Abstract
Minute granules of sporopollenin, called orbicules, can be observed on the innermost tangential and/or radial walls of secretory tapetum cells. Orbicules were investigated in 62 species (50 genera) of Apocynaceae s.l. using light microscopy, scanning electron microscopy and transmission electron microscopy. Orbicules were found in 43 species (34 genera) distributed amongst the subfamilies Rauvolfioideae, Apocynoideae, Periplocoideae, and in the genus Riocreuxia (Asclepiadoideae). Absence of orbicules is apparent in Secamonoideae and Asclepiadoideae (except Riocreuxia). The orbicule types described are based on observed morphological and ultrastructural variation. Of the six orbicule types previously described, Type I and Type II orbicules are lacking. In the majority of species, Type III orbicules were recorded in addition to Types IV, V and VI. In this study we suggest that embedded Type VI orbicules are more derived. A correlation between orbicule typology and evolutionary tendencies in Apocynaceae s.l. palynology was found. A trend was observed from the presence of Type III orbicules in the majority of species belonging to the basal group of genera characterized by colporate to porate single pollen grains, or 3–6‐porate tetrads, towards the more derived embedded Type VI orbicules in the more advanced Periplocoideae genera with multiporate tetrads or pollinia. Orbicule data have proven not to be useful for evaluating tribal delimitation within the Apocynaceae s.l. contrary to the Rubiaceae and Loganiaceae s.l.
Key words: Orbicules, Ubisch bodies, Apocynaceae s.l., SEM, TEM, morphology, ultrastructure, typology, tapetum, systematics
INTRODUCTION
Orbicules
In anthers of flowering plants, tiny (<4 µm) granules sometimes occur on the radial and innermost tangential wall of secretory tapetum cells. These granules are sometimes in close contact with pollen grains, and are called orbicules (Erdtman et al., 1961) or Ubisch bodies (Kosmath, 1927). The systematic distribution of orbicules in angiosperms and their possible functions have been reviewed by Huysmans et al. (1998, 2000).
Orbicules develop simultaneously with the growing pollen exine and are composed of sporopollenin, similar to the pollen exine. Pro‐orbicules, which are produced by the endoplasmic reticulum of secretory tapetum cells, are the progenitors of these orbicules (Echlin and Godwin, 1968). Pro‐orbicules are extruded through the radial and innermost tangential wall of the secretory tapetum cells at the beginning of the tetrad stage (Christensen et al., 1972; El‐Ghazaly and Jensen, 1986; Clément and Audran, 1993; Vijayaraghavan and Chaudry, 1993). Pro‐orbicules are spherical structures that are composed of a complex organic mixture. They are surrounded by a glycocalyx. The deposition of sporopollenin precursors, produced by the protoplast of the secretory tapetum cells, on the glycoprotein filaments of the glycocalyx and on the growing pollen exine, starts at the late tetrad stage. The wall of mature orbicules is composed of structural and filling elements (Clément and Audran, 1993). Proteins, pectins, polysaccharides and glycolipids, organized in a three‐dimensional polygonal frame (Rowley, 1990) are part of the structural elements. The filling elements consist of unsaturated lipids and polyphenols (Clément and Audran, 1993). Using transmission electron microscopy (TEM), Clément and Audran (1993) could distinguish four different zones of electron density in mature orbicules of Lilium L. ‘enchantment’: (1) an electron‐translucent orbicule cavity; (2) a thin and very electron‐dense orbicule cavity wall interface; (3) a homogeneous electron‐dense orbicule wall; and (4) a thin very electron‐dense peripheral layer.
Orbicules are considered to be a general feature of species characterized by a secretory tapetum. However, Huysmans et al. (1998) reported that orbicules are lacking in several taxa with such a tapetum. Species with an amoeboid tapetum, which is believed to be more derived than the secretory tapetum type, characteristically do not produce orbicules (Sporne, 1973; Pacini et al., 1985), although there are exceptions such as Gentiana acaulis L. (Lombardo and Carraro, 1976; Vinckier and Smets, 2002). Orbicules have been recorded in some Mesozoic (Cretaceous) seed plants (Taylor and Alvin, 1984; Osborn et al., 1991; Serbet and Stockey, 1991; Archangelsky and Taylor, 1993), which may indicate that the presence of orbicules is a primitive feature of seed plants.
Taxonomic usefulness of orbicules
In higher plants, there are often striking analogies between ornamentation of the pollen exine and that of the orbicule wall (El‐Ghazaly and Jensen, 1986; Hesse, 1986). These parallelisms are explained by Hesse (1985) as rooted in the homology of tapetum and sporogeneous tissue. Since ornamentation of the pollen exine offers useful characters for systematics, orbicules might also have taxonomic value. It was suggested that orbicule characteristics can be useful for systematics at the tribal level in Loganiaceae s.l. (Vinckier and Smets, 2002) and the Rubiaceous subfamilies Cinchonoideae (Huysmans et al., 1997) and Ixoroideae (Vinckier et al., 2000). Apart from these studies on Gentianales taxa, the taxonomic usefulness of orbicule characters has so far only been studied for two angiosperm taxa: the genus Euphorbia L. (El‐Ghazaly, 1989; El‐Ghazaly and Chaudhary, 1993); and the Chloanthaceae, now included in the Verbenaceae (Raj and El‐Ghazaly, 1987).
Apocynaceae s.l.
The family Apocynaceae s.l. consists of approx. 424 genera with about 3500 species (Endress and Bruyns, 2000). The distribution is global but species are mainly present in subtropical to tropical, rarely temperate regions. The representatives of this family are lianas, herbs, shrubs, small to large trees, or rheophytes (Kanahia R. Br.). They appear as non‐succulents or succulents (Stapelia L.). Flowers are usually showy, bisexual and actinomorphic or nearly so. Members of the family produce latex, diverse iridoids, cardioglycosides, and various alkaloids (notably indole and steroid groups), sometimes cyanogenic, rarely saponiferous. Certain genera are of economic importance, e.g. as a source of rubber (Mascarenhasia A. DC.), latex (Landolphia P. Beauv.), drugs such as cardioglycosides (Acokanthera G. Don), ouabin and cymarin (Strophanthus DC.), and the alkaloids reserpine and rescinnamine (Rauvolfia L.). Many genera are used in horticulture, some of these now being widespread, such as oleander (Nerium L.), frangipani (Plumeria L.), Hoya R. Br., Ceropegia L. and many of the succulent species (Nicholas and Baijnath, 1994).
In the opinion of Endress and Stevens (2001), a unified family Apocynaceae s.l. (including Asclepiadaceae) best reflects the phylogeny of the group. Endress and Bruyns (2000) proposed a unified classification based on morphological characters supplemented with molecular results. Their classification consists of 424 genera distributed among five subfamilies: Rauvolfioideae, Apocynoideae, Periplocoideae, Secamonoideae and Asclepiadoideae. In the present paper the unified classification proposed by Endress and Stevens (2001) based on Endress and Bruyns (2000) is used.
In flowers of Apocynaceae s.l. the major evolutionary trend is towards progressive efficiency of the pollination mechanism, which is mainly achieved by increasing synorganization of the androecium and gynoecium (Nilsson et al., 1993). In Rauvolfioideae, the most basal subfamily, pollen grains occur as single 3–4‐colporate pollen grains; however, in the tribe Alyxieae, 2–3‐porate pollen occurs, and tetrads seem to occur sporadically in Rauvolfioideae, e.g. in Melodineae (Endress and Bruyns, 2000), and Alyxieae (van der Ham et al., 2001). Representatives of Apocynoideae, which have many advanced traits in common with asclepiadaceous genera, are characterized by single 3–4‐porate (occasionally aperturate or polypantoporate) pollen grains, with the exception of Apocynum L. in which tetrads occur (Endress and Bruyns, 2000). In Periplocoideae, pollen is arranged in tetrads and even pollinia are recorded (Verhoeven and Venter, 1998, 2001). These pollinia are very similar to those present in Secamonoideae in that the pollinium consists of an aggregation of tetrads and is not covered by a pollinium wall (ectexine). However, the pollinia in Periplocoideae differ from those in Secamonoideae in that the four pollinia in Periplocoideae per anther are free, whereas in Secamonoideae they are attached to caudicles (Verhoeven and Venter, 1998). The wall structure of the tetrads forming the pollinium also differs. In Periplocoideae (except Camptocarpus Decne.) the wall consists of a tectum and a granular stratum subtended by an intine, whereas in Camptocarpus and Secamonoideae a three‐layered exine structure occurs, subtended by an intine (Verhoeven and Venter, 2001). The proximal walls between the tetrads are reduced in Secamonoideae and consist of a granular exine layer subtended by an intine (Civeyrel, 1995). In Asclepiadoideae only two pollinia occur, which consist of single pollen grains, presumably evolved from tetrads and representing an advanced character‐state, with an outer wall enclosing the pollinium. However, Fockea Endl. in Endlicher & Fenzl differs from other Asclepiadoideae genera because the pollinium consists of calymmate tetrads, which are not fused or surrounded by a pollinium wall (Verhoeven and Venter, 2001). The distal exine layer seems to be homologous to the tectum in Periplocoideae and Secamonoideae, the inner walls consist of a discontinuous granular exine layer subtended by an intine (Verhoeven and Venter, 2001).
Aims of this study
The present study has four major aims: (1) to investigate the presence of orbicules in Apocynaceae s.l.; (2) to provide detailed descriptions of the morphology and ultrastructure of orbicules present; (3) to construct an orbicule typology, based on morphological and ultrastructural variations; and (4) to investigate whether the orbicule typology is correlated with suggested trends in palynology of Apocynaceae s.l. and to discuss the sytematic usefulness of this typology.
MATERIALS AND METHODS
Herbarium material supplemented with living material was investigated in this study. Sixty‐two species of 50 Apocynaceae s.l. genera were selected for examination (Table 1 and Appendix). The aim of this selection was to cover all subfamilies recognized in the Apocynaceae s.l. (Endress and Bruyns, 2000; Endress and Stevens, 2001).
Table 1.
Orbicule morphology and ultrastructure
| Size (µm) | Pollen | Orbicule ultrastructure | |||||||||||
| Subfamily | Tribe | Species | (mean ± s.d.) | Shape | Indentations | Aggregates | exine | Density | ETC | EDC | EDW | EDP | Type |
| Rauvolfioideae | Alstonieae | Alstonia congensis | 0·599 ± 0·134 | ro | Scarce | + | perf | a | + | + | + | + | IIIa |
| Aspidosperma quebracho–blanco | – | – | – | – | psil | – | – | ||||||
| Aspidosperma melanocarpon | – | – | – | – | psil | – | – | ||||||
| Vinceae | Amsonia tabernaemontana | 1·043 ± 0·324 | ir, gr | + | + | perf | a | ? | |||||
| Catharanthus roseus | 0·749 ± 0·182 | ro | Scarce | + | psil–perf | a | IIIa | ||||||
| Kopsia fructicosa | 0·563 ± 0·117 | ro | Scarce | + | psil–perf | a | + | + | + | + | IIIa | ||
| Rauvolfia mattfeldiana | 0·321 ± 0·059 | ro | + | + | psil–perf | a | IIIa | ||||||
| Rauvolfia vomitoria | 0·364 ± 0·086 | ro | + | + | psil | a | IIIa | ||||||
| Willughbeeae | Dictyophleba lucida | 0·662 ± 0·119 | ro | Scarce | + | perf | a | IIIa | |||||
| Tabernaemon taneae | Molongum zschokkeiforme | 1·301 ± 0·869 | ir–ro | Scarce | + | perf | va | + | + | + | + | V | |
| Tabernaemontana calcarea | 0·545 ± 0·145 | ds | cent | + | psil–perf | a | IIIb | ||||||
| Tabernaemontana coronaria ‘flore pleno’ | 0·788 ± 0·119 | ds | cent | + | psil–perf | a | + | – | + | + | IIIb | ||
| Melodineae | Not sampled | ||||||||||||
| Hunterieae | Picralima nitida | 1·186 ± 0·223 | ro, gr | + | + | sc | va | + | + | + | + | ? | |
| Plumerieae | Allamanda cathartica | 0·322 ± 0·088 | ro | Scarce | + | psil–perf | a | + | + | + | + | IIIa | |
| Cerberiopsis candelabra | 0·653 ± 0·129 | ro, gr | + | + | perf | a | IIIa | ||||||
| Plumeria rubra var. acutifolia | 0·749 ± 0·268 | ro | Scarce | + | perf | a | IIIa | ||||||
| Thevetia bicornuta | 0·267 ± 0·086 | aif | Scarce | + | perf–mret | a | + | +/– | + | + | IV | ||
| Carisseae | Acokanthera oblongifolia | 0·955 ± 0·292 | ir, gr | + | + | psil–perf | va | +/– | +/– | + | + | ? | |
| Acokanthera laevigata | 0·811 ± 0·204 | ir, gr | + | + | psil–perf | a | ? | ||||||
| Carissa bispinosa | 0·593 ± 0·126 | ro | + | + | perf | a | IIIa | ||||||
| Alyxieae | Not sampled | ||||||||||||
| Apocynoideae | Wrightieae | Adenium obesum | 0·426 ± 0·101 | ds | cent | + | psil–perf | a | IIIb | ||||
| Nerium oleander | 1·074 ± 0·378 | aif | + | + | psil–perf | va | + | + | + | + | IV | ||
| Strophanthus courmontii | 0·521 ± 0·149 | ro | + | + | psil–perf | a | IIIa | ||||||
| Strophanthus cumingii | 0·479 ± 0·089 | ro | + | + | psil–perf | a | IIIa | ||||||
| Wrightia natalensis | 0·298 ± 0·068 | ir | + | – | psil | a | +/– | – | + | + | ? | ||
| Malouetieae | Funtumia africana | 0·085 ± 0·019 | ro | – | – | psil | a | IIIa | |||||
| Mascarenhasia arborescens | 0·257 ± 0·058 | ro | – | – | psil–perf | a | IIIa | ||||||
| Mascarenhasia lanceolata | 0·101 ± 0·028 | ro | – | – | psil–perf | a | IIIa | ||||||
| Pachypodium baronii | 0·305 ± 0·086 | ds | cent | + | psil–perf | a | IIIb | ||||||
| Pachypodium namaquanum | 0·578 ± 0·168 | ds | cent | + | psil–perf | a | + | – | + | + | IIIb | ||
| Apocyneae | Apocynum cannabinum | 0·263 ± 0·038 | ro | Scarce | + | tetrad: psil | a–va | + | – | + | + | IIIa | |
| Baissea baillonii | 0·775 ± 0·121 | ro | + | + | psil–perf | a | IIIa | ||||||
| Baissea leonensis | 0·289 ± 0·059 | ro | + | + | psil–perf | a | IIIa | ||||||
| Beaumontia grandiflora | 3·178 ± 0·485 | ir, gr | + | + | psil–supgr | a | ? | ||||||
| Trachelospermum jasminoides | 0·312 ± 0·071 | ds | cent | + | psil | a | IIIb | ||||||
| Mesechiteae | Mandevilla atroviolacea | 0·639 ± 0·162 | ro | Scarce | + | psil–perf | a | IIIa | |||||
| Echiteae | Parsonsia cumingiana | 0·262 ± 0·086 | ro | + | + | psil–perf | a | IIIa | |||||
| Prestonia portobellensis | 0·435 ± 0·063 | ro | + | + | perf | a | + | + | + | + | IIIa | ||
| Periplocoideae | Camptocarpus crassifolius | 0·193 ± 0·041 | ds | cent | + | tetrad: psil | a | IIIb | |||||
| Hemidesmus indicus | 0·345 ± 0·052 | r | – | – | mas: psil | a | VI | ||||||
| Periploca graeca | 0·374 ± 0·076 | ds | cent | + | tetrad: psil | a | + | +/– | + | + | IIIb | ||
| Periploca laevigata | 0·230 ± 0·044 | ds | cent | + | tetrad: psil | a | IIIb | ||||||
| Raphionacme brownii | 0·249 ± 0·057 | r | – | – | tetrad: psil | a | VI | ||||||
| Streptocaulon tomentosum | 0·287 ± 0·085 | r | – | – | mas: psil | a | VI | ||||||
| Secamonoideae | Secamone elliptica ssp. elliptica | – | – | – | – | mas: psil | – | – | |||||
| Secamone filliformis | – | – | – | – | mas: psil | – | – | ||||||
| Secamonopsis madagascariensis | – | – | – | – | mas: psil | – | – | ||||||
| Asclepiadoideae | Fockeeae | Fockea edulis | – | – | – | – | pol: psil | – | – | ||||
| Marsdenieae | Hoya sp. | – | – | – | – | pol: psil | – | – | |||||
| Telosma procumbens | – | – | – | – | pol: psil | – | – | ||||||
| Ceropegieae | Riocreuxia picta | 0·324 ± 0·065 | r | – | – | pol: psil | a | VI | |||||
| Asclepiadeae | Araujia sericifera | – | – | – | – | pol: psil | – | – | |||||
| Asclepias curassavica | – | – | – | – | pol: psil | – | – | ||||||
| Asclepias friesii | – | – | – | – | pol: psil | – | – | ||||||
| Cynanchum acutum | – | – | – | – | pol: psil | – | – | ||||||
| Ditassa capillaris | – | – | – | – | pol: psil | – | – | ||||||
| Matelea carolinensis | – | – | – | – | pol: psil | – | – | ||||||
| Pentarrhinum abyssinicum | – | – | – | – | pol: psil | – | – | ||||||
| Pergularia tomentosa | – | – | – | – | pol: psil | – | – | ||||||
| Tylophora sylvatica | – | – | – | – | pol: pil | – | – | ||||||
| Tylophora yunnanensis | – | – | – | – | pol: pil | – | – | ||||||
| Vincetoxicum nigrum | – | – | – | – | pol: perf–psil | – | – | ||||||
Species in bold possess orbicules.
Subfamily and Tribe sensu Endress and Stevens (2001).
r, regular; ir, irregular; ro, rounded oblate; aif, angular irregular folded; ds, doughnut‐shaped; gr, sporopollenin granulae present on the orbicule surface; sc, scabrate; cent, central; mas, massulae; pol, pollinium; perf, perforate; mret, microreticulate; supgr, supratectal granules; pil, pilate; psil, psilate; a, abundant; va, very abundant (locule‐surface totally covered); ETC, electron‐translucent cavity; EDC, electron‐dense cavity wall interface; EDW, homogeneous electron‐dense orbicule wall; EDP, very electron‐dense peripheral layer.
Type: Orbicule type sensu Huysmans et al. (1997) and Vinckier et al. (2000); ?, orbicule type difficult to define.
For scanning electron microscopy (SEM) of orbicules, dried flowers or buds were rehydrated in the wetting agent Agepon® (Agfa Gevaert, Leverkusen, Germany) for 1–2 h. The anthers were picked out from the flowers, and the tips of anthers were removed using a razor blade to facilitate rehydration of the locules. After dissection, anthers remained in the wetting agent for another hour. Following dehydration in a graded acetone series, the material was critical point dried (CPD 030, Balzers) and mounted on stubs using double‐sided adhesive tape prior to further preparation. The pollen grains were then removed from the opened locules using a fine cactus spine to facilitate observation of the locule surface.
Preparation of anthers from fresh flowers differed slightly from the description above. Immediately after collecting the flowers, the anthers were picked out and slit open using a razor blade. They were fixed for at least 4 h in 2 % glutaraldehyde at pH 7·4, buffered with 0·05 m sodium cacodylate. The next steps in the preparation, beginning with dehydration in acetone, were identical to those described above.
The stubs were sputter‐coated with gold (SPI‐MODULE™ Sputter Coater; SPI Supplies, West Chester, PA, USA). A Jeol JSM‐6400 microscope, at 10–20 kV, was used for morphological observations. Comparative size measurements of orbicules were ascertained from scanning electron micrographs using Carnoy 2·0 (Schols et al., 2002). For each anther investigated, 100 orbicules were measured, and mean values and standard deviations calculated (Table 1).
For TEM observations, anthers of selected flowers were fixed with 2 % glutaraldehyde at pH 7·4, buffered with 0·05 m sodium cacodylate. The majority of specimens was embedded in LR‐White Resin (Polysciences Inc., Warrington, PA, USA). Prior to embedding in LR‐White Resin the material was dehydrated in a graded ethanol series, followed by block‐staining in 1 % phosphotungstic acid (PTA) in 100 % ethanol. Anthers of Apocynum cannabinum L. and Plumeria rubra var. acutifolia (Poir) L. H. Bailey were embedded in Araldit Resin Grade 6005 (Polysciences Inc.). Prior to embedding in Araldit Resin the material was post‐fixed with 2 % OsO4, block‐stained in 2 % uranylacetate and dehydrated in a graded acetone series. Semi‐thin (±1 µm) sections were cut using a Reichert Jung Ultracut E microtome and stained with 0·1 % thionin–0·1 % methylene blue. Semi‐thin sections were observed using a Leica DM LB light microscope (LM). Ultra‐thin (±70 nm) sections, on copper grids, were stained with uranylacetate and lead citrate in an LKB 2168 Ultrostainer and were observed in a Zeiss EM 900 transmission electron microscope at 80 kV.
RESULTS
Orbicule characters
Orbicules were found in 43 species (34 genera) distributed among the subfamilies Rauvolfioideae, Apocynoideae, Periplocoideae, and in the genus Riocreuxia Decne. (Asclepiadoideae). Orbicules were absent in Secamon oideae and Asclepiadoideae (Table 1).
The size and shape of orbicules, the presence of indentations in the orbicule wall, and the occurrence of aggregated orbicules were recorded. Results are summarized in Table 1.
Size.
Orbicules in Funtumia africana (Benth.) Stapf (Fig. 2B) were the smallest observed (0·085 µm), whereas the largest orbicules (3·178 µm) occurred in Beaumontia grandiflora Wall. (Table 1). The apparently large orbicules are often aggregations of smaller orbicules.
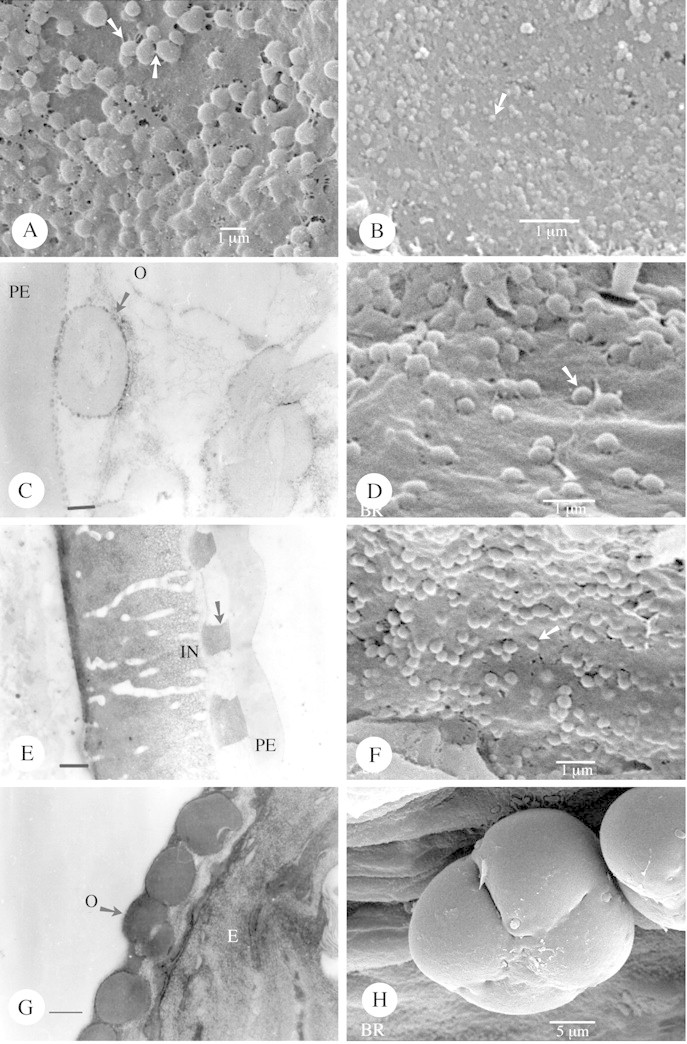
Fig. 2. Type IIIa orbicules as seen by SEM and TEM. A, Orbicules of Mandevilla atroviolacea (viewed under SEM) showing the thin threads connecting the orbicules or running between the orbicules and the locule wall surface (arrow). B, SEM observation of the very small orbicules of Funtumia africana (arrow). C–E, Prestonia portobellensis. C, TEM observation of an orbicule (arrow) in close contact with the pollen exine (PE). A thin electron‐dense (ED) cavity wall interface is present between the homogeneous ED orbicule wall and the electron‐translucent (ET) orbicule cavity, as is a granular ED peripheral layer. Bar = 0·15 µm. D, Spherical orbicules present in this species viewed under SEM. E, TEM observation of the structureless pollen exine (PE), a thick channelled intine (IN), and inbetween darkly stained inclusions (arrow). Bar = 0·6 µm. F–H, Apocynum cannabinum. F, SEM observation of small orbicules (arrow) lying on the locule wall surface. G, TEM observation of single orbicules. The ET orbicule cavity, surrounded by the ED orbicule wall, is visible in two orbicules. Bar = 0·25 µm. H, A porate tetrad lying on the locule wall surface inside the anther. O, Orbicle.
Shape.
There are three distinct shapes of orbicules: (1) more or less globose (Figs 1B, D, F and H and 2A, B, D and F); (2) rounded oblate with a central indentation (doughnut‐shaped) (Fig. 3A, B, D and F); and (3) irregular angular and ‘folded’ orbicules (Fig. 4A and C; Table 1). Apart from these clearly definable shapes, the shape of orbicular elements may be amorphous. Irregular layers of small to large aggregations of orbicules embedded in residual tapetal material occur in some species (Fig. 4E).
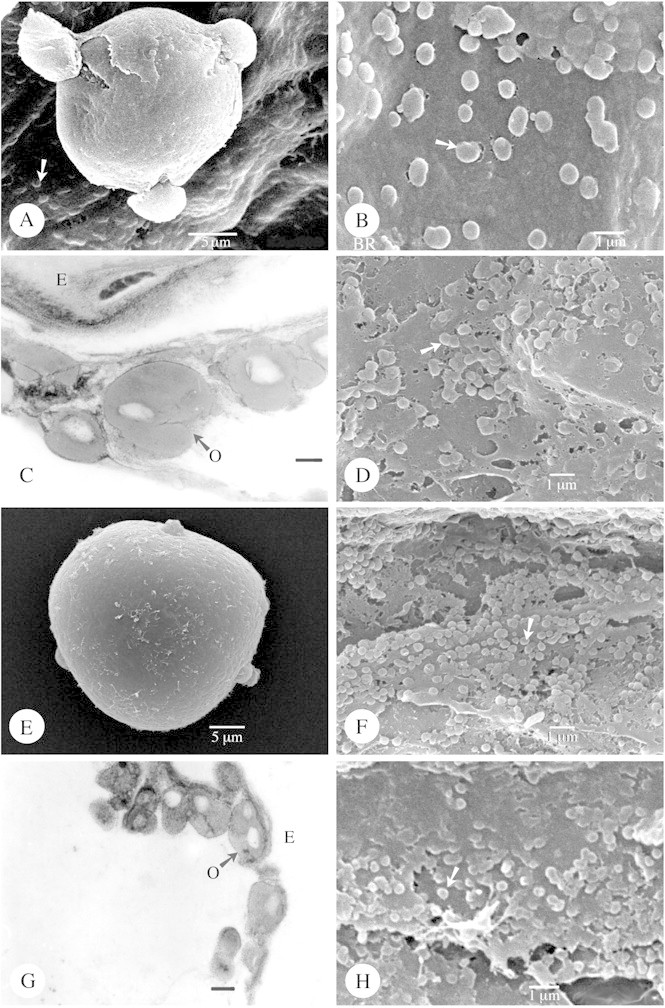
Fig. 1. Type IIIa orbicules viewed using SEM and TEM. A–C, Alstonia congensis. A, SEM observation of a tri‐colporate pollen grain (polar view) lying on the locule wall surface, covered with orbicules (arrow). B, Detail of the orbicules (arrow) covering the locule wall surface. C, Orbicules as viewed under TEM. Between the electron‐translucent (ET) orbicule cavity and the homogeneous electron‐dense (ED) orbicule wall a more ED orbicule cavity wall interface is visible. The orbicule wall is delimited by a very ED peripheral layer. Bar = 0·25 µm. D, SEM observation of the orbicules of Kopsia fructicosa covering the locule wall surface. Some aggregations of orbicules occur (arrow). E and F, Rauvolfia matfeldiana (SEM). E, Tri‐aperturate pollen grain in polar view. F, Many small orbicules (arrow) covering the locule wall surface. G and H, Allamanda cathartica. G, TEM observation of single or compound (arrow) orbicules. The different zones of electron density are present. Bar = 0·4 µm. H, SEM observation of the orbicules.
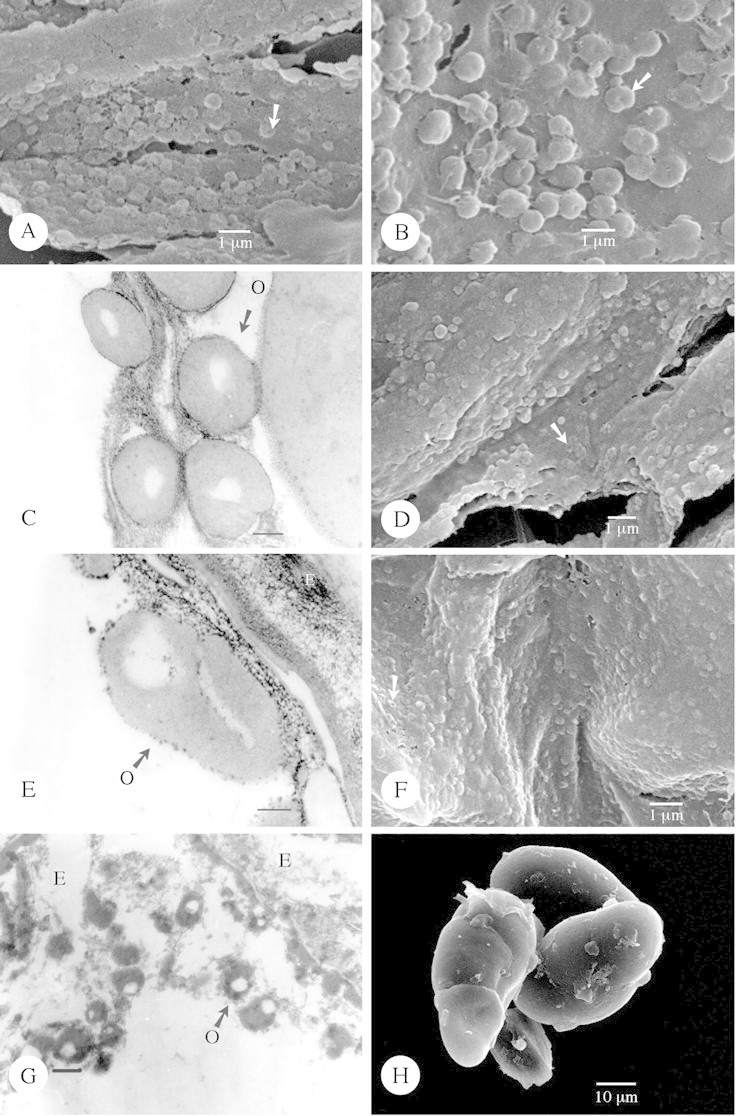
Fig. 3. Type IIIb orbicules viewed using SEM and TEM. A, Adenium obesum. SEM observation of the doughnut‐shaped orbicules, characterized by a central indentation (arrow) in the orbicule wall. B–C, Tabernaemontana coronaria ‘flore pleno’. B, SEM observation of the doughnut‐shaped orbicules (arrow). Thin interconnecting threads also occur. C, TEM observation of the single orbicules. A granular electron‐dense (ED) peripheral layer delimits the homogeneous ED orbicule wall. The electron‐translucent orbicule cavities are clearly visible. Bar = 0·25 µm. D, Small doughnut‐shaped orbicules (arrow) of Trachelospermum asiaticum viewed using SEM. E, Pachypodium namaquanum. Detailed TEM observation of an orbicule. The ED peripheral layer and ED cavity wall interface, lining the homogeneous ED orbicule wall, have a granular appeareance. Bar = 0·15 µm. F, Scanning electron micrograph of the doughnut‐shaped orbicules (arrow) of Camptocarpus crassifolius lying on the surface of the locule wall. G–H, Periploca graeca. TEM observation of single orbicules. Bar = 0·25 µm. H, A group of porate tetrads. O, Orbicule.
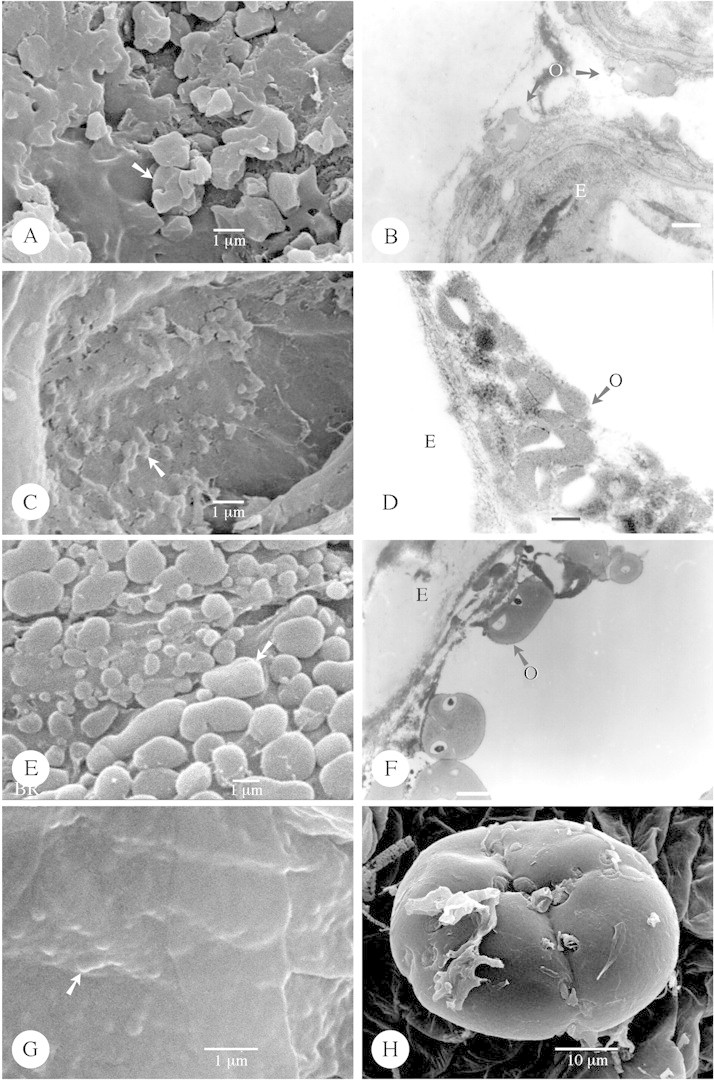
Fig. 4. Type IV (A–D), Type V (E and F) and Type VI orbicules (G and H). A and B, Nerium oleander. SEM observation of the irregular angular and folded orbicules (arrow) covering the locule wall surface. B, TEM observation of the irregular orbicules positioned on the remnants of tapetum cells. The orbicules posses an electron‐translucent (ET) cavity, and the homogeneous electron‐dense (ED) orbicule wall is delimited by an ED peripheral layer. Bar = 0·4 µm. C and D, Thevetia bicornuta. C, Small irregular angular and folded orbicules (arrow) viewed in an SEM. D, TEM observation of the irregular orbicules. The orbicules possess an ET cavity and the homogeneous ED orbicule wall is delimited by an ED peripheral layer. Bar = 0·15 µm. E and F, Molongum zschokkeiforme. E, SEM observation of compound orbicules (arrow) covering the locule wall surface. F, TEM observation of compound orbicules. In the ET orbicule cavities a very ED dot occurs. The homogeneous ED orbicule wall is surrounded by a thin very ED peripheral layer. Bar = 0·6 µm. G and H, Raphionacme brownii. G, SEM observation of the strongly embedded orbicules (arrow) present in this species. H, SEM observation of a multiporate tetrad. E, Endothelium; O, orbicule.
Surface.
In the majority of species orbicules have a smooth surface. However, in some species orbicules are covered by sporopolleninous granulae (Fig. 5E and G). Thin threads may occur, connecting orbicules to each other, or running between the tapetal membrane and orbicules (Fig. 2A).
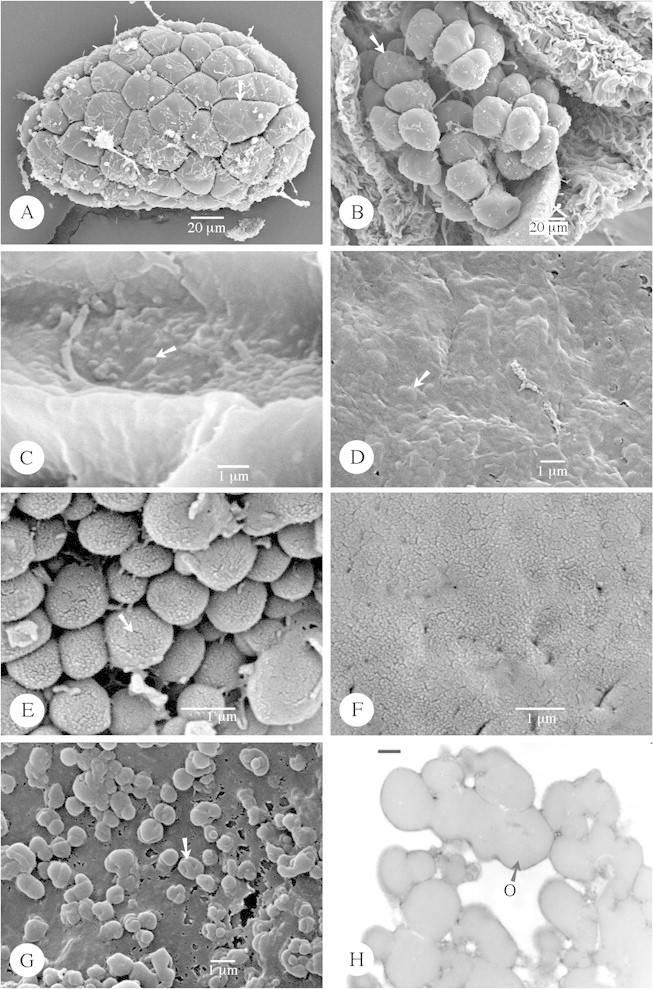
Fig. 5. Type VI (A–D) orbicules and those that are difficult to define (E–H). A, Hemidesmus indicus. Observation of one massula (pollinium) composed of porate tetrads (arrow). B and C, Streptocaulon tomentosum (SEM). B, Observation of the massulae (arrow) lying on the spoon‐like translator. C, Orbicules are flattened and embedded in this species. D, Flattened orbicules of Riocreuxia picta strongly embedded within the tapetal membrane. E and F, Picralima nitida. E, Detailed SEM observation of the orbicules present in this species. Short elongated elements occur (arrow) on the orbicule wall, similar to those present on the pollen exine (Fig. 5F). F, Detail of the pollen exine surface, which consists of short elongated elements. G and H, Acokanthera oblongifolia. G, SEM observation of the irregular orbicules (arrow). Indentations may be present on the orbicule wall. H, TEM observation of the irregular orbicules. The homogeneous electron‐dense (ED) orbicule wall is delimited by a very ED peripheral layer. O, Orbicule.
Distribution density and location of orbicules
When present, orbicules are dispersed over the entire locule surface. Occasionally they attach to the pollen exine surface (Fig. 6A). The position of orbicules relative to the tapetal membrane varies: they may be completely embedded within (Fig. 4G), or lightly attached to, the surface of the tapetal membrane (Fig. 1F). A continuum between both extremes may occur within one species (Fig. 3B).
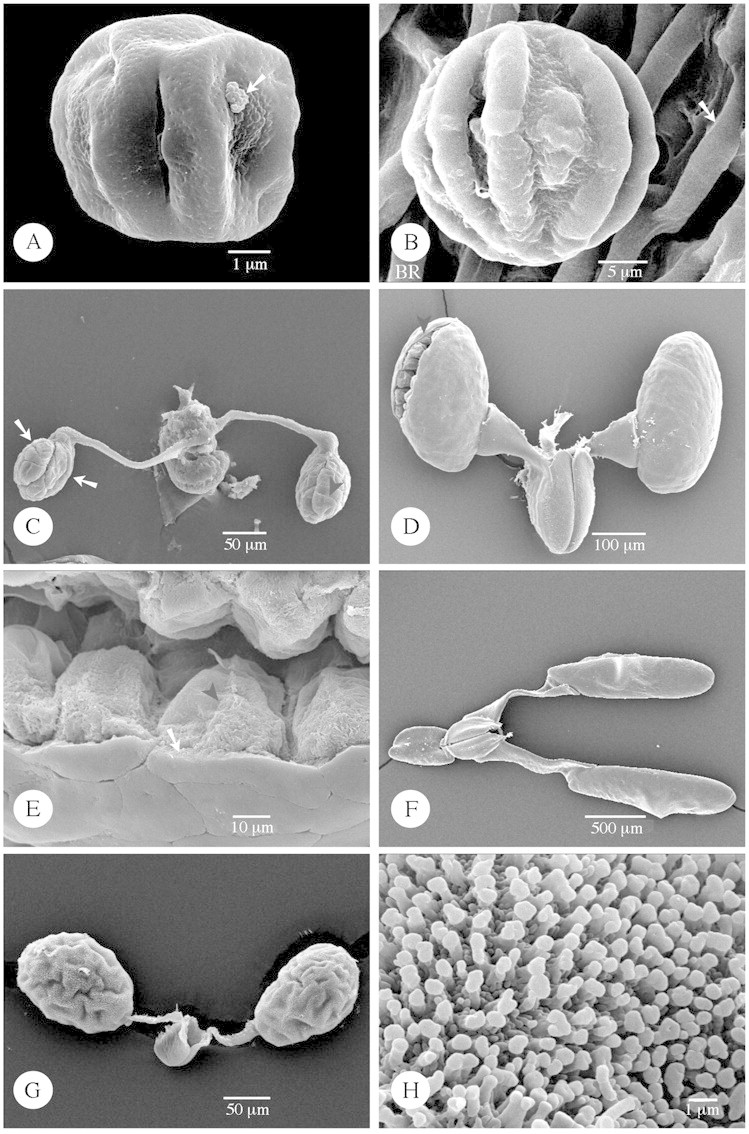
Fig. 6. SEM of Rauvolfioideae microspores (A and B), Secamonoideae (C), and Asclepiadoideae pollinia (D–H). A, Acokanthera oblongifolia. A group of orbicules (arrow) is attached on the pollen exine. B, Aspidosperma quebracho‐blanco. Pollen grain lying on the locule wall surface which consists of wavy ridges with deep, narrow grooves. The ridges on the locule surface form small loops (arrow), probably caused by underlying endothecial thickenings. C, Secamonopsis madagascariensis. Two massulae (arrows) are produced per locule and are composed of tetrads. Delimitations between tetrads are still visible as narrow grooves (black arrowhead). D and E, Cynanchum acutum. D, View of pollinia attached to caudicles and the corpusculum. One pollinium is torn open (arrowhead). E, Inner structure of the pollinium: single pollen grains (arrowhead) building up the pollinium, surrounded by a pollinium wall (ectexine) (arrow). F, Araujia sericifera. View of pollinia attached to caudicles and the corpusculum. G and H, Tylophora sylvatica. View of pollinia attached to caudicles and the corpusculum. H, Detail of the pilate ectexine.
Orbicules are always irregularly arranged. The number of orbicules present on the locule surface is well in excess of the number of pollen grains within the locule. Two terms are used (Table 1) to describe the distribution density: ‘very abundant’ denotes that the locule surface is more or less obscured by orbicules (Fig. 5E), while ‘abundant’ denotes that the orbicules are not in contact, or are connected only by thin threads or other tapetal debris so that the locule surface is visible between the orbicules (Fig. 2A).
Ultrastructure
Fourteen species (14 genera) were investigated at the ultrastructural level (TEM, Table 1). These species covered the subfamilies Rauvolfioideae, Apocynoideae and Periplocoideae. The selection was based on observed variations in morphological characters of the orbicules. In the descriptions of ultrastructure (Table 1), special attention is paid to the occurrence of the different zones of electron density, as defined by Clément and Audran (1993) (see Introduction).
In cross‐section, orbicules vary considerably in shape; they can be spherical (Fig. 3C), rounded oblate (Figs 1G, 2C and G and 3E), granular (Fig. 5H), irregular (Fig. 4B) or many‐sided (Fig. 4D).
The number of electron‐translucent orbicule cavities is indicative of whether the orbicules are single entities (Figs 1C, 2C and G and 3C and G) or compound (Figs 1G and 4F). Inside these cavities, very electron‐dense dots may occur (Fig. 4F). Between the orbicule cavity and the homogeneous electron‐dense orbicule wall, a thin electron‐dense orbicule cavity wall interface is sometimes present (Figs 1C, 2C and 3E). The presence of a very electron‐dense peripheral layer, delimiting the homogeneous electron‐dense orbicule wall, is a general feature of the species studied. This electron‐dense peripheral layer, as well as the orbicule cavity wall interface, sometimes has a granular appearance, especially in anthers treated with Phosphotungstic acid (PTA) (Figs 2C and 3C and E). PTA is a specific histochemical stain for proteins (Benedetti and Bertolini, 1963; Marinozzi, 1968), indicating the glycoprotein nature of these zones.
Pollen characteristics
Characteristics of the ornamentation of the pollen exine surface are summarized in Table 1. A small amount of variation of pollen ornamentation types was observed among the species studied. Most species possessed a perforate (Figs 1A, E and 6A) or psilate exine (Figs 2H, 3H, 4H and 5A and B). Apart from these ornamentation types, micro‐reticulate pollen was present in Thevetia L., and a scabrate exine consisting of short, randomly spaced exine elements was observed in Picralima Pierre (Fig. 5F) (Table 1). In Prestonia R. Br. (Fig. 2E), a one‐layered, structureless exine was observed using TEM. Between the exine and the prominent intine, darkly staining inclusions were present projecting inwards. Nilsson et al. (1993) and Endress et al. (1996) observed similar inclusions in Forsteronia acouci (Aubl.) A. DC. and Prestonia mollis Kunth., respectively.
In most Asclepiadoideae species studied, pollinia possessed a psilate ectexine (Fig. 6D and F). However, in Tylophora R. Br., a pilate ectexine was present (Fig. 6G and H). In Cynanchum acutum L., a pollinium was observed that was torn open (Fig. 6D, black arrowhead), giving us the opportunity to observe its inner structure. The pollinium was built up of single pollen grains (Fig. 6E, black arrowhead) and surrounded by the pollinium wall (ectexine) (Fig. 6E, arrow).
DISCUSSION
In addition to previous reports on the presence of orbicules in Catharanthus roseus (L.) G. Don (Cousin, 1979; Cerceau‐Larrival et al., 1981; El‐Ghazaly and Nilsson, 1991), Lepinia solomonensis Hemsl. and Plectaneia thouarsii Roem. & Schult. (van der Ham et al., 2001), an additional 61 species belonging to Apocynaceae s.l. (49 genera) were investigated in this study, of which 42 species (33 genera) possessed orbicules (Table 1).
Orbicule types
The orbicule types described are based on morphological and ultrastructural variations observed in the study group. The typology (Table 1) follows the treatment of orbicule types described for the rubiaceous subfamilies Cincho noideae (Huysmans et al., 1997) and Ixoroideae (Vinckier et al., 2000). In the Cinchonoideae, Huysmans et al. (1997) defined four orbicule types: spiny orbicules (Type I); microrugulate orbicules (Type II); smooth and rounded oblate orbicules (Type III); and irregular and folded orbicules (Type IV). Two additional orbicule types were defined by Vinckier et al. (2000) in the Ixoroideae: large flattened and irregular orbicules (Type V); and distinctly embedded orbicules (Type VI). In Ixoroideae (Vinckier et al., 2000), Type III orbicules were divided into two subtypes: rounded oblate orbicules (Subtype IIIa); and doughnut‐shaped orbicules (Subtype IIIb), which are characterized by a central indentation in the orbicule wall. For a more extensive description of the different orbicule types, see Huysmans et al. (1997), Vinckier et al. (2000) and Vinckier and Smets (2002).
Orbicule types are defined in this paper by the same types given above (Table 1). Of the six orbicule types previously described, Type I and Type II orbicules are lacking in the study group. In the majority of species (21 species), Type III orbicules are recorded (Table 1). Only four species possess Type VI orbicules, two species Type IV, and Molongum Pichon is the only representative with Type V orbicules (Table 1). Some species have orbicules that are difficult to classify into the defined types (Fig. 5E–H).
Correlations between pollen exine and orbicule surface ornamentation within the same species
Strong correlations between pollen exine and orbicule surface ornamentation within the same species have been recorded previously (Huysmans et al., 1997; Vinckier et al., 2000; Vinckier and Smets, 2002). In the present study, however, few examples of such correlations were present. The most remarkable correlation between exine and orbicule surface ornamentation was observed in Picralima (Figs. 5E and F). The orbicule wall ornamentation (Fig. 5E) resembles the ornamentation of the exine in that similar randomly spaced elements are present on the orbicule wall and the scabrate exine (Fig. 5F).
Correlation of orbicule typology with trends in Apocynaceae palynology and systematic usefulness of orbicules
At the species level, all specimens investigated belonging to the same species were characterized by the same orbicule type; no intraspecific variation was observed (Table 1).
Rauvolfioideae have been considered to be basal and more heterogeneous than Apocynoideae (Endress and Bruyns, 2000). This heterogeneity is also reflected in the orbicule typology. In Rauvolfioideae, Type IIIa, Type IIIb, Type IV and Type V orbicules are present, whereas in Apocynoideae only Type IIIa, Type IIIb and Type IV orbicules are recorded (Table 2). In Periplocoideae, a more derived group than Apocynoideae, species with 4–6‐porate tetrads possess Type IIIb doughnut‐shaped orbicules (Fig. 3F–H; Tables 1 and 2). Similar tetrads also occur in Apocynum (Apocynoideae) (Fig. 2H). However, Apocynum has Type IIIa orbicules, which could be interpreted as support for the hypothesis of Nilsson et al. (1993) that the similar tetrad type found in Apocynum and in Periplocoideae is the result of parallel evolution within two closely related lines with a common genetic background. In Raphionacme Harvey, multiporate tetrads occur (Fig. 4H) and distinctly embedded Type VI orbicules are present (Fig. 4G). The multiporate tetrads in Raphionacme can be regarded as more advanced than the 4–6‐porate tetrads found in the Periplocoideae genera (Nilsson et al., 1993). Embedded Type VI orbicules also occur in Hemidesmus R. Br. and Streptocaulon Wight & Arn. (Fig. 5C; Tables 1 and 2). These genera possess pollinia (Fig. 5A and B) and can be regarded as more advanced than Periplocoideae genera with single tetrads (Verhoeven and Venter, 2001). We suggest that the embedded Type VI orbicules are a more derived type of orbicule. In the more advanced subfamilies Secamonoideae and Asclepiadoideae (except Riocreuxia; Fig. 5D) orbicules are lacking (Tables 1 and 2).
Table 2.
Palynological trends in Apocynaceae s.l. based on Civeyrel (1995), Endress and Bruyns (2000), Nilsson et al. (1993) and Verhoeven and Venter (1998, 2001)
| Subfamily | Pollen | Most common orbicule type | Other orbicule types |
| Rauvolfioideae | 3–4‐colporate single pollen grains (Fig. 1A) | IIIa and IIIb | IV, V, and ? |
| Apocynoideae | 3–4‐porate, occasionally aperturate or polypantoporate single pollen grains (Fig. 1E) | IIIa and IIIb | IV and ? |
| Porate tetrads in Apocynum (Fig. 2H) | IIIa | – | |
| Periplocoideae | 4–6‐porate tetrads (Fig. 3H) | IIIb | – |
| Multiporate tetrads in Raphionacme (Fig. 4H) | Embedded type VI | – | |
| 2 pollinia (massulae) per locule composed of porate tetrads without outer wall enclosing the pollinium in Hemidesmus (Fig. 5A) and Streptocaulon (Fig. 5B) | Embedded type VI | – | |
| Secamonoideae | 2 pollinia (massulae) per locule composed of inaperturate tetrads without outer wall enclosing the pollinium (Fig. 6C) | Absence of orbicules | |
| Asclepiadoideae | 1 pollinium per locule composed of inaperturate single pollen grains with an outer wall enclosing the pollinium (Fig. 6D‐H) (excl. Fockea) | Absence of orbicules (except in Riocreuxia, type VI) |
We can conclude that orbicule typology is correlated with the evolutionary trends in Apocynaceae s.l. palynology. A trend is observed from the presence of Type III orbicules in the majority of species belonging to the basal group of genera characterized by colporate or porate single pollen grains, or 3–6‐porate tetrads, towards the more derived embedded Type VI orbicules in the more advanced genera of Periplocoideae with multiporate tetrads or pollinia (Table 2). Finally, orbicules disappear completely in Secamonoideae and the most advanced Asclepiadoideae (except Riocreuxia) (Table 2). These observations fit well with the suggested primitive character of the presence of orbicules (Introduction). Similar results were found in our study on the Rubiaceae subfamily Ixoroideae (Vinckier et al., 2000). Gardeniinae, which are considered to be the most derived group in Ixoroideae (Andreasen and Bremer, 1996), are also characterized by the absence of orbicules (Vinckier et al., 2000).
Orbicule data have proven to be useful for evaluating tribal delimitation within Rubiaceae (Huysmans et al., 1997; Vinckier et al., 2000) and Loganiaceae s.l. (Vinckier and Smets, 2002); however, they seem not to be useful for tribal delimitation in Apocynaceae (Table 1).
ACKNOWLEDGEMENTS
We thank Dr Eric Schoeters (TEM, Leuven), Koen Collart (TEM, Leuven), and Ir. Marcel Verhaegen (SEM, National Botanic Garden of Belgium) for excellent technical assistance. Thanks are due to the curator of The National Botanic Garden of Belgium (BR) for the generous supply of anther samples. This research was supported financially by projects No. 2.0038.91 (SEM), G.025496 (TEM), and G.0104.01 (general research project) from the Fund for Scientific Research‐Flanders (FWO) and a grant from the Research Council of K.U.Leuven (OT/97/23). We are grateful for the valuable suggestions of Dr Suzy Huysmans and Dr Mary Endress.
APPENDIX
Specimens are listed alphabetically on family level; living material is indicated by an asterisk.
Apocynaceae s.l.
Acokanthera laevigata F.K. Kupicha, Tanzania, J. Lovett 1605 (BR)
Acokanthera oblongifolia (Hochstetter) Codd, Democratic Republic of Congo, H. Breyne 3741 (BR)
Adenium obesum (Forssk.) Roem. & Schult., Oman, S.A. Ghazanfar & L. Evangelista 1642 (BR)
Allamanda cathartica L., Madagascar, A.J.M. Leeuwenberg 13788 (BR)
Alstonia congensis Engl., Gabon, A.J.M. Leeuwenberg 12495 (BR)
Amsonia tabernaemontana Walt. (*), cultivated in the botanical garden of the Institute of Botany and Microbiology (K.U.Leuven), S. Vinckier s.n.
Apocynum cannabinum L., USA, H.N. Moldenke 7954 (BR)
Araujia sericifera Brotero, Brazil, P.I. Oliveira 687 (BR)
Asclepias curassavica L. (*), cultivated in a glasshouse of the Institute of Botany and Microbiology (K.U.Leuven), S. Vinckier s.n.
Asclepias curassavica L., Democratic Republic of Congo, P. Sita 4007 (bis) (BR)
Asclepias friesii Schltr., Malawi, D.J. Goyder, A.J. Paton & E.J. Tawakali 3601 (BR)
Aspidosperma megalocarpon Müll. Arg., Costa Rica, B. Hammel 18896 (BR)
Aspidosperma quebracho‐blanco Schltdl., Argentina, T. Meyer 12·778 (BR)
Baissea baillonii Hua, Ivory Coast, A.J.M. Leeuwenberg 4109 (BR)
Baissjea leonensis Benth., Liberia, De Wilde, 3698 (BR)
Beaumontia grandiflora Wall., India, Dr. v. Barth s.n. (BR); India, Hortus Botanicus Calcutta 226 (BR)
Camptocarpus crassifolius Decne., Madagascar, P. Phillipson 1777 (BR)
Carissa bispinosa (L.) Desf. ex Brenan, Malawi, J.D. Chapman & E.J. Tolwakali 5984 (BR)
Catharanthus roseus (L.) G. Don, Zambia, B. Leteinturier, F. Malaisse & J. Matera 135 (BR)
Cerberiopsis candelabra Vieill. Ex Panch. & Sebert, New Caledonia, P. Bamps 5707 (BR)
Cynanchum acutum L., Spain, I. Aizpuru & F. Muñoz 17310 (BR)
Dictyophleba lucida Pierre, Democratic Republic of Congo, J. Lejoly 3762 (BR)
Ditassa capillaris E. Fourn., Brazil, G. Hatschbach, M. Hatschbach & D. Guimaraes 55065 (BR)
Funtumia africana (Benth.) Stapf, Democratic Republic of Congo, Nsola 1227 (BR)
Fockea edulis K. Schum., South Africa, R.D.A. Bayliss BS 7401 (BR)
Hemidesmus indicus (L.) R. BR. ex Schult., India (cultivated in Hortus Botanicus Calcuitensis), Major Prain, s.n. (BR)
Hoya sp., New Caledonia, P. Bamps 5783 (BR)
Kopsia fructicosa (Ker Gawl.) A. DC. (*), Malaya (cultivated in National Botanic Garden of Belgium, n° 07–4017), S. Vinckier s.n.
Mandevilla atroviolacea (Stadelm.) Woodson, Brazil, J. Cordeiro, 190 (BR)
Mascarenhasia arborescens A.DC., ex Hort. Kew, Hort. Bog IV.A.131 (BR)
Mascarenhasia lanceolata A. DC., Madagascar, A.J.M. Leeuwenberg & S.H.J.V. Rapanarivo 14755 (BR)
Matelea carolinensis (Jacq.) Woodson, USA, T. Daggy 4873 (BR); USA, ex herb. J.H. Wibbe, s.n. (BR)
Molongum zschokkeiforme (Markgr.) Pichon, Brazil, J.L. Zaucchi s.n. (BR)
Nerium oleander L., Morocco, Ch. Aurich, H. Förther s.n. (BR); Portugal, R. Dechamps 7219 (BR); Senegal, C. Vanden Berghen 10124 (BR)
Pachypodium baroniiCostantin & Bois, Madagascar, A.J.M. Leeuwenberg & S.H.J.V. Rapanarivo 14771 (BR)
Pachypodium namaquanum (Wyley ex Harvey) Welw., Namibia, C. Evrard 9186 (BR)
Parsonsia cumingiana A.DC., Papua New Guinea, P. Goetghebeur 3794 (BR)
Pentarrhinum abyssinicum Decne., Tanzania, Polhill & Paulo 1884 (BR)
Pergularia tomentosa L., Morocco, J. Lewalle 11613 (BR)
Periploca graeca L. (*), Belgium, cultivated by Dr L. Ronse de Craene, S. Vinckier s.n.
Periploca graeca L., Corsica, J. Lambinon & R. Deschatres 86/10/78 (BR); Morocco, J. Lewalle 11613 (BR); Russia, V. Vâsàk & A. Vêzda s.n. (BR)
Periploca laevigata Aiton, Canaries, J. Bouharmont 25465 (BR)
Picralima nitida (Stapf) T. Durand & H. Durand, Gabon, G. Le Testu 5898 (BR)
Plumeria rubra var. acutifolia (Poir.) L. H. Bailey, Costa Rica, P. Hoet 18 (BR)
Prestonia portobellensis (Beurl.) R.E. Woodson, Mexico, J.H. Beaman 6204 (BR); Costa Rica, H. Pittier & TH. Durand 9889 (BR)
Raphionacme brownii Scott‐Elliot, Ivory Coast, P. Poilecot 2457 CI (BR)
Rauvolfia mattfeldiana Markgr., Brazil, Hallard 13 (BR)
Rauvolfia vomitoria Afzel., Sierra Leone, J. Bouharmont 15076 (BR)
Riocreuxia picta Schltr., South Africa, E.S. Kemp 652 (BR)
Secamone elliptica R.Br. ssp. elliptica, New Caledonia, Mac Kee 41131 (BR)
Secamone filiformis (Linnaeus f.) J.H. Ross, South Africa, R.D.A. Bayliss BS7400 (BR)
Secamonopsis madagascariensis Jum., Madagascar, S.T. Malcomber 1140 (BR)
Streptocaulon tomentosum Wight & Arn., India, J.W. Helfer 59 (BR)
Strophanthus courmontii Sacleux ex Franch., Kenya, A. Paulo 810 (BR)
Strophanthus cumingii A. DC., Philippines, M. Celestino & J. Ramos (Phil. nat. herb.) 23074 (BR)
Tabernaemontana calcarea Pichon, Madagascar, A.J.M. Leeuwenberg 14708 (BR)
Tabernaemontana coronaria ‘flore pleno’ (Jacq.) Willd. (*), cultivated in National Botanic Garden of Belgium no 38–1341, S. Vinckier s.n. (BR)
Telosma procumbens (Blanco) Merr., Philippines, G.E.E. Dano 7342 (BR)
Thevetia bicornuta Müll. Arg., Paraguay, T.M. Pedersen 4137 (BR)
Trachelospermum jasminoides (Lindl.) Lem., Kenya, S. G. Mathenge 727 (BR)
Tylophora yunnanensis Schltr., China, Sino‐Amer. Bot. Exped. 802 (BR)
Tylophora sylvatica Decne, Liberia, J.W.A. Jansen 1972 (BR)
Vincetoxicum nigrum (L.) Moench, Spain, C. Evrard 12730 (BR)
Wrightia natalensis Stapf, Zimbabwe, R.B. Dreumond 10403 (BR)
Supplementary Material
Received: 8 April 2002; Returned for revision: 30 July 2002; Accepted: 15 August 2002 Published electronically: 2 October 2002
References
- AndreasenK, Bremer B.1996. Phylogeny of the subfamily Ixoroideae (Rubiaceae). In: Robbrecht E, Puff C, Smets E, eds. Proceedings of the 2nd International Rubiaceae Conference, Meise1995. Opera Botanica Belgica7: 119–138. Meise: National Botanic Garden of Belgium. [Google Scholar]
- ArchangelskyS, Taylor TN.1993. The ultrastructure of in situ Clavatipollenites pollen from the early Cretaceous of Patagonia. American Journal of Botany 80: 879–885. [Google Scholar]
- BenedettiEL, Bertolini B.1963. The use of phosphotungstic acid as a stain for the plasma membrane. Journal of the Royal Microscopical Society London 81: 219–222. [Google Scholar]
- Cerceau‐LarrivalM‐Tet al.1981. Relations sporophyte‐gamétophyte: assise tapétale‐pollen.Annales de Sciences Naturelles et Botanique de Paris 13: 69–92. [Google Scholar]
- ChristensenJE, Horner HT Jr, Lersten NR.1972. Pollen wall and tapetal orbicular wall development in Sorghum bicolor (Gramineae). American Journal of Botany 59: 43–58. [Google Scholar]
- CiveyrelL.1995. Pollen morphology and ultrastructure of the genus Secamone in Africa. In: Le Thomas A, Roche E, eds. 2nd Symposium on African Palynology, Tervuren, Belgium. Orleans: CIFEG. [Google Scholar]
- ClémentC, Audran J‐C.1993. Cytochemical and ultrastructural evolution of orbicules in Lilium Plant Systematics and Evolution 7: 63–74. [Google Scholar]
- CousinM‐T.1979. Tapetum and pollen grains of Vinca rosea (Apocynaceae). Ultrastructure and investigations with scanning electron microscope. Grana 18: 115–128. [Google Scholar]
- EchlinP, Godwin H.1968. The ultrastructure and ontogeny of pollen in Helleborus foetidus L. I. The development of the tapetum and ubisch bodies. Journal of Cell Science 3: 161–174. [DOI] [PubMed] [Google Scholar]
- El‐GhazalyG.1989. Pollen and orbicule morphology of some Euphorbia species. Grana 28: 243–259. [Google Scholar]
- El‐GhazalyG, Chaudhary R.1993. Morphology and taxonomic application of orbicules (Ubisch bodies) in the genus Euphorbia Grana Supplement 2: 26–32. [Google Scholar]
- El‐GhazalyG, Jensen WA.1986. Studies of the development of wheat (Triticum aestivum) pollen. Grana 25: 1–29. [Google Scholar]
- El‐GhazalyG, Nilsson S.1991. Development of tapetum and orbicules of Catharanthus roseus (Apocynaceae). In: Blackmore S, Barnes SH, eds. Pollen and spores. Systematic association Oxford: Clarendon Press, 317–329. [Google Scholar]
- EndressME, Bruyns PV.2000. A revised classification of the Apocynaceae s.l. TheBotanical Review 66: 1–56. [Google Scholar]
- EndressME, Stevens WD.2001. The renaissance of the Apocynaceae s.l.: recent advances in systematics, phylogeny, and evolution: Introduction. Annals of the Missouri Botanical Garden 88: 517–522. [Google Scholar]
- EndressME, Sennblad B, Nilsson S, Civeyrel L, Chase MW, Huysmans S, Grafström E, Bremer B.1996. A phylogenetic analysis of Apocynaceae s. str. and some related taxa in Gentianales: a multidisciplinary approach. In: Robbrecht E, Puff C, Smets E, eds. Proceedings of the 2nd International Rubiaceae Conference, Meise1995. Opera Botanica Belgica 7: 59–102. Meise: National Botanic Garden of Belgium. [Google Scholar]
- ErdtmanG, Berglund B, Praglowski J.1961. An introduction to a Scandinavian pollen flora. Stockholm: Almqvist and Wiksell. [Google Scholar]
- HesseM.1985. Hemispheric surface processes of exine and orbicules in Calluna (Ericaceae). Grana 24: 93–98. [Google Scholar]
- HesseM.1986. Orbicules and the ektexine are homologous sporopollenin concretions in Spermatophyta.Plant Systematics and Evolution 153: 37–48. [Google Scholar]
- HuysmansS, El‐Ghazaly G, Smets E.1998. Orbicules in angiosperms. Morphology, function, distribution, and relation with tapetum types. Botanical Review 64: 240–272. [Google Scholar]
- HuysmansS, El‐Ghazaly G, Smets E.2000. Orbicules: still a well hidden secret of the anther. In: Nordenstam B, El‐Ghazaly G, Kassas M, eds. Plant systematics for the 21st century. London: Portland Press. [Google Scholar]
- HuysmansS, El‐Ghazaly G, Nilsson S, Smets E.1997. Systematic value of tapetal orbicules: a preliminary survey of the Cinchonoideae (Rubiaceae). Canadian Journal of Botany 75: 815–826. [Google Scholar]
- KosmathL.1927. Studie über das Antherentapetum.Österrechische Botanische Zeitschrift 76: 235–241. [Google Scholar]
- LombardoG, Carraro L.1976. Tapetal ultrastructural changes during pollen development. III. Studies on Gentiana acaulis Caryologia 29: 345–349. [Google Scholar]
- MarinozziV.1968. Phosphotungstic acid (PTA) as a stain for polysaccharides and glycoproteins in electron microscopy. In: Bocciarelli DS, ed. Electron microscopy1968. Pre‐Congress Abstracts 4th European Regional Conference on Electron Microscopy. Rome 1968 Rome: ERCEM Organizing Committee Tipogratia Poliglota Vaticana, 55–56. [Google Scholar]
- NicholasA, Baijnath H.1994. A consensus classification for the order Gentianales with additional details on the suborder Apocynineae. Botanical Review 60: 440–482. [Google Scholar]
- NilssonS, Endress ME, Grafström E.1993. On the relationship of the Apocynaceae and Periplocaceae. Grana Supplement 2: 3–20. [Google Scholar]
- OsbornJM, Taylor TN, Crane PR.1991. The ultrastructure of Sahnia pollen (Pentoxylales). American Journal of Botany 78: 1560–1569. [Google Scholar]
- PaciniE, Franchi GG, Hesse M.1985. The tapetum: its form, function and possible phylogeny in embryophyta. Plant Systematics and Evolution 149: 155–185. [Google Scholar]
- RajB, El‐Ghazaly G.1987. Morphology and taxonomic application of orbicules (Ubisch bodies) in Chloanthaceae. Pollen and Spores 29: 151–166. [Google Scholar]
- RowleyJR.1990. The fundamental structure of the pollen exine. Plant Systematics and Evolution Supplement5: 13–29. [Google Scholar]
- ScholsP, Dessein S, D’Hondt C, Huysmans S, Smets E.2002. Carnoy: a new digital measurement tool for palynology. Grana 41: 124–126. [Google Scholar]
- SerbetR, Stockey RA.1991. Taxodiaceous pollen cones from the Upper Cretaceous (Horseshoe Canyon Formation) of Drumheller, Alberta, Canada. Review of Palaeobotany and Palynology 70: 67–76. [Google Scholar]
- SporneKR.1973. A note on the evolutionary status of tapetal types in dicotyledons. New Phytologist 72: 1173–1174. [Google Scholar]
- TaylorTN, Alvin KL.1984. Ultrastructure and development of Mesozoic pollen, Classopollis American Journal of Botany 71: 575–587. [Google Scholar]
- van der HamR, Zimmermann Y‐M, Nilsson S, Igersheim A.2001. Pollen morphology and phylogeny of the Alyxieae (Apocynaceae). Grana 40: 169–191. [Google Scholar]
- VerhoevenRL, Venter HJT.1998. Pollinium structure in Periplocoideae (Apocynaceae). Grana 37: 1–14. [Google Scholar]
- VerhoevenRL, Venter HJT.2001. Pollen morphology of the Periplocoideae, Secamonoideae, and Asclepiadeae (Apocyn aceae). Annals of the Missouri Botanical Garden 88: 569–582. [Google Scholar]
- VijayaraghavanMR, Chaudhry B.1993. Structure and development of orbicules in the tapetum of Prosopsis juliflora (Leguminosae, Mimosoideae). Phytomorphology 43: 41–48. [Google Scholar]
- VinckierS, Smets E.2002. Morphology, ultrastructure and typology of orbicules in family Loganiaceae s.l. and related genera, in relation to systematics. Review of Paleobotany and Palynology 119: 161–189. [Google Scholar]
- VinckierS, Huysmans S, Smets E.2000. Morphology and ultrastructure of orbicules in the subfamily Ixoroideae (Rubiaceae). Review of Palaeobotany and Palynology 108: 151–174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


