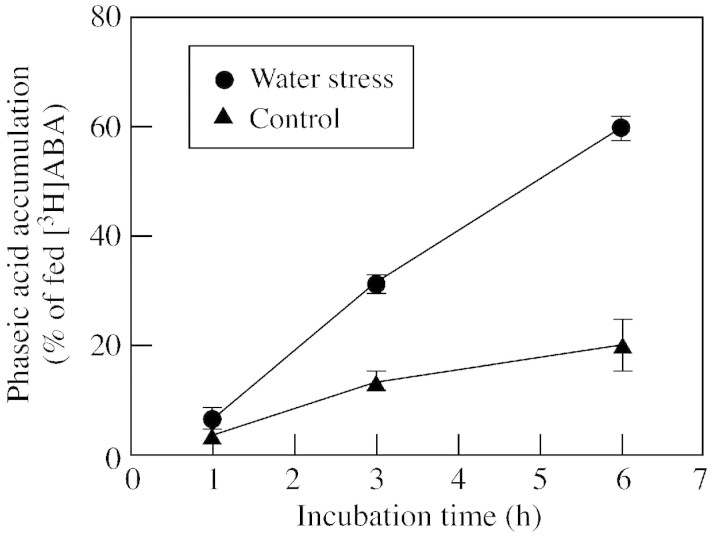
Studies of kernels fed (+)‐[3H]ABA of high specific radioactivity revealed that kernels from water‐stressed plants had substantially higher rates of ABA catabolism than those from well‐watered controls (Fig. 4). Water‐stressed kernels steadily converted ABA to PA, reaching about 60 % of radioactivity in PA at 6 h, compared with about 20 % in control kernels. Catabolism rates estimated with this system are affected by treatment differences in the size of endogenous ABA pools with which the introduced radiotracer mixes. This creates uncertainty of specific radioactivity in the pools accessed by the catabolic enzymes. To estimate ABA catabolism activity with comparable ABA concentration and specific radioactivity in both treatments, kernels were fed (+)‐[3H]ABA containing 200 µmol l–1 ABA, thereby introducing sufficient total moles of ABA into endogenous pools so that specific radioactivity in control and stress treatments was essentially the same. This method of radiotracer analysis indicated that ABA catabolism activity in water‐stressed kernels was about three‐fold higher per gram fresh weight than that in well‐watered kernels in both apical and basal kernels (Fig. 5A). However, if calculated on a per‐kernel basis, catabolism rates in basal kernels were higher than those in apical kernels (Fig. 5B), due to the much larger size of basal kernels in both treatments.
Fig. 4. Effects of water‐deficit stress on [3H]phaseic acid accumulation in maize kernels. Basal kernels incubated in 10 µl (+)‐[3H]ABA medium for 1–6 h. Means ± s.e.m. for three replicates are shown.
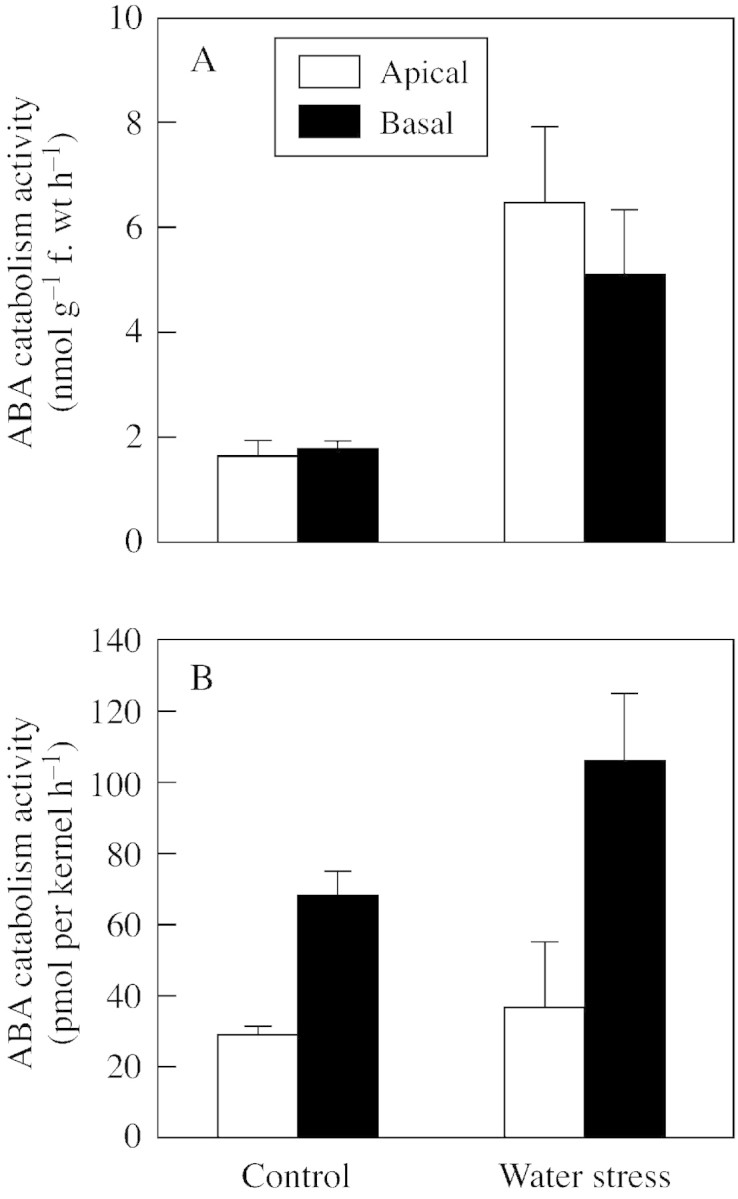
Fig. 5. ABA catabolism in whole‐kernel explants from apical and basal region of maize ears from plants subjected to control and water stress treatments. ABA catabolism activity per gram fresh weight (A) and per kernel (B). Kernels were incubated for 6 h in 10 µl medium containing 200 µm (±)‐ABA with (+)‐[3H]ABA tracer. The sum of radioactivity in ABA catabolites from HPLC separations was used to calculate ABA catabolism rate, as described in Materials and Methods. Means ± s.e.m. for four replicates are shown.
Studies of maize plants subjected to water stress in the early post‐pollination stage have indicated that maternal tissues are the source of ABA which accumulates in endosperm (Ober and Setter, 1992), and that ABA is transported from leaves to sink organs via phloem (Ober et al., 1990; Zhang et al., 1996). Hence, it is possible that tissues in the transport pathway between the site of phloem unloading in placenta to the site of net dry matter accumulation in endosperm might play a role in modulating the import and accumulation of ABA in endosperms. To examine this pathway, kernels were dissected into three tissue parts: placenta, pericarp and endosperm. Compared with apical kernels, basal kernels retained a higher proportion of introduced radiolabel in the placenta, such that a considerably lower proportion of fed radiolabel was transported into endosperm (Table 1). There was no difference in the percentage of radiolabel from [3H]ABA transported into pericarp tissue in apical and basal kernels.
Table 1.
Distribution of radiolabel in ABA and metabolites after 6 h of feeding (+)‐[3H]ABA via exposed placenta tissue immersed in media
| Ear region | Placenta (%) | Pericarp (%) | Endosperm (%) |
| Apical | 45·8 ± 0·6a | 38·6 ± 0·8a | 15·6 ± 1·3a |
| BasalLSD0·05 | 51·3 ± 1·6b4·3 | 38·6 ± 1·2a5·1 | 10·1 ± 1·0b3·0 |
Kernels were used at 6 d after pollination.
Means ± s.e.m., n = 3.
Means within a column with the same superscript letter are not significantly (P > 0·05) different.
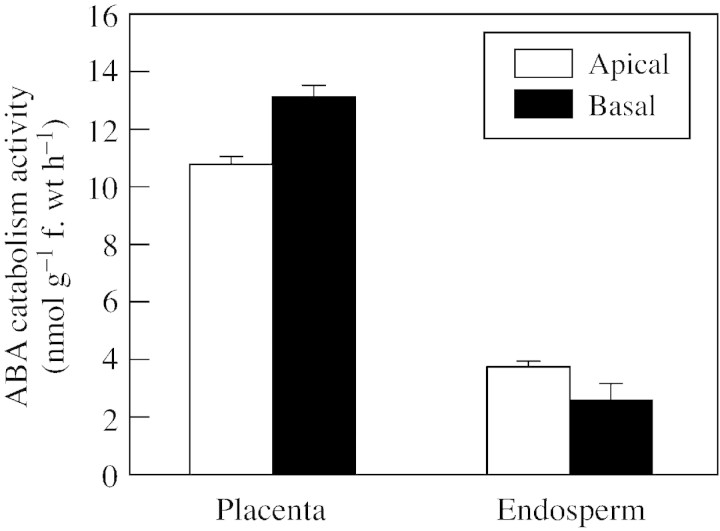
A factor that may have contributed to the greater retention of radiolabel in placenta of basal than apical kernels was the greater activity of ABA catabolism in basal‐kernel placenta. In addition to the larger size of basal‐kernel placenta, (+)‐[3H]ABA catabolism activity per gram fresh weight of isolated basal‐kernel placenta was higher than that of apical‐kernel placenta (Fig. 6). Although ABA catabolism activity per gram fresh weight in endosperm was higher in apical than basal kernels (Fig. 6), on a whole kernel basis it was higher in endosperms of basal kernels (data not shown). Moreover, the activity in placenta predominated because at 6 DAP placenta were larger than endosperm, and placenta had three to four‐fold higher activity per gram fresh weight than endosperm (Fig. 6). This suggests that placenta could play a pivotal role in ABA supply to endosperm and other kernel tissues.
Fig. 6. ABA catabolism activity in placenta and endosperm tissues from apical and basal regions of ears on water‐stressed maize plants. Isolated tissues were incubated for 6 h in 200 µm (±)‐ABA with (+)‐[3H]ABA tracer. The sum of radioactivity in ABA catabolites from HPLC separations was used to calculate ABA catabolism rate, as described in Materials and Methods. Means ± s.e.m. for two replicates are shown.
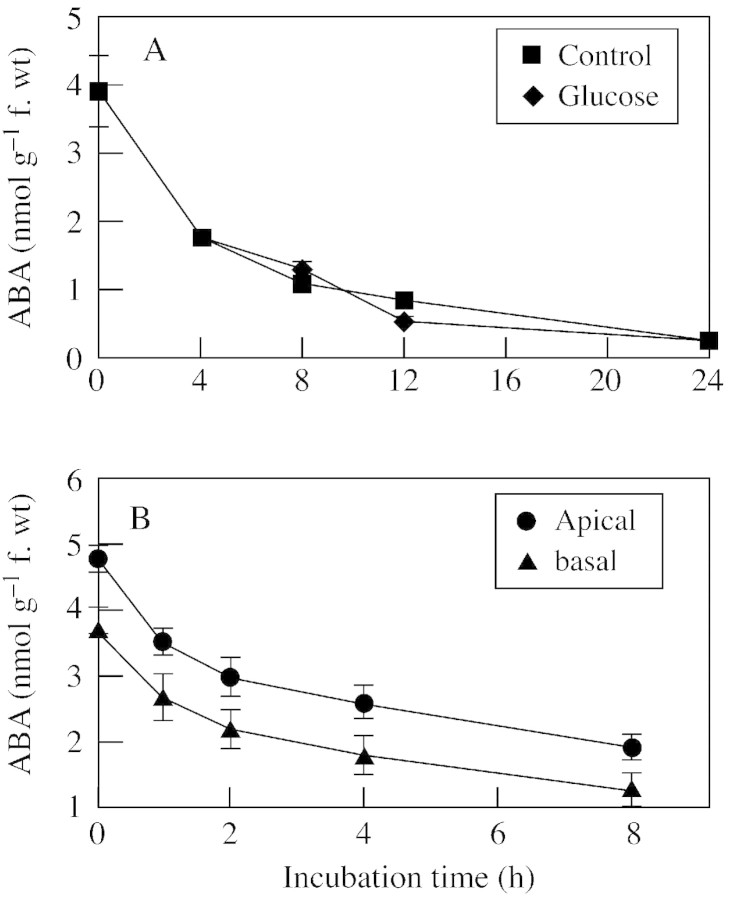
To examine the catabolism of endogenous ABA in kernels from water‐stressed plants, kernels were explanted at 6 DAP to in vitro medium and ABA was quantified during the time course of net ABA decrease (Fig. 7). Preliminary studies with this system indicated that endogenous ABA decrease was a function of the relative rates of synthesis and catabolism. These studies showed that inclusion of tetcyclasis, an inhibitor of the P450 ABA catabolism enzyme ABA 8′‐hydroxylase (Krochko et al., 1998), blocked ABA decrease, and kernels responded to low water potential osmoticum with enhanced ABA synthesis that could be blocked with protein synthesis inhibitors (data not shown).
Fig. 7. Net catabolism of endogenous ABA during incubation of explanted water‐stressed maize kernels on in vitro media. A, Kernels from water‐stressed plants were explanted at 6 DAP to 50 µl medium containing control or 50 mmglucose treatments. B, Kernels from apical and basal regions of ears from water‐stressed plants were explanted at 6 DAP to in vitro medium. Means ± s.e.m. for eight replicates are shown.
Water deficit decreases photosynthesis and phloem transport to kernels, so it is plausible that stress decreases kernel sugar status (Zinselmeier et al., 1999; Setter et al., 2001). To determine whether carbohydrate status modulates the rate of ABA synthesis, kernels were explanted to in vitro wells with their placenta immersed in control or glucose‐containing media. These tests indicated that net ABA decrease was unaffected by supplemental carbohydrate supply (Fig. 7A).
ABA decreased at about the same rate during in vitro incubation in both apical and basal kernels (Fig. 7B); however, apical kernels had a higher initial ABA concentration and this gap between apical and basal kernels was not closed even after 8 h of incubation. Thus, apical kernels had substantial capacity to deplete ABA, although not sufficient to prevent ABA accumulation to high levels during stress.
DISCUSSION
When maize plants were subjected to water deficit, kernels in the apical region of the ear inflorescence accumulated more ABA than basal kernels (Fig. 1), as previously reported (Ober et al., 1990; Artlip et al., 1995; Setter et al., 2001). However, after prolonged drought, ABA levels plateaued, and after rewatering at day 5 ABA levels rapidly returned to normal (Fig. 1). This suggests that ABA catabolism might be critical in maintaining steady‐state ABA levels in kernels.
ABA catabolism pathways
There are several metabolic pathways by which ABA can be catabolized to inactive compounds in plant tissues (Walton and Li, 1995; Zeevaart, 1999). The two major metabolic pathways are oxidation to PA and DPA, and conjugation of ABA to its glucose ester (ABA‐GE) (Zeevaart and Creelman, 1988). Although both pathways exist in maize kernels, the present data showed that with natural (+)‐ABA, catabolism to PA and DPA is the dominant pathway, and conjugation to ABA‐GE is minor. This contrasts with the situation in roots of maize and sunflower where ABA‐GE formation predominates such that it represents a major transport form in xylem (Hansen and Dörffling, 1999; Sauter and Hartung, 2000). However, in several other tissues of grass‐family plants, catabolism to PA predominates, including developing barley grains (Naumann and Doerffling, 1982) and maize suspension‐cultured cells (Balsevich et al., 1994).
ABA catabolism in apical vs. basal kernels
Water stress was accompanied by an enhanced ABA catabolism rate in kernels (Fig. 5A), consistent with previous reports in barley and wheat aleurones (Uknes and Ho, 1984), in potato and arabidopsis suspension cultures (Windsor and Zeevaart, 1997), in bean leaves (Gergs et al., 1993), and in embroyonic axes of chickpea (Babiano, 1995). (+)‐ABA 8′‐hydroxylase catalyses the first step in the oxidative catabolism of ABA and is considered to be the pivotal enzyme controlling the rate of ABA catabolism. Krochko et al. (1998) showed that (+)‐ABA 8′‐hydroxylase is induced by its substrate, (+)‐ABA, which is elevated in response to water stress. However, multiple responses may be operating that affect the steady‐state level of ABA. Cutler et al. (1997) showed that, whereas ABA 8′‐hydroxylase was induced by (+)‐ABA, such induction was suppressed if maize suspension cells were pre‐treated with mannitol osmotic treatments. In the current work, water stress, which substantially elevated kernel ABA contents (Fig. 1), increased ABA catabolism rates to about three times those of the controls (Fig. 5). In our explant system for assaying ABA catabolism, kernels were placed in a buffer containing fed [3H]ABA without osmoticum. Thus, partial kernel rehydration during assay may have prevented the stressed kernels from experiencing negative effects on catabolism due to low water potential. By employing exogenous ABA feeding, the assay system may have induced ABA 8′‐hydroxylase beyond what was present in situ, and partially masked treatment differences. Consistent with this interpretation, ABA 8′‐hydroxylase was induced progressively in maize suspension cells over a 15 h exposure to 100 µmol l–1 ABA in cell suspension medium, with an intermediate level of induction at 6 h (Cutler et al., 1997; Krochko et al., 1998). However, ABA uptake in whole kernel explants and in whole tissue segments was gradual (data not shown), and hence the assay time frame may have been sufficiently short to avoid substantial induction of ABA 8′‐hydroxylase by the [3H]ABA fed to kernels. Our ability to detect substantially higher ABA catabolism activity in water‐deficit kernels relative to controls (Fig. 5), and the observation that comparisons of water stress vs. control were similar when the assay system involved feeding 200 µmol l–1 (±)‐ABA (Fig. 5) vs. 0·3 pmol l–1 (+)‐ABA (Fig. 4) supports this interpretation.
In maize ears, kernels in apical regions accumulate ABA to a greater extent than those in middle and basal regions (Ober and Setter, 1990; Ober et al., 1991; Setter et al., 2001). Due to their import of solutes and water via the phloem rather than xylem, kernel tissues remain at high water potential during whole‐plant water deficit treatments that substantially induce ABA synthesis in leaves and other plant parts (Westgate and Thompson Grant, 1989; Ober and Setter, 1990). Studies with ABA synthesis mutants indicated that ABA in endosperms of stressed kernels is imported from maternal tissues (Ober and Setter, 1992), yet the faster growth and influx of phloem solutes in basal than apical kernels would, in turn, be expected to deliver a greater flux of maternally derived ABA to basal kernels. This led us to postulate that ABA catabolism activity may be greater in basal than in apical kernels. However, on a fresh weight basis, ABA catabolism activity was essentially the same in apical and basal kernels (Fig. 5A). Nor did the present study find evidence that sugar supply affects the rate of ABA catabolism (Fig. 7A). Indeed, ABA catabolism activity was high relative to endogenous ABA levels (Figs 1, 5 and 6). Thus, when ABA synthesis was decreased by rewatering intact plants (Fig. 1) or incubating explants on in vitro media (Fig. 7), the ABA concentration declined rapidly. But despite the high capacity for ABA catabolism, the rapid decline did not eliminate the difference between apical and basal kernels in the first 8 h of in vitro incubation (Fig. 7). A similar situation was observed by Jia et al. (1996) in maize leaves leading them to suggest that the rate of ABA catabolism is partly a function of compartmentation and the rate at which ABA is transported to the sites of ABA catabolism enzymes. In the present system we found that the placenta had particularly high ABA catabolism activity (Fig. 6) and noted that it is positioned along the transport pathway between phloem unloading and net importing endosperm cells. It is possible that in its transport role, the placenta may control ABA delivery to ABA catabolic enzymes, and a high rate of catabolism in the placenta may decrease the flux of ABA reaching the endosperm. How these opposing forces of ABA synthesis, catabolism and compartmentation interact in determining ABA levels in various tissues merits further investigation.
ACKNOWLEDGEMENTS
This project was supported by grants 00‐35100‐9279 and 95‐37100‐1606 from the NRI‐CGP Program of the US Department of Agriculture.
Supplementary Material
Received: 24 June 2002; Returned for revision: 24 July 2002; Accepted: 2 August 2002 Published electronically: 2 October 2002
References
- ArtlipTS, Madison JT, Setter TL.1995. Water deficit in developing endosperm of maize: Cell division and nuclear DNA endoreduplication. Plant Cell and Environment 1: 1034–1040. [Google Scholar]
- BabianoMJ.1995. Metabolism of (2‐14C)‐abscisic acid by a cell free system from embryonic axes of Cicer arietinum L. seeds. Journal of Plant Physiology 145: 374–376. [Google Scholar]
- BalsevichJJ, Cutler AJ, Lamb N, Friesen LJ, Kurz EU, Perras MR, Abrams SR.1994. Response of cultured maize cells to (+)‐abscisic acid, (–)‐abscisic acid and their metabolites. Plant Physiology 106: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CorbineauF, Benamar A, Come D.2000. Changes in sensitivity to abscisic acid of the developing and maturing embryo of two wheat cultivars with different sprouting susceptibility. Israel Journal of Plant Science 48: 189–197. [Google Scholar]
- CutlerAJ, Krochko JE.1999. Formation and breakdown of ABA. Trends in Plant Science 4: 472–478. [DOI] [PubMed] [Google Scholar]
- CutlerAJ, Squires TM, Loewen MK, Balsevich JJ.1997. Induction of (+)‐abscisic acid 8′‐hydroxylase by (+)‐abscisic acid in cultured maize cells. Journal of Experimental Botany 48: 1787–1795. [Google Scholar]
- DunstanDI, Bock CA, Abrams GD, Abrams SR 1992. Metabolism of (+)‐abscisic and (–)‐abscisic acid by somatic embryo suspension‐cultures of white spruce. Phytochemistry 31: 1451–1454. [Google Scholar]
- GergsU, Hagemann K, Zeevaart JAD, Weiler EW.1993. The determination of phaseic acid by monoclonal antibody‐based enzyme immunoassay. Botanica Acta 106: 404–410. [Google Scholar]
- GuerreroF, Mullet J.1986. Increased abscisic acid biosynthesis during dehydration requires transcription. Plant Physiology 80: 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HansenH, Dörffling K.1999. Changes of free and conjugated abscisic acid and phaseic acid in xylem sap of drought‐stressed sunflower plants. Journal of Experimental Botany 50: 1599–1605. [Google Scholar]
- JeschkeWD, Peuke AD, Pate JS, Hartung W 1997. Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. Journal of Experimental Botany 48: 1737–1747. [Google Scholar]
- JiaW, Zhang J, Zhang DP.1996. Metabolism of xylem‐delivered ABA in relation to ABA flux and concentration in leaves of maize and Commelina communis.Journal of Experimental Botany 47: 1085–1091. [Google Scholar]
- KrochkoJE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ.1998. (+)‐abscisic acid 8′‐hydroxylase is a cytochrome P450 mono oxygenase. Plant Physiology 118: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LabelP, Lelu MA 2000. Exogenous abscisic acid fate during maturation of hybrid larch (Larixxlepto europaea) somatic embryos. Physiologia Plantarum 109: 456–462. [Google Scholar]
- MambelliS, Setter TL.1998. Inhibition of maize endosperm cell division and endoreduplication by exogenously applied abscisic acid. Physiologia Plantarum 104: 266–277. [Google Scholar]
- MyersPN, Setter TL, Madison JT, Thompson JF.1990. Abscisic acid inhibition of endosperm cell division in cultured maize kernels. Plant Physiology 94: 1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NaumannR, Doerffling K.1982. Variation of free and conjugated abscisic acid, phaseic acid and dihydrophaseic acid levels in ripening barley grains. Plant Science Letters 27: 111–118. [Google Scholar]
- OberES, Setter TL.1990. Timing of kernel development in water stress maize: Water potentials and abscisic acid concentrations. Annals of Botany 66: 665–672. [Google Scholar]
- OberES, Setter TL.1992. Water deficit induces abscisic acid accumulation in endosperm of maize viviparous mutants. Plant Physiology 98: 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OberES, Setter TL, Madison JT, Thompson JF, Shapiro PS.1991. Influence of water deficit on maize endosperm development. Enzyme activities and RNA transcripts of starch and zein synthesis, abscisic acid and cell division. Plant Physiology 97: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SauterA, Hartung W 2000. Radial transport of abscisic acid conjugates in maize roots: its implication for long distance stress signals. Journal of Experimental Botany 51: 929–935. [PubMed] [Google Scholar]
- SchmitzN, Abrams SR, Kermode AR 2000. Changes in abscisic acid content and embryo sensitivity to (+)‐abscisic acid during the termination of dormancy of yellow cedar seeds. Journal of Experimental Botany 51: 1159–1162. [DOI] [PubMed] [Google Scholar]
- SetterTL, Flannigan BA, Melkonian J.2001. Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Science 41: 1530–1540. [Google Scholar]
- SuzukiT, Matsuura T, Kawakami N, Noda K.2000. Accumulation and leakage of abscisic acid during embryo development and seed dormancy in wheat. Plant Growth Regulation 30: 253–260. [Google Scholar]
- TianLN, Brown DCW 2000. Improvement of soybean somatic embryo development and maturation by abscisic acid treatment. Canadian Journal of Plant Science 80: 271–276. [Google Scholar]
- UknesSJ, Ho THD.1984. Mode of action of abscisic acid in barley aleurone layers. Plant Physiology 75: 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WaltonDC, Li Y.1995. Abscisic acid biosynthesis and metabolism. In: Davies PJ, ed. Plant hormones: physiology, biochemistry and molecular biology. Dordrecht: Kluwer Academic Publishers, 140–157. [Google Scholar]
- WestgateME, Thomson Grant DL.1989. Water deficits and reproduction in maize. Response of the reproductive tissue to water deficits at anthesis and mid‐grain fill. Plant Physiology 91: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WindsorML, Zeevaart JAD.1997. Induction of ABA 8′‐hydroxylase by (+)‐S‐, (–)‐R‐ and 8′,8′8′‐trifluoro‐S‐abscisic acid in suspension cultures of potato and Arabidopsis. Phytochemistry 45: 931–934. [DOI] [PubMed] [Google Scholar]
- ZeevaartJAD.1999. Abscisic acid metabolism and its regulation. In: Hooykaas PJJ, Hall MAK, Libbenga R, eds. Biochemistry and molecular biology of plant hormones. Amsterdam: Elsevier, 189–207. [Google Scholar]
- ZeevaartJAD, Creelman RA.1988. Metabolism and physiology of abscisic acid. Annual Review of Plant Physiology and Plant Molecular Biology 39: 439–473. [Google Scholar]
- ZhangW, Hartung W, Komor E, Schobert C.1996. Phloem transport of abscisic acid in Ricinus communis L. seedlings. Plant Cell and Environment 19: 471–477. [Google Scholar]
- ZinselmeierC, Jeong B‐R, Boyer JS.1999. Starch and the control of kernel number in maize at low water potentials. Plant Physiology 121: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.