Abstract
Continuous exposure of tomato ‘Trust’ to high temperatures (day/night temperatures of 32/26 °C) markedly reduced the number of pollen grains per flower and decreased viability. The effect of heat stress on pollen viability was associated with alterations in carbohydrate metabolism in various parts of the anther during its development. Under control, favourable temperature conditions (28/22 °C), starch accumulated in the pollen grains, where it reached a maximum value 3 d before anthesis; it then diminished towards anthesis. During anther development, the concentration of total soluble sugars gradually increased in the anther walls and in the pollen grains (but not in the locular fluid), reaching a maximum at anthesis. Continuous exposure of the plants to high temperatures (32/26 °C) prevented the transient increase in starch concentration and led to decreases in the concentrations of soluble sugars in the anther walls and the pollen grains. In the locular fluid, however, a higher soluble sugar concentration was detected under the high‐temperature regime throughout anther development. These results suggest that a major effect of heat stress on pollen development is a decrease in starch concentration 3 d before anthesis, which results in a decreased sugar concentration in the mature pollen grains. These events possibly contribute to the decreased pollen viability in tomato.
Key words: Anther development, carbohydrate, heat stress, pollen, starch, temperature stress
INTRODUCTION
Tomato (Lycopersicon esculentum Mill.) production is limited by high daytime temperatures and, especially, by high night‐time temperatures (Moore and Thomas, 1952). Peet et al. (1997) demonstrated that daily mean temperature is more critical than night‐time temperature per se. At daily mean temperatures of 29 °C, fruit number, fruit weight per plant and seed number per fruit were markedly decreased compared with those at 25 °C. Peet et al. (1998) and Sato et al. (2000) concluded that impairment of pollen and anther development by elevated temperature contributes to decreased fruit set in tomato and, possibly, also in other crops.
Simple sugars are the principal metabolic substrates used by germinating pollen (Stanley, 1971). Speranza et al. (1997) found that starch reserves stored during pollen development give rise to carbohydrates at maturity. According to Pacini (1996), during pollen development soluble carbohydrates of sporophytic origin may be consumed immediately, or may be polymerized or be transformed into other molecules. In the pollen of Lycopersicon peruvianum, for example, some of the products of starch hydrolysis can be transformed in spherosomes (Pacini and Viegi, 1995).
The mature pollen grain of tomato is starchless (Buchmann, 1986). Nevertheless, amylolytic activity, accompanied by a decrease in starch concentration and an increase in soluble sugar concentration, has been detected in developing tomato anthers (Bhadula and Sawhney, 1989). Bhadula and Sawhney (1989) concluded that a deficiency in carbohydrate metabolism in the tomato anther leads to abnormal pollen development. According to Saini (1997), stress‐induced arrest of male gametophyte development is preceded by disturbances in carbohydrate metabolism and distribution within anthers. The affected pollen grains failed to accumulate starch, which is a major constituent of fertile grass pollen. In tomato, the failure of viable pollen grain production under high temperature conditions may also be associated with hindered sugar metabolism. The heat stress‐related biochemical events that diminish pollen grain viability are not known.
The objectives of the present work were (1) to investigate the effect of high temperatures on carbohydrate concentration in the developing tomato anthers; and (2) to relate any changes in pollen grain carbohydrates to viability.
MATERIALS AND METHODS
Plant material
Tomato (Lycopersicon esculentum Mill. ‘Trust’; De Ruiter Seed Company, Bergschenoek, The Netherlands) plants were grown in a glasshouse of the Southeastern Plant Environment Laboratory (NCSU phytotron, Raleigh, NC, USA) at day/night temperatures of 26/22 °C, under natural illumination for 3 months. At this time, plants had fruits, flowers and buds.
Plants were transferred into 3 m3 growth chambers (15 plants per chamber) with one of two temperature regimes: (1) high temperature: day/night temperatures of 32/26 °C; (2) control: day/night temperatures of 28/22 °C. Cool‐white fluorescent tubes and 100 W incandescent lamps provided a photosynthetic photon flux density of 500 µmol m–2 s–1 for 12 h d–1. Under both temperature regimes, plants produced flowers continuously for the next 4 months. Mature pollen grains were sampled 12 d after transfer to determine their number and viability. It has been shown that the critical period for development of functional pollen under heat stress is approx. 10 d before anthesis (Sato et al., 2002). Thus, flowers present 12 d after treatment at 32/26 °C would have been exposed to heat stress during development. Flower buds 9, 7, 5, 3 (A‐3), 1 (A‐1) and 0 (A) days before anthesis were sampled and their anther components were separated for carbohydrate analysis. Buds lengths were 4–5, 7–8, 9–10, 11–12, 12, and 12–13 mm, respectively, corresponding to the flower development stages ii, iii–iv, v–vi, v, vii and viii, respectively, of Sawhney and Bhadula (1988). Meiosis occurs in stage ii and antheis at stage viii [see Sawhney and Bhadula (1988) for further description of developmental events occurring at each of these stages].
Mature pollen was extracted, stained and counted according to Pressman et al. (1998). Six flowers at the first day of anthesis were sampled from each treatment. One anther was removed from each flower and placed in a microfuge tube containing 0·5 ml germination solution consisting of 100 g l–1 sucrose, 2 mm boric acid, 2 mm calcium nitrate, 2 mmmagnesium sulfate and 1 mm potassium nitrate. Tubes were shaken well to release pollen grains. Tubes were then placed in an incubator at 25 °C for 4 h, after which a drop of Alexander dye (Alexander, 1980) was added to the solution. Numbers of germinated, non‐germinated but viable (stained purple), and non‐germinated and non‐viable (stained green) pollen grains were recorded in eight fields per sample for each flower. This procedure was repeated three times, and average results calculated.
Analysis of anther components
Anthers were dissected according to Aouali et al. (2001). Three components (anther walls, locular fluid and microspores/pollen grains) were obtained by slicing the anthers transversely and vortexing them in cold, sucrose‐free germination solution. The solution was then filtered through cheesecloth to remove the anther walls, and the pollen grains (or microspores) were separated from the locular fluid by centrifugation for 10 min at 4000 r.p.m. The anther wall, locular fluid and pollen grain/microspore samples were then stored at –20 °C and subsequently freeze‐dried.
Carbohydrate analysis
Carbohydrate concentrations of the fresh anthers or freeze‐dried pollen were analysed according to Hubbard et al. (1990) and Stoop and Pharr (1994). Fresh samples were extracted three times in hot 80 % (v/v) ethanol. The supernatant was dried in vacuo at 40 °C and re‐solubilized in water. Soluble sugars were determined by HPLC with a Fast Carbohydrate column (Bio‐Rad, Hercules, CA, USA) operated at 85 °C, with de‐ionized, degassed water as eluent (Stoop and Pharr, 1994) and detected using a refractometer.
The insoluble residue that remained after ethanolic extraction was resuspended in 2 ml 30 mm HCl and boiled for 30 min. After cooling, the pH was adjusted to 4·5 with KOH. The gelatinized starch was digested for 60 min at 50 °C using approx. 36 units of amyloglucosidase from Aspargillus oryza (Hubbard et al., 1990). The reaction mixture was incubated at 25 °C for 30 min and absorbance at 340 nm was measured.
Originally, the concentration of each of three soluble sugars (sucrose, glucose and fructose) was evaluated separately. However, following a report by Aloni et al. (2001) of a high acid invertase activity at the late stages of pollen development in pepper, we checked our procedure which included storage of pollen at –20 °C and freeze‐drying of the samples, and concluded that it did not prevent the hydrolysis of sucrose into the monosaccharides (presumably by acid invertase), even at earlier stages of pollen development. Therefore, the measured concentration of the three sugars was summed and the result presented as the total soluble sugar concentration. Calculations of the results presented in this work and more recent, additional, experiments revealed that sucrose comprises about 70 % of the sugars, with the remainder divided evenly between the two monosaccharides, glucose and fructose. Starch was not hydrolysed during –20 °C storage of the samples so it is reported.
RESULTS
Compared with flowers developing at 28/22 °C, buds that developed at 32/26 °C had only half the number of pollen grains. In addition, at 32/26 °C the number of germinated pollen grains fell by a factor of about 13 (2·2 × 104 vs. 29 × 104, respectively), the number of viable pollen grains decreased by two‐thirds, and the number of non‐viable grains increased by almost a factor of four (Fig. 1).
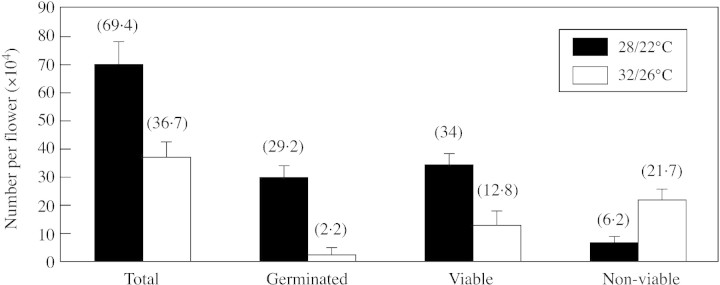
Fig. 1. Total number of pollen grains per flower and their classification into germinated, viable and non‐viable grains in tomato plants exposed to 32/26 or 28/22 °C throughout anther development. Bars represent standard error.
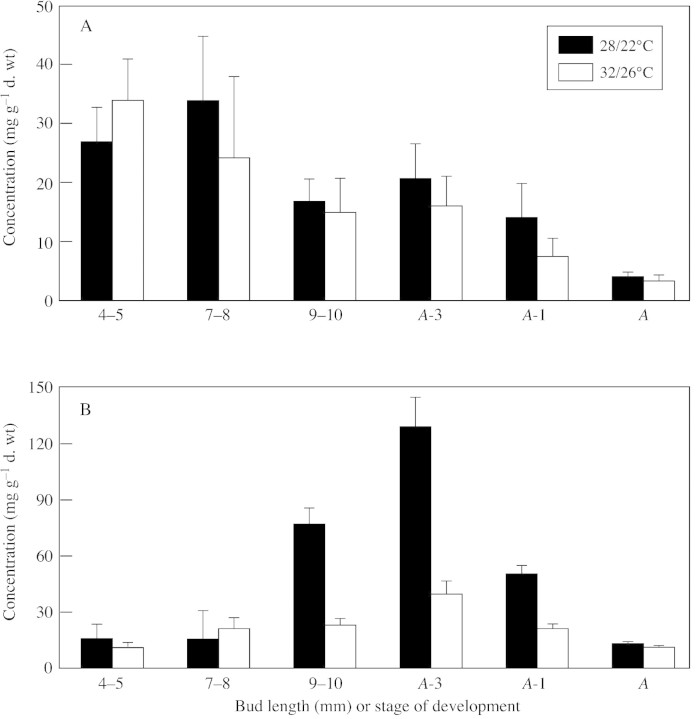
To elucidate the parts of the anther most susceptible to high temperature in terms of changes in sugar concentration, anther components were analysed separately. Figure 2 shows changes in carbohydrate concentration in anther walls and pollen grains. No starch was detected in the locular fluid. Significant amounts of starch were present in anther walls in the early (4–5 and 7–8 mm) stages, after which starch decreased and remained low until anther maturation, at which time little or no starch was detected (Fig. 2A). Starch declined in both temperature treatments, with no significant differences in concentration. The pattern of starch accumulation was markedly different in pollen grains, where it rose from initially low levels to a peak 3 d before anthesis, followed by a sharp decline, so that by anthesis levels were low again (Fig. 2B). Whilst both heat‐stressed and control pollen grains showed a similar rise and fall of starch concentration, the peak levels (9–10 mm bud length, A‐3 and A‐1) were significantly lower in heat‐stressed pollen.
Fig. 2. Changes in starch concentration in two constituents of the anthers of tomato exposed to 32/26 or 28/22 °C throughout anther development. A, Anther walls; B, pollen grains. The locular fluid does not contain starch. Bars represent standard error.
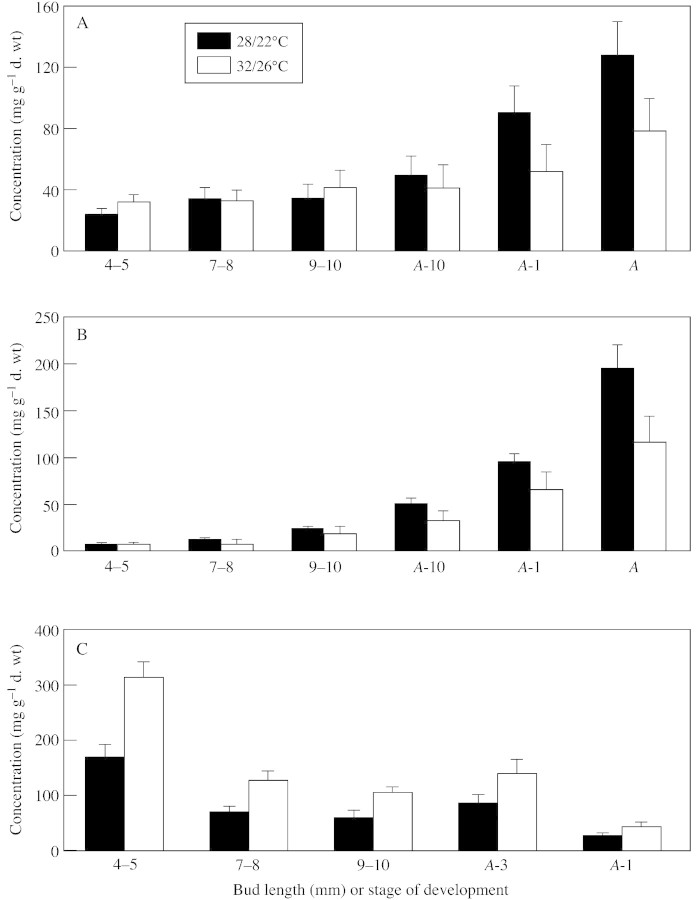
Figure 3 shows changes in soluble sugars (sucrose + glucose + fructose) in the separate anther components during anther development in both treatments. A gradual increase in concentration of the sugars was detected in the anther walls (Fig. 3A), with the highest concentration at A‐1 and anthesis. At these stages high temperatures were found to decrease the soluble sugar concentration. Note that the walls of the mature anther contain a remarkably high concentration of soluble sugars. A very similar pattern of sugar accumulation and a similar effect of high temperature were found in developing pollen grains (Fig. 3B). However, at maturation, concentrations in pollen were almost twice those in the anther walls. The locular fluid, on the other hand, contained significant amounts of sugars only at the early stage (4–5 mm; Fig. 3C), after which the sugar concentration decreased by half and remained constant until 1 d before anthesis. In contrast to its effects in the anther walls and pollen, high temperature caused an increase in the soluble sugar concentration of the locular fluid.
Fig. 3. Changes in total soluble sugar concentration in three major components of the developing anthers of tomato continuously exposed to 32/26 or 28/22 °C. A, Anther walls; B, pollen grains; C, locular fluid. Bars represent standard error.
DISCUSSION
The results of the present study show that continuous exposure of tomato ‘Trust’ plants to high temperatures has two major effects on pollen grains. First, it reduces the total number of grains and, secondly, it leads to a marked reduction in germination and a more moderate reduction in the viability of those grains. Iwahori (1965) demonstrated that tomato microspore mother cells in meiosis, 8–9 d before anthesis, were very sensitive to high temperatures, with almost all pollen grains being morphologically abnormal after exposure to temperatures above 40 °C for a few hours.
Saini (1997) suggested that a stress‐induced reduction in sugar delivery to reproductive tissue leads to failure of gametophyte development. Data in the present study do not support this suggestion in terms of carbohydrate availability 8–9 d before anthesis. No significant decrease in starch (Fig. 2) or total soluble sugars (Fig. 3) was detected in the high temperature‐treated 4–5 mm buds (8–9 d before anthesis). Rather, the results of the present study show that reduction in viability of the pollen grains was associated with variations in carbohydrate status at later stages of development of the anthers and, to an even greater extent, the pollen grains. Using periodic acid Schiff’s staining, Pacini and Viegi (1995) detected a single peak of insoluble polysaccharides during the binucleate stage (2–3 d before anthesis) in Lycopersicum peruvianum pollen grains. Similarly, in L. esculentum, starch accumulated in the pollen grains at the A‐3 stage, followed by a rapid decrease towards pollen maturation (Fig. 2B). Under heat stress, the concentration of starch and soluble sugar in the pollen grains (and that of sugars in the anther walls) was markedly lower than that under control conditions (Figs 2 and 3). These findings partially resemble those obtained with whole anthers of rice (Sheoran and Saini, 1996) and wheat (Dorion et al., 1996) where water stress during meiosis induced male sterility and inhibited starch accumulation, but enhanced the accumulation of soluble sugars. It has been suggested that carbohydrate starvation in those grains was probably not responsible for the stress‐induced pollen abortion or sterility.
The reason for decreased starch concentration in tomato pollen grains developing under high temperatures (Fig. 2B) is still not known. Wallwork et al. (1998) found that barley grains from heat‐treated plants accumulated less starch than grains from control plants due to reduced conversion of sucrose to starch. Sheoran and Saini (1996) suggested that stress could inhibit starch deposition in pollen, either by decreasing the availability of assimilates or by impairing the activities of enzymes involved in starch biosynthesis. The fact that assimilate import by tomato flower buds has been found to be inhibited by heat stress (Dinar and Rudich, 1985) may support the former possibility. In contrast, Sheoran and Saini (1996) demonstrated that not only did levels of both reducing and non‐reducing sugars in whole rice anthers not decline during stress, they actually increased substantially. This led to the suggestion that accumulation of sugars could be the result of disturbances in carbohydrate metabolism or of an inhibition of sugar utilization (Saini, 1997). The results of Wallwork et al. (1998), showing that high temperatures caused a reduction in the activity of enzymes involved in starch synthesis, may, in part, support these suggestions. Hoekstra and van Roekel (1988) indicated that pollen maturation during the last 3 d of development occurred independently from the parent plant. Therefore, the accumulation of sugars that took place in the locular fluid of heat‐stressed tomato anthers (Fig. 3C) may be attributed to a reduced demand by the affected pollen grains, mainly at the early stages of their development.
In conclusion, it is suggested that a major effect of heat stress is to prevent the build‐up of starch which, in turn, may be associated with lower levels of soluble sugars derived from the hydrolysed starch in mature pollen grains. These phenomena may contribute to the decrease in pollen viability in tomato.
ACKNOWLEDGEMENTS
This is a contribution from the Agricultural Research Organization, the Volcani Center, Bet Dagan, Israel, No. 125/2001. We are grateful to the Director, Dr Judith Thomas, and the staff of the North Carolina State University Phytotron for their excellent assistance in plant‐growing and chamber maintenance. We would also like to acknowledge Mrs Rachel Shaked for her technical assistance.
Supplementary Material
Received: 25 June 2002; Returned for revision: 30 July 2002; Accepted: 8 August 2002
References
- AlexanderMP.1980. A versatile stain for pollen, fungi, yeast and bacteria. Stain Technology 55: 13–18. [DOI] [PubMed] [Google Scholar]
- AloniB, Peet M, Pharr M, Karni L.2001. The effect of high temperature and high atmospheric CO2 on carbohydrate changes in bell pepper (Capsicum annuum) pollen in relation to its germination. Physiologia Plantarum 112: 505–512. [DOI] [PubMed] [Google Scholar]
- AoualiN, Laporte P, Clement C.2001. Pectin secretion and distribution in the anther during pollen development in Lilium Planta 213: 71–79. [DOI] [PubMed] [Google Scholar]
- BhadulaSK, Sawhney VK.1989. Amylolytic activity and carbohydrate levels during the stamen ontogeny of a male fertile, and a ‘gibberellin sensitive’ male sterile mutant of tomato (Lycopersicon esculentum). Journal of Experimental Botany 40: 789–794. [Google Scholar]
- BuchmannSL.1986. Vibratile pollination in Solanum and Lycopersicon: a look at pollen chemistry. In: D’Arey WG, ed. Solanaceae, biology and systematics New York: Columbia University Press, 237–252. [Google Scholar]
- DinarM, Rudich J.1985. Effect of heat stress on assimilate partitioning in tomato. Annals of Botany 56: 239–248. [Google Scholar]
- DorionS, Lalonde S, Saini HS.1996. Induction of male sterility in wheat by meiotic‐stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiology 111: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HoekstraFA, Van Roekel T.1988. Desiccation tolerance of Papaver dubium L. pollen during its development in the anther. Plant Physiology 88: 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HubbardNL, Pharr DM, Huber SC.1990. Role of sucrose phosphate synthase in sucrose biosynthesis in ripening bananas and its relationship to the respiratory climacteric.Plant Physiology 94: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IwahoriS.1965. High temperature injuries in tomato. IV. Development of normal flower buds and morphological abnormalities of flower buds treated with high temperature. Journal of the Japanese Society for Horticultural Science 34: 33–41. [Google Scholar]
- MooreEL, Thomas WO.1952. Some effects of shading and para‐chlorophenoxy acetic acid on fruitfulness of tomatoes. Proceedings of the American Society for Horticultural Science 60: 289–294. [Google Scholar]
- PaciniE.1996. Types and meaning of pollen carbohydrate reserves. Sexual Plant Reproduction 9: 362–366. [Google Scholar]
- PaciniE, Viegi L.1995. Total polysaccharide concentration of developing pollen in two angiosperm species. Grana 34: 237–241. [Google Scholar]
- PeetMM, Sato S, Gardner RG.1998. Comparing heat stress effects on male‐fertile and male‐sterile tomatoes. Plant, Cell and Environment 21: 225–231. [Google Scholar]
- PeetMM, Willits DH, Gardner RG.1997. Response of ovule development and post‐pollen production processes in male‐sterile tomatoes to chronic, sub‐acute high temperature stress. Journal of Experimental Botany 48: 101–111. [Google Scholar]
- PressmanE, Moshkovitch H, Rosenfeld K, Shaked R, Gamliel B, Aloni B.1998. Influence of low night temperatures on sweet pepper flower quality and the effect of repeated pollinations, with viable pollen, on fruit setting. Journal of Horticultural Science and Biotechnology 73: 131–136. [Google Scholar]
- SatoS, Peet MM, Thomas JF.2000. Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic high temperature stress. Plant, Cell and Environment 23: 719–726. [Google Scholar]
- SatoS, Peet MM, Thomas JF.2002. Determining critical pre‐ and post‐anthesis periods and physiological processes in Lycopersicon esculentum Mill. exposed to moderately elevated temperatures. Journal of Experimental Botany 53: 1187–1195. [DOI] [PubMed] [Google Scholar]
- SainiHS.1997. Effects of water stress on male gametophyte development in plants.Sexual Plant Reproduction 10: 67–73. [Google Scholar]
- SawhneyVK, Bhadula SK.1988. Microsporogenesis in the normal and male‐sterile stamenless‐2 mutant of tomato (Lycopersicon esculentum). Canadian Journal of Botany 66:2013–2021. [Google Scholar]
- SheoranIS, Saini HS.1996. Drought‐induced male sterility in rice: changes in carbohydrate levels and enzyme activities associated with the inhibition of starch accumulation in pollen. Sexual Plant Reproduction 9: 161–169. [Google Scholar]
- SperanzaA, Calzoni GL, Pacini E.1997. Occurrence of mono‐ or disaccharides and polysaccharide reserves in mature pollen grains. Sexual Plant Reproduction 10: 110–115. [Google Scholar]
- StanleyRG.1971. Pollen chemistry and tube growth. In: Heslop‐Harrison J, ed. Pollen: development and physiology London: Butterworths, 131–155. [Google Scholar]
- StoopJMH, Pharr DM.1994. Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiology 106: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WallworkMAB, Logue SJ, MacLeod LC, Jenner CF.1998. Effect of high temperature during grain filling on starch synthesis in the developing barley grain. Australian Journal of Plant Physiology 25: 173–181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.