Abstract
Acclimation of winter oilseed plants in the cold (i.e. at temperatures >0 °C) followed by short exposure to sub‐lethal freezing temperatures resulted in pronounced ultrastructural changes of leaf epidermal and mesophyll cells. The following major changes were observed upon acclimation at 2 °C: increased thickness of cell walls; numerous invaginations of plasma membranes; the appearance of many large vesicles localized in the cytoplasm in close proximity to the central vacuole; the occurrence of abundant populations of microvesicles associated with the endoplasmic reticulum (ER) cisternae or located in the vicinity of dictyosomes; and the occurrence of paramural bodies and myelin‐like structures. In addition, large phenolic deposits were observed in the vicinity of the plasma membrane and membrane‐bound organelles such as chloroplasts, large vesicles or cytoplasm/tonoplast interfaces. Transient freezing (–5 °C for 18 h) of the cold‐acclimated leaves led to reversible disorganization of the cytoplasm and to pronounced structural changes of the cellular organelles. Chloroplasts were swollen, with the stroma occupying one half of their volume and the thylakoid system being displaced to the other half. Large phenolic aggregates disappeared but distinct layers of phenolic deposits were associated with mitochondrial membranes and with chloroplast envelopes. In frost‐thawed cells recovered at 2 °C for 24 h, dictyosomes and dictyosome‐ or ER‐derived small vesicles reappeared in the ribosome‐rich cytoplasm. Aberrations in the structure of chloroplasts and mitochondria were less pronounced. Few phenolic deposits were seen as small grains associated with chloroplast envelopes and vesicle membranes. These observations demonstrate that plants undergo different changes in cell ultrastructure depending on whether they are subjected to chilling or freezing temperatures. Results are discussed in relation to membrane recycling and the possible role of phenolics during the first and second stages of plant acclimation at low temperature.
Key words: Brassica napus var. oleifera, cell ultrastructure, cold acclimation, phenolics, freezing, thawing, recovery
INTRODUCTION
Growth of chilling‐resistant biennial plants, such as winter oilseed rape (Brassica napus L. var. oleifera L.), in the cold (>0 °C) brings about adjustments in plant growth and cellular metabolism to cope with low temperature stress, and results in increased resistance of cells to extracellular freezing (Kacperska, 1989). The latter effect is further increased by a short exposure of plants to sub‐zero temperatures (Kacperska, 1989). Such treatment has been shown to affect the properties of plasma membranes and to induce specific signalling pathways (for a review, see Kacperska, 1999).
Numerous authors have reported that exposure of chilling‐resistant plants to low temperatures brings about noticeable changes in cell ultrastructure. Such changes have been observed in mesophyll and root cells of herbaceous plants exposed to chilling temperatures (e.g. Ristic and Ashworth, 1993; Čiamporová and Trgiňová, 1999) and in the cortical and xylem parenchyma cells of woody perennials during acclimation under natural environmental conditions (e.g. Siminovitch et al., 1975; Niki and Sakai, 1981; Wiśniewski and Ashworth, 1986; Ristic and Asworth, 1996). Despite the different functions of the cells studied, many of the modifications in cell fine structure induced by low temperature are strikingly similar. In particular, large vacuoles are subdivided into smaller compartments, the cells often become enriched with ‘microvacuoles’, and numerous invaginations of the plasma lemma and the multivesicular (paramural) or myelin‐like bodies are often observed. However, data on modifications in cell fine structure brought about by subfreezing temperatures, necessary to induce the second stage of frost hardening in biennial and perennial plants, are very scarce. The only work describing freezing‐induced alterations in cell ultrastructure focused on the effects of temperatures as low as –17 and –80 °C (Ristic and Ashworth, 1996).
In previous studies we have observed that cold acclimation of plants results in a pronounced increase in phenylalanine ammonia‐lyase (PAL) activity (Solecka and Kacperska, 1995), which was associated with a marked accumulation of phenolic compounds in leaves (Solecka et al., 1999). The increase in PAL activity as well as the rate of accumulation of phenolics depended on the range of low temperatures to which plants were subjected: freezing treatment being more effective than chilling. Despite the important role of phenolics in stress‐affected tissues (Dixon and Paiva, 1995), data on their cellular localization in these tissues are very scarce. Compounds such as anthocyanins and hydrocinnamoyl esters, which were identified in low temperature‐affected winter oilseed rape leaves (Solecka et al., 1999), could be expected to localize in the vacuoles (Mueller and Greenwood, 1978; Waldhauser and Baumann, 1996). It is possible that other soluble phenolics, possibly polyphenols, can be localized in the cytoplasm and in the nuclei and/or apoplast (Zobel et al., 1989; Kuraś et al., 1999). However, it is likely that the localization of phenolics in cold‐ or cold and frost‐affected cells depends on the low temperature‐induced modifications in cell ultrastructure.
The present study was undertaken to determine the effects of an extended cold treatment followed by transient sub‐lethal freezing on cell ultrastructure and the distribution of phenolics in winter oilseed rape leaves. Phenolics were localized using caffeine, which forms electron‐dense deposits in plant cells (Mueller and Beckman, 1974, 1976; Mueller and Greenwood, 1978; Zobel et al., 1989; Wronka et al., 1994, 1995).
MATERIALS AND METHODS
Plant material
Winter oilseed rape plants (Brassica napus L. var. oleifera L. ‘Jantar’) were grown in sand under a 16‐h photoperiod and 20/15 °C (day/night) temperatures for 6 weeks (non‐acclimated plants) as described previously (Stefanowska et al., 1999). The level of photosynthetically active radiation was 200 µmol m–2 s–1. After 3 weeks of growth, half of the plants were transferred to an acclimation chamber and kept at 2 °C for 3 weeks with the light conditions unchanged. Cold‐grown plants were then subjected to freezing at –5 °C for 18 h in darkness in a freezing chamber (Dual Program Illuminated Incubator 818; Precision Scientific, Dublin, OH, USA) and allowed to recover at 2 °C for 24 h in darkness. Samples for microscopic analyses were taken from leaf blades of the fourth leaf from the plant base from non‐acclimated (NA), cold‐acclimated (CA), cold‐acclimated and frost‐thawed (CAF), and cold‐acclimated and frost‐recovered (CAF1) plants. Sampling took place 3 h after the lights were turned on, with the exception of CAF and CAF1 leaves, which were examined immediately after the treatment to avoid secondary effects of photoinhibition (Powles, 1984).
Microscopic analyses
Rectangular segments (1 mm × 2 mm) were cut from the leaf between the third and the fourth principal vein, 5 mm from the midvein. The shorter axis of the segment was always situated along the vein. The segments were fixed for 2 h in 2 % glutaraldehyde at pH 7·2, prepared in 0·1 m cacodylate buffer with the addition of 1 % caffeine, and post‐fixed in buffered 2 % OsO4, as described earlier (Wronka et al., 1995; Kuraś et al., 1999). Caffeine was added for precipitation of phenolics (Mueller and Beckman, 1974; Mueller and Greenwood, 1978). After fixation and dehydration, leaf samples were embedded in Spurr’s resin (Spurr, 1969). Ultrathin sections were stained with uranyl acetate followed by lead citrate and examined in a JEOL JEM 100C transmission electron microscope. To determine the effects of temperature on cell ultrastructure, three leaves were collected from different plants subjected to the specified temperature treatments. Five segments were cut from each leaf and several cells in each segment were analysed. The ultrastructure of adaxial epidermis and palisade parenchyma cells was examined and the presence of electron‐dense caffeine/phenolic deposits in different cell compartments was recorded.
RESULTS
Epidermis and palisade parenchyma cells of NA leaves (Fig. 1A–C) contained large central vacuoles. A thin layer of parietal cytoplasm contained numerous ribosomes, long cisternae of endoplasmic reticulum (ER) and active dictyo somes (Fig. 1C). A distinct tonoplast was seen (Fig. 1B and C). Numerous, oblong chloroplasts with stacked grana, unstacked thylakoids, starch grains and small plastoglobuli were present (Fig. 1B). In the nuclei, heterochromatin was dispersed uniformly (Fig. 1B). No phenolic deposits were found in NA cells.
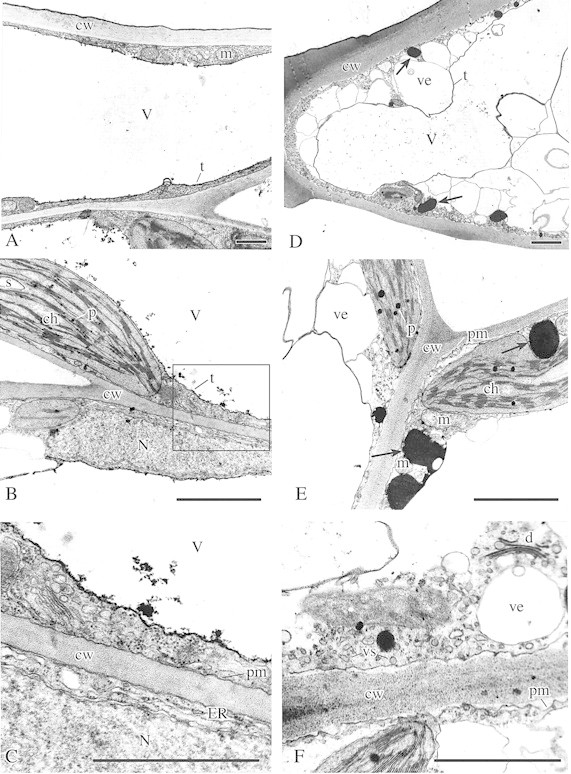
Fig. 1. Ultrastructure of leaf cells in non‐acclimated (NA; A–C) and cold acclimated (CA; D–F) plants. Fixation: GA + caffeine and OsO4; contrasting: uranyl acetate and lead citrate, according to Reynolds (1963). A, General view of upper epidermis cell in NA leaves. Note large regular central vacuole (V) with distinct tonoplast (t), mitochondria (m) and cell wall (cw). Bar = 1 µm. B, Palisade parenchyma cells in NA leaves showing nucleus (N), regular chloroplast (ch) with small starch grains (s) and small plastoglobuli (p), a thin layer of cytoplasm, part of the central vacuole (V) with distinct tonoplast (t), and thin cell wall (cw). Bar =1 µm. C, Higher magnification of the section marked in B. Note cell wall (cw) with rather smooth and closely adjacent plasmalemma (pm), cytoplasm with long endoplasmic reticulum (ER) cisternae, active dictyosome (d), fragment of nucleus (N) and central vacuole (V). Bar = 0·5 µm. D, General view of an upper epidermis cell in CA leaves (contrast with A). Note the irregular shape of the central vacuole (V), numerous vesicles (ve) of different sizes at the border of the tonoplast (t) and cytoplasm, thick cell walls (cw), and phenolic aggregations in the cytoplasm (arrows). Bar = 1 µm. E, Palisade parenchyma cells in CA leaves (contrast with B) showing thick cell walls (cw), folded plasmalemma (pm), chloroplasts (ch) with numerous large plastoglobuli (p), numerous large vesicles (ve), mitochondria (m) and big phenolic aggregations in the cytoplasm (arrows). Bar = 1 µm. F, Palisade parenchyma cells in CA leaves (compare with C) showing thick cell wall (cw) with folded, detached plasmalemma (pm), numerous vesicles of different size (ve, vs) in the cytoplasm and active dictyosome (d). Bar = 0·5 µm.
In CA leaves, pronounced modifications in the ultrastructure of the epidermis and palisade parenchyma cells were found. The thickness of cell walls increased markedly (Fig. 1D–F) and the central vacuoles became irregular in shape (Fig. 1D). Many vesicles of different size appeared between the peripheral layer of the cytoplasm and the tonoplast (Figs 1D, E and 2B and F). Within the cytoplasm, dictyosomes generating microvesicles, numerous single microvesicles or dilatated cisternae of ER (Figs 1F and 2B and F) were noted. The plasma membrane showed numerous invaginations (Figs 1F and 2B and F). In some cells, paramural bodies (groups of polyvesicular elements situated between the cytoplasm and the vacuole or protruding into the vacuoles; Fig. 2E) occurred, as well as complex membrane structures (Fig. 2F) associated with the plasmalemma (myelin‐like bodies). Also, in some epidermal cells, a fusion of large vesicles (filled with cell wall‐like material) with the cell wall was observed (Fig. 2C). Cold acclimation did not significantly affect chloroplast structure. Chloroplast envelopes, stacked grana and unstacked stroma thylakoids were well‐defined (Figs 1E and 2A). Within the stroma, numerous large plastoglobuli and some starch grains were present (Fig. 2A). In the nuclei, a few chromatin aggregations and numerous pores in the nuclear envelope could be seen (Fig. 2D).
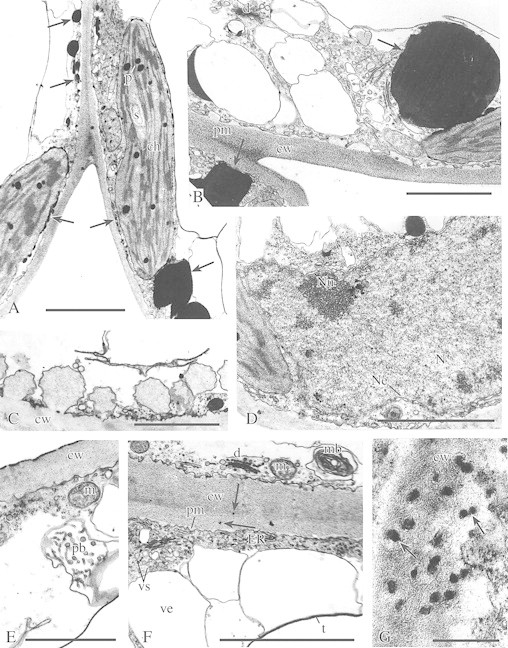
Fig. 2. Ultrastructure of cells of the upper epidermis (C, E and F) and palisade parenchyma (A, B, D and G) in cold‐acclimated plants (CA). A, Palisade parenchyma showing chloroplasts (ch) with numerous large plastoglobuli (p) and single, small starch grains (s). Numerous phenolic deposits (arrows) are associated with membranes of the chloroplast envelopes or are in the vicinity of the plasmalemma and large phenolic aggregations in the cytoplasm. Bar = 1 µm. B, Palisade parenchyma. Note the cell wall (cw) with folded, non‐adjacent plasmalemma (pm), the cytoplasm containing many empty vesicles of different size, active dictyosomes (d) and large phenolic aggregations (arrows) adjacent to chloroplasts or to the plasmalemma. Bar = 1 µm. C, Outer cell wall (cw) of upper epidermis. Note large vesicles with wall material, fused with plasmalemma (pm). Bar = 1 µm. D, Ultrastructure of nucleus (N) in a palisade parenchyma cell showing the nucleolus (Nu), scarce agregations of chromatin, and envelope (Ne) of the nucleus with pores. Bar = 1 µm. E, Upper epidermis cell showing thick outer cell wall (cw), cytoplasm with ribosomes and vesicles, mitochondrion (m) and paramural body (pb). Bar = 1 µm. F, Thick cell wall (cw) between two cells of the upper epidermis, folded plasmalemma (pm), numerous small vesicles (vs), dictyosomes (d), ER structures, mitochondrion (m), myelin structure (mb), large vesicles (ve) at the border of the tonoplast (t) and cytoplasm, and small phenolic deposits in the cell wall (arrows). Bar = 1 µm. G, Cell wall (cw) in palisade parenchyma showing build‐up of phenolic deposits (arrows). Bar = 0·1 µm.
In CA cells, large electron‐dense phenolic deposits were observed in the cytoplasm (Figs 1D and E and 2A and B). Many phenolic aggregations were located in close proximity to chloroplast envelopes (Fig. 2A and B) or other membrane systems, such as the cytoplasmic vesicles (Fig. 2A) or tonoplasts (Fig. 1E). Smaller deposits could be seen along the plasma membranes (Fig. 2A). Numerous small phenolic deposits were also observed in cell walls (Fig. 2F and G). No phenolic deposits were found in the vacuoles.
In CAF leaves (i.e. CA tissue subjected to a transient freezing), shrinkage of some of the cells was observed and the cellular ultrastructure was altered drastically (Fig. 3). Two groups of cells could be distinguished. In most of the cells, the plasma membrane and tonoplast were well preserved (Fig. 3A). However, almost the entire area of the cytoplasm was occupied by swollen chloroplasts (Fig. 3A, C and E) with their stroma filling almost half of the chloroplast volume and the thylakoid system being displaced to the other half (Fig. 3A, C and E). Numerous plastoglobuli were present in the thylakoid zone (Fig. 3C and E). In some chloroplasts single starch grains were also present (Fig. 3E). There were no large vesicles that were originally located in the cytoplasm of CA cells in close proximity to the central vacuole (Fig. 3A and E). However, many smaller, electron‐lucent vesicles were present in the peripheral part of the cytoplasm (Fig. 3A, C and E). There were no dictyosomes. In the swollen mitochondria, the cristae system practically disappeared (Fig. 3B). In the nuclei, numerous aggregations of chromatin occurred (Fig. 3A).
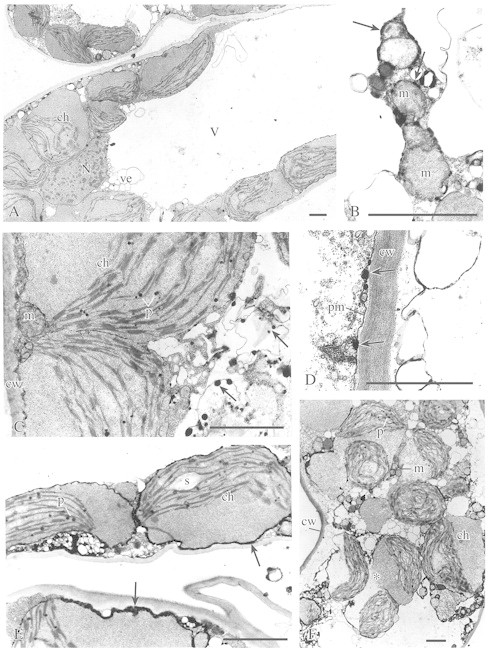
Fig. 3. Ultrastructure of upper epidermis (D) and palisade parenchyma (A–C, E and F) cells in leaves of cold‐acclimated plants exposed to a transient freezing (CAF). A, General view of a palisade parenchyma cell showing large central vacuole (V), swollen chloroplasts (ch) with thylakoid‐free part of the stroma, nucleus (N) with condensed chromatin, and numerous empty vesicles (ve) in the peripheral part of the central vacuole. Bar = 1 µm. B, Ultrastructure of mitochondria (m) in palisade parenchyma cell showing phenolic deposits associated with membranes of the mitochondrial envelope (arrows). Bar = 1 µm. C, Palisade parenchyma cell showing swollen chloroplasts (ch) with plastoglobuli (p), mitochondrion (m) and cell wall (cw). Note the phenolic deposits associated with vesicle‐like structures (arrows). Bar = 1 µm. D, Cell well (cw) between two cells of the upper epidermis. Phenolic deposits (arrows) are associated with the plasmalemma (pm). Bar = 1 µm. E, Palisade parenchyma showing the ultrastructurally altered chloroplasts (ch) with plastoglobuli (p) and a single grain of starch (s). Numerous phenolic deposits (arrows) often form a homogeneous layer associated with chloroplast envelopes or connected with membranes of numerous groups of small, electron‐empty vesicles. Bar = 1 µm. F, Disorganized cell of the palisade parenchyma. Note the chloroplasts (ch) with swollen stroma, dilatated thylakoids, plastoglobuli (p) and sometimes broken chloroplast envelopes (asterisk). The cell wall (cw), numerous electron‐empty vesicles, and mitochondria (m) can also be seen. Bar = 1 µm.
In some palisade cells the tonoplast structure had disintegrated (Fig. 3D and F). The plasma membranes seemed to be detached from the cell wall and numerous electron‐empty vesicles could be observed in the cytoplasm (Fig. 3F). In the chloroplasts, broken envelope membranes, swollen stroma, dilatated thylakoids and numerous small plastoglobuli within the thylakoid zone were noticed (Fig. 3F). Many round mitochondria were also present (Fig. 3F).
In CAF cells, there were no large phenolic deposits (Fig. 3). However, smaller phenolic aggregations were still present associated with the plasmalemma and other membrane systems (Fig. 3B–E). Distinct layers of phenolic deposits were also found in association with the mitochondrial membranes (Fig. 3B) and chloroplast envelopes (Fig. 3E).
In CAF1 cells (allowed to recover for 24 h from the frost‐induced stress; Fig. 4), the contraction of some cells was still observed (Fig. 4A). However, these cells had a continuous layer of smooth plasma membrane, and central vacuoles had distinct tonoplasts (Fig. 4A and B). In some vacuoles, ER cisternae‐like fragments were noted (Fig. 4D). In the cytoplasm, numerous small vacuoles of irregular shape were present (Fig. 4A and B). The cytoplasm was rich in ribosomes, dictyosomes, dilatated rER cisternae and dictyosome‐ or ER‐derived small vesicles (Fig. 4C and D). In the chloroplasts, swelling of the stroma decreased and the thylakoid arrangement became more regular than that in CAF cells (Fig. 4B). Between stacked grana thylakoids, small areas of lower electron density and single starch grains could be seen (Fig. 4A and B). The mitochondria were still round or irregular in shape (Fig. 4B and C) but the cristae system reappeared (Fig. 4C).
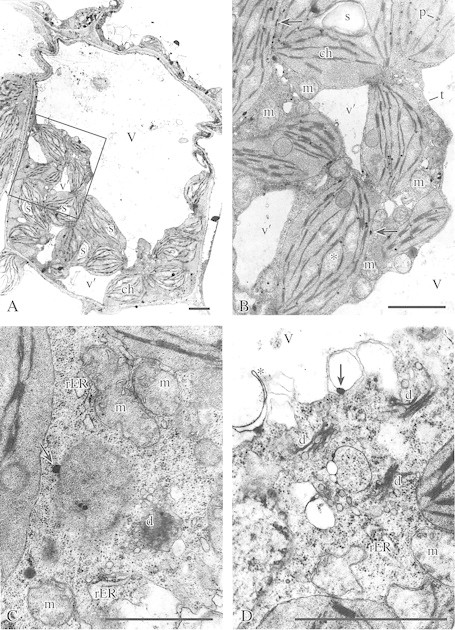
Fig. 4. A–C, Ultrastructure of palisade parenchyma cells in leaves of cold‐acclimated plants recovered from freezing stress (CAF1). A, General view of palisade parenchyma cell showing chloroplasts (ch) with numerous starch grains (s) and a normal arrangement of thylakoids, central vacuole (V) and small vacuoles of irregular shape (v′). Bar = 1 µm. B, Higher magnification of the section marked in A showing chloroplasts (ch) with plastoglobuli (p), starch (s) and small areas of lower electron density (asterisk), numerous mitochondria (m), irregularly shaped small vacuoles (v′) and part of the central vacuole (V) with tonoplast (t). Small phenolic deposits are often connected with the chloroplast envelope (arrows). Bar = 1 µm. C, Ultrastructure of palisade parenchyma cell. Note the mitochondria (m) with recovered cristae system, active dictyosome (d), dilatated cisternae of rough ER (rER), numerous dictyosomes, and dictyosome‐ and ER‐derived small vesicles. Phenolic deposits are indicated by arrows. Bar = 1 µm. D, Ultrastructure of palisade parenchyma showing numerous active dictyosomes (d), mitochondria (m), dilatated cisternae of rER, numerous vesicles, and ER cisternae‐like fragment (asterisk) within the vacuole (V). Phenolic deposits are associated with the vesicle membrane (arrow). Bar = 1 µm.
Twenty‐four hours after the freezing treatment phenolic deposits were very scarce. They were seen as small grains associated with the chloroplast envelope (Fig. 4B) and vesicle membranes (Fig. 4D) or located in the cytoplasm (Fig. 4C).
DISCUSSION
Low temperature‐induced alterations in cell ultrastructure
The present results show that cold‐acclimation of winter oilseed plants is associated with pronounced modifications in the ultrastructure of leaf cells. The modifications predominantly concern the cytoplasm, although thickening of cell walls was also observed, as previously reported (Stefanowska et al., 1999). Changes in cell ultrastructure consisted of numerous invaginations of the plasma membrane, the occurrence of paramural bodies or myelin‐like structures, and the occurrence of abundant populations of microvesicles associated with the dictyosomal or ER cisternae or located in the vicinity of the dictyosomal cisternae. Similar alterations in cell ultrastructure have been observed during seasonal acclimation in bark parenchyma cells of black locust (Pomeroy and Siminovitch, 1971) and mulberry twigs (Niki and Sakai, 1981), and in cortical xylem ray parenchyma cells of peach stems (Wiśniewski and Ashworth, 1986), as well as in leaves of Arabidopsis thaliana rapidly acclimated in the cold (Ristic and Ashworth, 1993).
Paramural and myelin‐like bodies have often been observed both in animal and plant cells. Despite their common occurrence, they have often been ignored or considered to be fixation artefacts. Marchant and Robards (1968) carried out an extensive study of the vesicular and membranous structures associated with the plasmalemma in higher and lower plants using different methods of fixation, and concluded that these formations are not artefacts. This opinion is supported by the observation of a connection between these structures and other cell organelles (Baur and Walkinshaw, 1974). It has been proposed that they have a role in transporting precursors and enzymes to outside the plasmalemma (Robards and Kidwai, 1969). Thus, the appearance of paramural and myelin‐like bodies in cold‐acclimated cells, and their absence in non‐acclimated material subjected to identical fixation procedures suggest that the effects of cold acclimation on the ultrastructure of the cytoplasm are real.
All cold‐induced modifications in cytoplasm ultrastructure are likely to represent processes related to plasma membrane turnover during acclimation. Such turnover occurs as a consequence of secretion (exocytosis), which incorporates new membranes into the plasma membrane surface, and endocytosis which internalizes the surface membrane (Steer, 1988). The new membrane material is delivered to the plasma membrane by Golgi secretory vesicles and by the formation of ER secretory vesicles (Steer, 1988). Exocytosis is also responsible for delivery of non‐cellulosic cell wall polysaccharides synthesized in the Golgi apparatus (Buchanan et al., 2000). This process was actually observed in cells of the adaxial epidermis in cold‐acclimated leaves of winter oilseed rape. The observation is consistent with results of our earlier studies that showed a pronounced thickening of cell walls in cold‐acclimated leaves (Stefanowska et al., 1999). The return flow of the excess membrane material to the cell vacuole is believed to occur via the multivesicular bodies, and to the endomembrane system via the dictyosome cisternae and coated vesicles (Steer, 1988). The presence of multivesicular bodies and myelin‐like structures in cold‐acclimated mesophyll cells indicates that the return flow of membrane material to the vacuoles has actually occurred in these cells. Therefore, the results of our studies indicate that prolonged treatment of winter oilseed rape plants with cold results in the activation of plasma membrane turnover, as proposed for leaves of Arabidopsis thaliana (Ristic and Ashworth, 1993). The results are also in agreement with the early observations of Siminovitch et al. (1968) who proposed that ‘membrane replication in association with whole protoplasmic augmentation’ is an essential factor in the frost hardening of living parenchymatic cells of trees.
The fact that similar modifications in protoplast ultrastructure occur in cells capable of developing extreme freezing resistance (to below –70 °C; Siminovitch et al., 1968) as well as in leaf mesophyll cells capable of withstanding freezing temperatures not lower than –10 to –14 °C (Sikorska and Kacperska‐Palacz, 1979; Ristic and Ashworth, 1993) implies that factors other than membrane augmentation may also be involved in the development of cell resistance to freezing. It appears that apart from differences in the structure and properties of cell walls, which may affect patterns of water freezing in tissue (Reaney and Gusta, 1999), low temperature‐induced modifications in the size and morphology of vacuoles may be important for frost survival. Cells with small vacuoles may experience less volumetric collapse when exposed to freezing‐induced dehydration (Pearce et al., 1998). In trees, the large, centrally located vacuoles in non‐acclimated parenchyma cells during summer were replaced by numerous small vacuoles during winter (Siminovitch et al., 1968; Wiśniewski and Ashworth, 1986; Sauter and Cleve, 1991). In mesophyll cells of cold‐acclimated leaves, the central vacuole was well preserved, although restriction of its volume associated with the appearance of numerous membrane‐bound vesicles in the cytoplasm could be observed. Such ultrastructural modification may be advantageous to frost‐hardened cells. Interactions between water molecules and polar head groups of membrane lipids might result in the lowering of water potential in these compartments. This, in turn, is an important factor for avoidance of excessive dehydration of cells during formation of extracellular ice.
The freezing‐induced modifications of cell fine ultrastructure were, in many respects, similar to those described for cortical cells of Prunus persica at the peak of frost hardiness (Wiśniewski and Ashworth, 1986). Apart from the fact that the central vacuole was present in leaf cells only, features such as the relatively smooth appearance of the plasma membranes, the high number of microvacuoles in the cytoplasm and the distended chloroplasts with greatly swollen stroma could be found in both cortical and mesophyll cells. It seems that these modifications reflect the frost‐induced changes in the osmotic properties of cell membranes associated with increased water absorption by the hydrophilic components of the chloroplast stroma or mitochondrial matrix upon thawing. The physiological role of the freezing‐induced modifications in cell ultrastructure and membrane properties during plant hardening is not clear. Frost‐dependent modifications in properties of the plasma membrane were proposed to be important for the induction of signalling and metabolic pathways operating during the second, frost‐dependent stage of plant hardening (Kacperska, 1989, 1999). However, the low temperature‐induced disorganization of chloroplast ultrastructure observed both in cold‐treated cells of chilling‐sensitive species (Niki et al., 1978; Čiamporová and Trgiňová, 1996, 1999), and in frost‐exposed cells of chilling‐tolerant tissues (Čiamporová and Trgiňová, 1999), is commonly discussed in terms of injury rather than acclimation. Since such disorganization is associated with an enormous swelling of the chloroplast stroma rather than with disruption of the thylakoid systems, we propose that the phenomenon might reflect the ability of the chloroplasts to accommodate the excess water entering cells during thawing in the frost‐pretreated cold‐acclimated cells.
The lack of dictyosomes and paramural bodies in the frost‐thawed cells may imply that membrane recycling was arrested in these cells. However, the reappearance of numerous dictyosomes and dictyosome‐ or ER‐derived vesicles in leaf cells collected 24 h after leaf thawing indicates an efficient recovery of the membrane turnover pathways in these cells. The presence of ER cisternae‐like fragments in the vacuoles also points to the active membrane recycling process. In addition, the recovery of chloroplast and mitochondria structures in these cells indicates that the osmotic properties of chloroplast and mitochondrial membrane systems have been regained.
Phenolic localization in low temperature‐treated leaf cells
The results of this study show that distribution and possibly the properties of phenolics may depend on the stage of plant acclimation to low temperature. The observed pronounced accumulation of phenolic deposits within the cytoplasm of cold‐acclimated cells is in accordance with the results of Solecka et al. (1999) showing that low temperature promotes significant accumulation of water‐soluble phenolics in winter oilseed rape leaves. However, the lack of phenolic deposits in vacuoles, despite a large accumulation of esterified hydroxycinnamic acids in the cold‐acclimated tissues (Solecka et al., 1999) requires explanation since the derivatives of hydroxycinnamic acids are known to be stored in the vacuoles (for a review, see Dixon and Paiva, 1995). One possible reason is that caffeine used to precipitate epicatechin‐like flavonoids (Mueller and Beckman, 1974) was not effective in the reaction with non‐polymerized phenolics such as hydrocinnamic acid derivatives. Alternatively, phenolics may have leaked out of the vacuoles during the fixation procedure, as suggested for banana root cells (Mueller and Greenwood, 1978). If this is the case, the lack of phenolic deposits in the vacuoles could be an artefact, caused by cold‐induced modifications of the membrane. Both possibilities need verification, especially given that phenolic deposits were found previously in vacuoles of Brassica napus radicles during seed maturation and germination (Zobel et al., 1989; Iwanowska et al., 1994).
Phenolic deposits in the cytoplasm probably reflect accumulation of flavonoids. These deposits were located in the vicinity of the plasma membranes and membrane‐bound organelles such as chloroplasts, small vacuoles and cytoplasm/tonoplast interfaces. Such a localization is in accordance with subcellular sites of phenylpropanoid biosynthesis (Dixon and Paiva, 1995). It is also in agreement with the proposed role of flavonoids as powerful antioxidants (Rice‐Evans et al., 1997). As hydrogen‐ or electron‐donating agents, phenolics protect cells against reactive oxygen species formed during redox reactions and during incomplete reduction of oxygen or oxidation of water by the mitochondrial and chloroplast electron transport chains. One of the major targets for oxygen radicals produced in low temperature‐affected plant cells is undoubtedly the phospholipid bilayers of cellular and subcellular membranes. Flavonoids, such as epicatechin and epicatechin gallate, localized near the surface of membranes have been shown to be powerful antioxidants against lipid peroxidation when the phospholid bilayers are exposed to aqueous oxygen radicals (Terao et al., 1994).
The free radical‐dependent oxidation of polyphenols might result in a higher degree of their condensation. This may be reflected by phenolic deposition at the surface of the chloroplast and mitochondria membranes, as observed in frost‐thawed mesophyll cells. A decrease in the frequency and in the size of phenolic aggregations in frost‐thawed cells following recovery for 24 h is difficult to explain, especially in light of the observation of the increased soluble phenolics in the frost‐recovered leaves (Solecka et al., 1999). It is possible that caffeine penetration to such tissues was decreased for an unknown reason or that polyphenols underwent modifications that rendered them inaccessible to caffeine.
In summary, the present observations indicate that low temperature‐induced modifications in cell ultrastructure in winter oilseed rape leaves depend on the range of temperatures to which plants are exposed. The issue of phenolic localization in cold‐acclimated and frost‐pretreated tissues requires further investigation.
ACKNOWLEDGMENTS
The authors thank Dr D. Solecka of Plant Resistance Lab, Institute of Experimental Plant Biology for providing plant material and for helpful discussions.
Supplementary Material
Received: 20 May 2002; Returned for revision: 1 July 2002; Accepted: 5 August 2002 Published electronically: 2 October 2002
References
- BaurPS, Walkinshaw CH.1974. Fine structure of tannin accumulations in callus cultures of Pinus elliotii (slash pine). Canadian Journal of Botany 52: 615–619. [Google Scholar]
- BuchananBB, Gruissem W, Jones RL.2000. Biochemistry and molecular biology. Rockville, USA: American Society of Plant Physiologists, 22–23. [Google Scholar]
- ČiamporováM, Trgiňová I.1996. Ultrastructure of chloroplast in leaves and of plastids in root tips of two maize lines differing in chilling tolerance. Biologia, Section Botany, Bratislava 51: 357–364. [Google Scholar]
- ČiamporováM, Trgiňová I.1999. Modifications of plant cell ultrastructure accompanying metabolic responses to low tempera tures. Biologia, Section Botany, Bratislava 54: 349–360. [Google Scholar]
- DixonRA, Paiva NL.1995. Stress‐induced phenylpropanoid metabolism. The Plant Cell 7: 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IwanowskaA, Tykarska T, Kuraś M, Zobel AM.1994. Localization of phenolic compounds in the covering tissues of the embryo of Brassica napus (L.) during different stages of embryogenesis and seed maturation. Annals of Botany 74: 313–320. [Google Scholar]
- KacperskaA.1989. Metabolic consequences of low temperature stress in chilling‐insensitive plants. In: Li PH, ed. Low temperature stress physiology in crops. Boca Raton: CRS Press, 27–40. [Google Scholar]
- KacperskaA.1999. Plant responses to low temperature: signaling pathways involved in plant acclimation. In: Margesin R, Schinner F, eds. Cold‐adapted organisms Ecology, physiology, enzymology and molecular biology. Berlin: Springer, 79–103. [Google Scholar]
- KuraśM, Stefanowska‐Wronka M, Lynch JM, Zobel AM.1999. Cytochemical localization of phenolic compounds in columella of root cap in seeds of Brassica napus. Changes in phenolic compounds localization during germination. Annals of Botany 84: 135–143. [Google Scholar]
- MarchantR, Robards AW.1968. Membrane system associated with the plasmalemma of plant cells. Annals of Botany 32: 457–471. [Google Scholar]
- MuellerWC, Beckman CH.1974. Ultrastructure of phenol‐storing cells in the roots of bananas. Physiological Plant Pathology 4: 187–190. [Google Scholar]
- MuellerWC, Beckman CH.1976. Ultrastructure and development of phenolic‐storing cells in cotton roots. Canadian Journal of Botany 54: 2074–2082. [Google Scholar]
- MuellerWC, Greenwood AW.1978. The ultrastructure of phenolic‐storing cells fixed with caffeine. Journal of Experimental Botany 29: 757–764. [Google Scholar]
- NikiT, Sakai A.1981. Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry twigs. Plant Cell Physiology 22: 171–183. [Google Scholar]
- NikiT, Yoshida S, Sakai A.1978. Studies on chilling injury in plant cells. I. Ultrastructural changes associated with chilling injury in callus tissues of Cornus stolonifera.Plant Cell Physiology 19: 139–146. [Google Scholar]
- PearceRS, Houlston CE, Atherton KM, Rixon JE, Harrison P, Hughes MA, Dunn MA.1998. Localization of expression of three cold‐inducible genes, blt101, blt4.9 and blt14, in different tissues of the crown and developing leaves of cold‐acclimated cultivated barley. Plant Physiology 117: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PomeroyMK, Siminovith D.1971. Seasonal cytological changes in secondary phloem parenchyma cells in Robinia pseudoacacia in relation to cold hardiness. Canadian Journal of Botany 49: 787–795. [Google Scholar]
- PowlesSB.1984. Photoinhibition of photosynthesis induced by visible light. Annual Review of Plant Physiology 35: 15–44. [Google Scholar]
- ReaneyMJT, Gusta LV,1999. Modeling sequential responses of plant cells to freezing and thawing. In: Margesin R, Schinner F, eds. Cold‐adapted organisms Ecology, physiology, enzymology and molecular biology. Berlin: Springer, 119–135. [Google Scholar]
- Rice‐EvansCA, Miller NJ, Paganga G.1997. Antioxidant properties of phenolic compounds. Trends in Plant Science 2: 152–159. [Google Scholar]
- RisticZ, Ashworth EN.1993. Changes in leaf ultrastructure and carbohydrates in Arabidopsis thaliana L. (Heyn) cv. Columbia during rapid cold acclimation. Protoplasma 172: 111–123. [Google Scholar]
- RisticZ, Ashworth EN.1996. Response of xylem ray parenchyma cells of red osier dogwood (Cornus sericea L.) to field freezing stress, and to freeze‐thaw cycle. Journal of Plant Physiology 149: 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RobardsAW, Kidwai P.1969. Vascular involvement in differentiating plant vascular cells. New Phytologist 68: 343–349. [Google Scholar]
- SauterJJ, Cleve BV.1991. Biochemical and ultrastructural results during starch‐sugar‐conversion in ray parenchyma cells of Populus during cold adaptation. Journal of Plant Physiology 139: 19–26. [Google Scholar]
- SikorskaE, Kacperska‐Palacz A.1979. Phospholipid involvement in frost tolerance. Physiologia Plantarum 47: 144–150. [Google Scholar]
- SiminovitchD, Singh J, De La Roche I.1975. Studies on membranes in plant cells resistant to extreme freezing. I. Augmentation of phospholipids and membrane substance without changes in unsaturation of fatty acids during hardening of black locust bark. Cryobiology 12: 144–153. [DOI] [PubMed] [Google Scholar]
- SiminovitchD, Rheaume B, Pomeroy K, Lepage M.1968. Phospholipid, protein, and nucleic acid increases in protoplasm and membrane structures associated with development of extreme freezing resistance in black locust tree cells. Cryobiology 5: 202–225. [DOI] [PubMed] [Google Scholar]
- SoleckaD, Kacperska A.1995. Phenylalanine ammonia‐lyase activity in leaves of winter oilseed rape plants as affected by acclimation of plants to low temperature. Plant Physiology and Biochemistry 33: 585–591. [Google Scholar]
- SoleckaD, Boudet AM, Kacperska A.1999. Phenylpropanoid and anthocyanin changes in low temperature treated winter oilseed rape leaves. Plant Physiology and Biochemistry 37: 491–496. [Google Scholar]
- SpurrAR.1969. A low‐viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- SteerMW.1988. Plasma membrane turnover in plant cells. Journal of Experimental Botany 39: 987–996. [Google Scholar]
- StefanowskaM, Kuraś M, Kubacka‐Zȩbalska M, Kacperska A.1999. Low temperature affects of growth and structure of cell walls in winter oilseed rape (Brassica napus L. var. oleifera L.) plants. Annals of Botany 84: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeraoJ, Piskuła M, Yao Q.1994. Protective effect of epicatechin, epicatecin gallate and quercetin on lipid peroxidation in phospholipid bilayers. Archives of Biochemistry and Biophysics 308: 278–284. [DOI] [PubMed] [Google Scholar]
- WaldhauserSSM, Baumann TW.1996. Compartmentation of caffeine and related purine alkaloids depends exclusively on the physical chemistry of their vacuolar complex formation with chlorogenic acids. Phytochemistry 42: 985–996. [Google Scholar]
- WiśniewskiM, Ashworth EN.1986. A comparison of seasonal ultrastructural changes in stem tissues of peach (Prunus persica) that exhibit contrasting mechanisms of cold hardiness. Botanical Gazette 147: 407–417. [Google Scholar]
- WronkaM, Kuraś M, Tykarska T, Podstolski A. Zobel AM.1995. Inhibition of the production of phenolic compounds in Brassica napus 2‐amino‐oxyacetic acid. Annals of Botany 75: 319–324. [Google Scholar]
- WronkaM, Lewak S, Tykarska T, Kuraś M, Zobel AM.1994. Localization of phenolic compounds in the root cap columella of six‐year‐old dry seeds of Brassica napus during imbibition and germination. Annals of Botany 74: 321–326. [Google Scholar]
- ZobelAM, Kuraś M, Tykarska T.1989. Cytoplastic and apoplastic location of phenolic compounds in the covering tissue of the Brassica napus radicle between embryogenesis and germination. Annals of Botany 64: 149–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


