Abstract
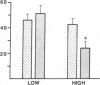
To determine the role of dietary sodium intake in the reduction in beta-adrenergic sensitivity in hypertension, lymphocyte beta-receptors from 8 borderline hypertensive and 16 normotensive subjects were studied after 5 d on a high sodium diet (400 meq/d) and also following a low sodium diet (10 meq/d). During the high sodium diet, lymphocyte beta-receptor-stimulated adenylate cyclase activity, expressed as the relative increase over basal levels stimulated by the beta-agonist isoproterenol, was significantly (P less than 0.025) decreased in hypertensive (24 +/- 5%, mean +/- SE) compared with normotensive (42 +/- 4%) subjects. Neither beta-receptor density nor the proportion of nonsequestered beta-receptors differed between groups. A low sodium diet significantly increased beta-receptor-stimulated adenylate cyclase activity in hypertensives (low sodium, 51 +/- 7%; high sodium, 24 +/- 5%, P less than 0.025) to a level not different than that of normotensives (46 +/- 5%). Thus, reduced lymphocyte beta-receptor responsiveness in hypertensive subjects is not due to beta-receptor sequestration and is corrected on a low sodium diet. Dietary sodium may be an important factor in the beta-receptor defect in early hypertension.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosioni E., Costa F. V., Borghi C., Montebugnoli L., Giordani M. F., Magnani B. Effects of moderate salt restriction on intralymphocytic sodium and pressor response to stress in borderline hypertension. Hypertension. 1982 Nov-Dec;4(6):789–794. doi: 10.1161/01.hyp.4.6.789. [DOI] [PubMed] [Google Scholar]
- Amer M. S., Gomoll A. W., Perhach J. L., Jr, Ferguson H. C., McKinney G. R. Aberrations of cyclic nucleotide metabolism in the hearts and vessels of hypertensive rats. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4930–4934. doi: 10.1073/pnas.71.12.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla R. C., Sharma R. V. Characteristics of hormone-stimulated adenylate cyclase in vascular smooth muscle: altered activity in spontaneously hypertensive rat. Blood Vessels. 1982;19(3):109–116. [PubMed] [Google Scholar]
- Bhalla R. C., Sharma R. V., Ramanathan S. Ontogenetic development of isoproterenol subsensitivity of myocardial adenylate cyclase and beta-adrenergic receptors in spontaneously hypertensive rats. Biochim Biophys Acta. 1980 Nov 3;632(4):497–506. doi: 10.1016/0304-4165(80)90326-8. [DOI] [PubMed] [Google Scholar]
- Borkowski K. R., Porter M. An altered beta-adrenoreceptor-mediated modulation of noradrenaline-induced vasoconstriction in spontaneously hypertensive rat mesenteric arteries. J Auton Pharmacol. 1984 Mar;4(1):27–31. doi: 10.1111/j.1474-8673.1984.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Brodde O. E., Daul A. E., O'Hara N., Khalifa A. M. Properties of alpha- and beta-adrenoceptors in circulating blood cells of patients with essential hypertension. J Cardiovasc Pharmacol. 1985;7 (Suppl 6):S162–S167. doi: 10.1097/00005344-198500076-00028. [DOI] [PubMed] [Google Scholar]
- Brodde O. E., Kretsch R., Ikezono K., Zerkowski H. R., Reidemeister J. C. Human beta-adrenoceptors: relation of myocardial and lymphocyte beta-adrenoceptor density. Science. 1986 Mar 28;231(4745):1584–1585. doi: 10.1126/science.3006250. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carpentieri U., Minguell J. J., Gardner F. H. Adenylate cyclase and guanylate cyclase activity in normal and leukemic human lymphocytes. Blood. 1981 May;57(5):975–978. [PubMed] [Google Scholar]
- Cohen M. L., Berkowitz B. A. Decreased vascular relaxation in hypertension. J Pharmacol Exp Ther. 1976 Feb;196(2):396–406. [PubMed] [Google Scholar]
- Colucci W. S., Alexander R. W., Williams G. H., Rude R. E., Holman B. L., Konstam M. A., Wynne J., Mudge G. H., Jr, Braunwald E. Decreased lymphocyte beta-adrenergic-receptor density in patients with heart failure and tolerance to the beta-adrenergic agonist pirbuterol. N Engl J Med. 1981 Jul 23;305(4):185–190. doi: 10.1056/NEJM198107233050402. [DOI] [PubMed] [Google Scholar]
- De Blasi A., Cotecchia S., Fratelli M., Lipartiti M. Agonist-induced beta-adrenergic receptor internalization on intact human mononuclear leukocytes: effect of temperature of mononuclear leukocyte separation. J Lab Clin Med. 1986 Jan;107(1):86–94. [PubMed] [Google Scholar]
- Feldman R. D., Limbird L. E., Nadeau J., FitzGerald G. A., Robertson D., Wood A. J. Dynamic regulation of leukocyte beta adrenergic receptor-agonist interactions by physiological changes in circulating catecholamines. J Clin Invest. 1983 Jul;72(1):164–170. doi: 10.1172/JCI110954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. D., Limbird L. E., Nadeau J., Robertson D., Wood A. J. Leukocyte beta-receptor alterations in hypertensive subjects. J Clin Invest. 1984 Mar;73(3):648–653. doi: 10.1172/JCI111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. D., Park G. D., Lai C. Y. The interaction of verapamil and norverapamil with beta-adrenergic receptors. Circulation. 1985 Sep;72(3):547–554. doi: 10.1161/01.cir.72.3.547. [DOI] [PubMed] [Google Scholar]
- Feldman R. D. Physiological and molecular correlates of age-related changes in the human beta-adrenergic receptor system. Fed Proc. 1986 Jan;45(1):48–50. [PubMed] [Google Scholar]
- Fraser J., Nadeau J., Robertson D., Wood A. J. Regulation of human leukocyte beta receptors by endogenous catecholamines: relationship of leukocyte beta receptor density to the cardiac sensitivity to isoproterenol. J Clin Invest. 1981 Jun;67(6):1777–1784. doi: 10.1172/JCI110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P. A., Motulsky H. J. A hypothesis linking intracellular sodium, membrane receptors, and hypertension. Life Sci. 1984 Mar 12;34(11):1009–1013. doi: 10.1016/0024-3205(84)90013-4. [DOI] [PubMed] [Google Scholar]
- Katovich M. J., Soltis E. E., Iloeje E., Field F. P. Time course alterations in vascular adrenergic responsiveness in the DOCA/NaCl-treated rat. Pharmacology. 1984;29(3):173–180. doi: 10.1159/000138009. [DOI] [PubMed] [Google Scholar]
- Lima D. R., Turner P. Beta-blocking drugs increase responsiveness to prostacyclin in hypertensive patients. Lancet. 1982 Aug 21;2(8295):444–444. doi: 10.1016/s0140-6736(82)90475-5. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Nordberg J. Adenylate cyclase in thymus-derived and bone marrow-derived lymphocytes from normal donors and patients with chronic lymphocytic leukemia. J Clin Invest. 1979 Jun;63(6):1124–1132. doi: 10.1172/JCI109405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Cunningham E. M., DeBlasi A., Insel P. A. Desensitization and redistribution of beta-adrenergic receptors on human mononuclear leukocytes. Am J Physiol. 1986 May;250(5 Pt 1):E583–E590. doi: 10.1152/ajpendo.1986.250.5.E583. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schütz W., Steurer G., Tuisl E., Kraupp O. The cardiac and brain microvessel adenylate cyclase system in deoxycorticosterone acetate-hypertensive rats. J Cardiovasc Pharmacol. 1984 Mar-Apr;6(2):325–330. doi: 10.1097/00005344-198403000-00018. [DOI] [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Triner L., Vulliemoz Y., Verosky M., Manger W. M. Cyclic adenosine monophosphate and vascular reactivity in spontaneously hypertensive rats. Biochem Pharmacol. 1975 Mar 15;24(6):743–745. doi: 10.1016/0006-2952(75)90253-1. [DOI] [PubMed] [Google Scholar]