Abstract
Background
Southeast Asian populations are increasingly affected by allergic airway diseases. Etiology and specific causes, however, are still unknown. The aim of this study is therefore to identify allergens and risk factors for the high prevalence of allergic airway disease in the tropical urban environment.
Methods
Symptoms of allergic rhinitis (AR), asthma, and allergic dermatitis were recorded in two independent cohorts of 576 and 7373 ethnic Chinese individuals living in Singapore. Reactivity against common allergens was determined by skin prick tests (SPT); specific immunoglobulin E (sIgE) titers against 12 common allergens, as well as total serum IgE (tIgE), were measured in the smaller cohort.
Results
Immunoglobulin E sensitization was almost exclusively directed against house dust mite (HDM) allergens. More than 80% of individuals were HDM-sIgE positive. Of these, less than 30% also had sIgE for other allergens, and similarly, few of the HDM-sIgE-negative individuals reacted to other allergens. Titers for HDM-sIgE were 8–30 times higher than other non-HDM allergen titers and correlated directly with total serum tIgE levels. Migrants from nontropical countries typically arrived with low or undetectable HDM-sIgE but developed substantial titers in a time-dependent fashion. Importantly, prolonged stay in Singapore also resulted in the manifestation of AR and asthma symptoms, contributing to some of the highest national prevalence rates worldwide.
Conclusion
In a tropical urban environment, the allergic response is dominated by a single allergen class. The mono-specific IgE sensitization against HDM translates into increased prevalence of allergic airway diseases, which now impact a large proportion of the population in Singapore.
Keywords: allergens, asthma, atopy, immunoglobulin E, rhinitis
The prevalence of allergic airway phenotypes has increased dramatically in most countries in recent decades. By 2025, an estimated 400 million people will suffer from asthma, and nearly 500 million will be affected by allergic rhinitis (AR) 1,2. These allergic conditions often exist as co-morbidities, with close to two-thirds of asthmatics concurrently displaying AR symptoms. Interestingly, marked geographic differences are evident in the prevalence of allergic disease, with westernized and developed cities suffering most. The factors underlying these variations in the prevalence of allergic disease, however, are still largely unknown.
The primary pathological process in the development of allergic disease is production of immunoglobulin E (IgE). Studies from Europe and North America revealed a heterogeneous set of allergic compounds able to drive IgE production, including pollens, pet dander, fungus, molds, house dust mites, and many others 3–5. While IgE is clearly the mediator, the complexity of the allergen-set usually results in a poor correlation between the allergen-specific IgE (sIgE) titer and the total serum IgE (tIgE) 6. As a consequence, levels of tIgE do not necessarily correlate here with allergic symptoms, and hence, the relevance of tIgE as a risk factor for asthma has been brought into question 4,7.
While allergies have traditionally been associated with industrial countries, they are also endemic in the developing world. Recent reports indicate that in the Asia-Pacific region, allergic disease prevalence has reached the highest levels in 50 years 8, a trend also observed in tropical Southeast Asia 9,10. Although a similar set of allergens to those eliciting responses from populations in western regions has been proposed 9,11–14, the course, specificity, and complexity of the allergic response in tropical countries may differ substantially from that in temperate zones. Therefore, the objective of this study was to identify allergens and risk factors for the high prevalence of allergic airway disease in the tropical urban environment.
Methods
Ethics statement
This study is in compliance with the Helsinki declaration and has been performed with the approval of the Institutional Review Board of the National University of Singapore (IRB reference – NUS07-023 and NUS10-343). Samples used in this study were collected from ethnic Chinese participants following written informed consent.
Study populations
The two independent prospective cohorts were described below (discovery and validation). Ethnic Chinese subjects were recruited at the National University of Singapore as a part of an ongoing epidemiological collection for the study of allergic diseases. Volunteers were then classified as individuals with AR, with asthma, and healthy controls using a questionnaire collecting information on demographics and medical history, and based on the Allergic Rhinitis Impact on Asthma (ARIA) 2,15 and International Study of Asthma and Allergies in Childhood (ISAAC) guidelines. The volunteers also underwent a skin prick test (SPT) using a panel of 4 allergens common in Singapore (Dermatophagoides pteronyssinus, Blomia tropicalis, Elaeis guineensis, and Curvularia lunata). The initial discovery cohort consists of a total of 576 ethnic Chinese volunteers. Blood was collected for measurement of total and allergen-specific IgE levels against 12 common allergens. For validation, we used an independent prospective cohort of 7373 ethnic Chinese individuals similarly recruited. As in the discovery cohort, the study subjects filled a questionnaire and underwent SPT against the four allergens listed above. The demographics of these study populations are summarized in Table 1.
Table 1.
Demographics of the two study populations (Discovery cohort and Validation cohort)
| Country of birth | Gender | Discovery cohort | Validation cohort | ||
|---|---|---|---|---|---|
| Count | Age (years), Mean (range) | Count | Age (years), Mean (range) | ||
| Singapore | F | 119 | 20.81 (18.00–50.00) | 3136 | 21.00 (9.00–59.00) |
| M | 176 | 22.19 (19.00–42.00) | 2382 | 22.55 (17.00–65.00) | |
| Unknown | 11 | 20.82 (19.00–25.00) | 4 | 19.75 (19.00–21.00) | |
| Total | 360 | 21.60 (18.00–50.00) | 5522 | 21.67 (9.00–65.00) | |
| China | F | 31 | 25.42 (19.00–55.00) | 546 | 21.92 (17.00–46.00) |
| M | 35 | 27.03 (20.00–52.00) | 318 | 22.83 (18.00–56.00) | |
| Unknown | 4 | 21.25 (21.00–22.00) | |||
| Total | 75 | 26.27 (19.00–55.00) | 868 | 22.25 (17.00–56.00) | |
| Malaysia | F | 52 | 21.88 (18.00–31.00) | 541 | 21.88 (18.00–57.00) |
| M | 67 | 21.04 (18.00–35.00) | 441 | 21.90 (18.00–56.00) | |
| Unknown | 11 | 22.36 (20.00–31.00) | 1 | 21.00 (21.00–21.00) | |
| Total | 141 | 21.49 (18.00–35.00) | 983 | 21.89 (18.00–57.00) | |
| Total | F | 202 | 21.79 (18.00–55.00) | 4223 | 21.24 (9.00–59.00) |
| M | 278 | 22.52 (18.00–52.00) | 3141 | 22.49 (17.00–65.00) | |
| Unknown | 22 | 21.59 (19.00–31.00) | 9 | 20.56 (19.00–22.00) | |
| Total | 576 | 22.19 (18.00–55.00) | 7373 | 21.77 (9.00–65.00) |
Allergy testing
IgE levels measured in the discovery cohort
Levels of total immunoglobulin E (tIgE) and specific IgE (sIgE) against Dermatophagoides pteronyssinus (DP), Blomia tropicalis (BT), Aspergillus, Acacia, Cladosporum herbarium, Mugwort, Alternaria, Common ragweed, Bermuda grass, Dog dander, Cat dander, and German cockroach were measured using the USFDA-approved ImmunoCAP system (Phadia AB, Uppsala, Sweden).
Skin prick testing (SPT)
The volunteers were subjected to a SPT using a panel consisting of common allergens in Singapore such as DP (house dust mite), BT (house dust mite), Elaeis guineensis (pollen), and Curvularia lunata (fungi). A SPT response is considered positive when the wheal diameter is 3 mm or greater, when compared to positive (histamine) and negative (saline) controls.
Statistical methods
All data used in the analysis were processed using Accelrys Pipeline Pilot (Accelrys, San Diego, CA, USA) with statistical analysis in the R statistical language (version 2.12.1). P-values less than 0.05 were considered statistically significant. A one-way anova using logarithm-transformed IgE levels to ensure normality was used to determine whether the levels of specific IgE were significantly different for Singaporean Chinese. A Welch’s t-test was used to compare the logarithm-transformed total IgE levels of atopics and nonatopics. Pearson correlation was used to compare the logarithm-transformed BT, DP, and total IgE levels in a pairwise fashion. One-way anovas were used to determine whether BT, DP-specific, and total logarithm-transformed IgE levels were statistically different between countries of birth. Subjects born in Malaysia and China were divided into three groups by the number of years of residence in Singapore (3 years or less, 3–8 years, and over 8 years). One-way anovas were used to determine whether BT, DP, and total logarithm-transformed IgE levels were statistically different between groups of subjects born in China according to years of residence in Singapore. Fisher’s exact tests were used separately for Chinese and Malaysians to determine whether there were any associations between the groups and either house dust mite (HDM) sensitization or allergic phenotypes.
Results
Dominance of HDM-sIgE
To determine the allergen sensitization profile, 206 individuals born in Singapore were screened for responses to the 12 most common allergens reported to be present in the tropical environment. The sIgE titers against these allergens were determined using the ImmunoCAP system (Fig. 1). Sensitization to HDM allergens was markedly strongest and most common, with more than 70% of individuals possessing measurable titers against extracts from either one of the two HDM species Dermatophagoides pteronyssinus (DP) or Blomia tropicalis (BT). In most cases, sIgE against both mites was detected. Less than 4% of the HDM-positive individuals reacted exclusively to only one of the HDM species (Fig. S1A). Among the other allergens tested, a substantial sensitization rate was evident only for German cockroach (14.56%) and Bermuda grass (6.8%). sIgE titers to other allergens were detected only sporadically, that is, in less than 3% of the study subjects. Their median sIgE titers were generally low. In comparison, the titers of HDM-sIgE were 8- to 30-fold higher than the non-HDM-specific IgE.
Figure 1.
Levels of specific immunoglobulin E (IgE) against the 12 common allergens tested in a cohort of 206 Chinese individuals born in Singapore. Allergens are grouped into indoor allergens (house dust mite, pet dander, and cockroach) and outdoor allergens (fungus and pollens). Specific immunoglobulin E (sIgE) titers are expressed as U/ml. The strength of IgE reactivity toward the specific allergens tested is categorized in classes from 0 to 6 according to ImmunoCAP standards. Titers < 0.35 U/ml were considered negative. The species of the allergen source is listed below together with the percentage of individuals positive for sIgE to each allergen tested. The median sIgE titer including minimum (min) and maximum (max) is calculated for reactive individuals. Dermatophagoides pteronyssinus (Der p) or Blomia tropicalis (Blo t).
Presence of HDM-sIgE alone is indicative of ‘atopy’
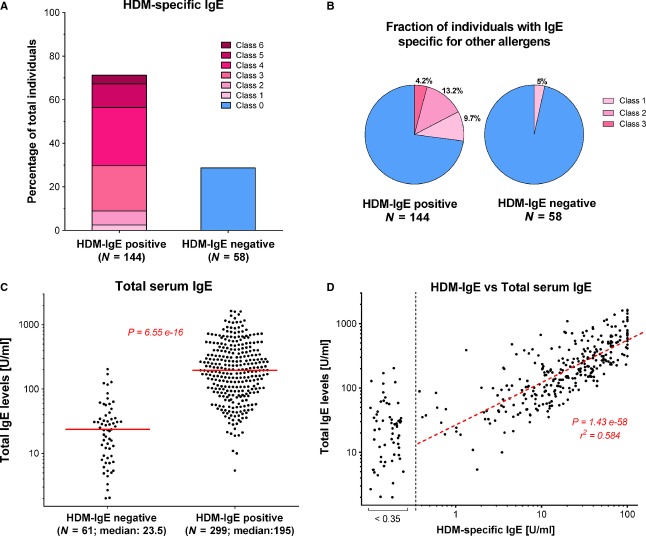
‘Atopy’ is a genetic predisposition toward the development of immediate hypersensitivity reactions against environmental allergens 16. Experimentally, atopy is defined by the presence of allergen-specific IgE or positive SPT to common allergens. In western countries, this test requires a set of four or more allergens, which typically reveals prevalence rates between 25% and 45% 6. The Singapore cohort showed a far higher rate. Based on the HDM response, 71% could be defined here as ‘atopic’ (Fig. 2A). While more than a quarter of the HDM-reactive individuals were sensitized also against other allergens, HDM-sIgE-negative individuals were also mostly negative for other sIgE (Fig. 2B). Thus, HDM sensitization alone could separate ‘atopic’ from ‘nonatopic’ individuals.
Figure 2.
Association of house dust mite (HDM) sensitization with ‘atopy’ and serum tIgE. Data shown are for Chinese individuals born in Singapore. (A) Percentage of HDM-specific immunoglobulin E (sIgE)-positive and immunoglobulin E (sIgE)-negative individuals. Distribution of individuals is shown based on sIgE titers specific for HDM. Color code refers to the sIgE titer class shown in Fig. 1. Individuals with sIgE titer < 0.35 U/ml were defined as HDM-sIgE negative. (B) Fraction of individuals with sIgE against non-HDM allergens among either HDM-sIgE-positive or HDM-sIgE-negative individuals. The relative fraction is shown for the two groups defined in Fig. 2A. The class of sIgE titer for non-HDM allergens is indicated by the color code. (C) Serum tIgE levels of HDM-sIgE-positive and HDM-sIgE-negative individuals in an extended cohort of 360 Singapore-born Chinese. Serum tIgE was determined by ImmunoCAP ELISA and plotted for HDM-sIgE-negative and HDM-sIgE-positive individuals (Median value indicated). Using a t-test with Welch correction, total immunoglobulin E (IgE) was found to be significantly different between the two groups (P = 6.55 × 10−16) (D) Correlation of tIgE levels with HDM-sIgE levels. The titers of HDM-sIgE were plotted against the serum tIgE. P and r2 values for the linear regression are indicated.
Direct correlation between serum HDM-sIgE and tIgE levels
While for western countries, only weak associations to the total IgE was reported 6, striking differences became apparent when an extended cohort of 360 Singaporean Chinese was grouped according to their atopy status (Fig. 2C). The median tIgE titer of HDM-sensitized individuals was more than eight times higher than in nonsensitized individuals (195.0 vs 23.5 U/ml, P-value of 6.55 × 10−16). Moreover, in a direct plot of HDM-sIgE vs tIgE, the levels of HDM-sIgE strongly correlated with tIgE (Fig. 2D, Fig. S1B,C). Thus, high serum tIgE titers are here directly indicative of HDM sensitization.
Extended exposure to the tropical urban environment drives the HDM-sIgE response and HDM sensitization
To evaluate whether the dominance of HDM-sIgE was an environmental phenomenon, the sIgE titers of Singapore-born Chinese were compared to the titers of migrant Chinese who arrived in Singapore either from other tropical or nontropical countries. For this, HDM-sIgE titers were determined in the extended discovery cohort of 576 individuals, which, in addition to Singapore-born Chinese, also included Singapore residents who had migrated from neighboring Malaysia or China. While the fraction of HDM-sIgE-positive individuals was around 80% for both Singapore-born and Malaysia-born individuals, less than 50% of the migrants from Mainland China tested positive (Fig. 3A). This difference was also reflected in the serum tIgE levels. Comparably high levels were measured in Singapore- and Malaysia-born individuals (median 151 vs 129 U/ml), whereas individuals from Mainland China had substantially lower tIgE titers (median 64 U/ml; Fig. 3B).
Figure 3.
Influence of time of exposure to tropical urban environment on house dust mite (HDM) sensitization within the discovery cohort (Table 1). (A) Percentage of HDM-specific immunoglobulin E (sIgE)-positive (titer > 0.35 U/ml) individuals among Singapore residents born in tropical and nontropical countries. (B) tIgE levels stratified by country of birth. tIgE titer for Singapore-born ethnic Chinese and Chinese migrants from China and Malaysia is shown. Median and P-values are indicated. (C) HDM-sIgE levels stratified by country of birth. (D) HDM-sIgE levels in migrants from China stratified by number of years’ residence in Singapore. (E) Distribution of HDM-sIgE levels in migrants from Malaysia stratified by number of years in Singapore.
Marked differences were similarly evident in the magnitude of the HDM-specific response (Fig. 3C, Fig. S2A). While most Singapore- and Malaysia-born individuals had high levels of HDM-sIgE (16.2 vs 13.8 U/ml), the majority of migrants from China were negative. This discrepancy was even more evident when we evaluated the sensitization based on the number of years the migrants had spent in Singapore (Fig. 3D, Fig. S2B). While for Malaysia-born individuals the move to Singapore was without any effect on the HDM-sIgE titer (Fig. 3E), Mainland Chinese were strongly affected by the duration of stay in Singapore (Fig. 3D). After 0–3 years in Singapore, less than 30% of China-born individuals were HDM-sIgE positive. The number increased to 50% for the 3–8 years group and reached 60% in the long-term residents (>8 years).
These data were further validated in a prospective study employing a large cohort of 7373 ethnic Chinese, where SPT was used to determine the prevalence of HDM sensitization. Of these, 5522 were born in Singapore, 983 migrated from Malaysia, and 868 from mainland China (Table 1). 70% of the Singapore-born as well as the Malaysia-born Chinese were HDM-SPT positive, while less than 30% of the migrants from Mainland China reacted against HDM (Fig. 4A), and this fraction dropped to less than 20% when considering the Chinese migrants in Singapore for less than 3 years (Fig. 4B, upper left panel). The HDM reactivity of China-born migrants increased over time, reaching nearly 50% in the group of migrants who had lived more than 8 years in Singapore (Fig. 4B, upper left panel). No significant difference was observed between Singapore-born individuals and migrants from neighboring Malaysia (Fig. 4A, Fig. S3B).
Figure 4.
Influence of time of exposure to tropical urban environment on the prevalence of allergic airway diseases in the prospective validation cohort (Table 1). (A) Percentage of house dust mite (HDM)-sensitized individuals stratified by country of birth. HDM sensitization was determined by skin prick test (SPT) on ethnic Chinese born in Singapore, or on migrants from China or Malaysia. (B) HDM sensitization, allergic rhinitis (AR), asthma, and allergic dermatitis in migrants from China stratified by number of years of residence in Singapore. The percentage of individuals from China with positive SPT or allergic symptoms is shown in reference to the duration of exposure to the tropical urban environment.
Thus, longer exposure time directly correlates with an increase in HDM-sIgE levels and HDM sensitization.
HDM sensitization translates into increased risk of allergic airway diseases
Statistical analysis confirmed that HDM reactivity, defined either by the presence of HDM-sIgE, tIgE, or a positive HDM-SPT, was significantly associated with the frequency of self-reported AR and asthma (Tables S1 and S2). Definitions of AR, asthma, and allergic dermatitis are detailed in the Supplementary Information. The percentage of individuals with symptoms of AR, as determined by questionnaire, proportionately reflected the HDM reactivity determined by SPT (Fig. 4B, upper panel). For both Singapore- and Malaysia-born Chinese, the >70% sensitization rate translates into an AR rate of more than 40% (Fig. S3). In comparison, migrants from China who spent 0–3 years in Singapore have an AR prevalence of only 9% (Fig. 4B). In line with increasing HDM reactivity, the AR prevalence climbs to 22% after over 8 years’ exposure to the tropical urban environment (Fig. 4B). The same trend is also observed for asthma, albeit to a lesser degree (lower left panel), but not for allergic dermatitis (AD; lower right panel). Notably, migrants born in Malaysia did not show this trend. HDM sensitization and disease incidence were in the same range as for the Singaporean-born cohort (Fig. S3).
Discussion
This study focused on identifying the main sensitizing agents responsible for allergic airway diseases in a tropical urban environment. Allergic diseases can be caused by a number of common allergens that are present in considerable concentrations in Singapore 17. Surprisingly, we found that the response is in fact largely dominated by a single allergen, the house dust mite (HDM). A total of 70–80% of the population reacted against this allergen. The serum tIgE levels of sensitized individuals were nearly eight times higher than in nonsensitized individuals, and tIgE titers correlated directly with HDM-sIgE titers. Apparently, the environmental penetrance by the allergen is so strong that anybody who can mount an IgE response will react against mite allergens. On the other hand, individuals who were not sensitized by HDM usually do not develop specific IgE against any other allergen. Hence, ‘HDM sensitization’ is here both a true and potentially useful measure of ‘atopy’.
The cause of this massive HDM response is clearly environmental. Based on our data collected from migrants, sensitization prevalence is as high in Singapore as in the neighboring tropical country of Malaysia, but much lower in the temperate climate of China: 70–80% of Singapore- or Malaysia-born Chinese react against mites, while less than 20% of the recent migrants from mainland China tested positive. Based on our previous work, Chinese individuals from Singapore and mainland China are genetically similar 18, thus excluding a major role of genetics in our observations. Instead, ‘time spent in Singapore’ was the strong confounding factor, with Chinese migrants who spent more than 8 years in the city-state reaching a high HDM reactivity prevalence of ∼50%.
House dust mite exposure not only results in staggering specific IgE titers, it also has serious implications for disease progression. The high prevalence of HDM reactivity in Singapore- and Malaysia-born Chinese is associated with an unusually high prevalence of AR and asthma. The prevalence of 40% and 18% for AR and asthma, respectively, rank at the top worldwide 8,9,19. Additionally, these symptoms were present throughout the year, with 65% of the AR individuals having persistent AR as defined by ARIA guidelines 2,15. The causal connection between airway disease and mite-allergen exposure is implied by the increase in AR prevalence in the Chinese migrant group over time. To a weaker extent, this also applies for asthma, but not for AD; here, the rate remains unchanged, suggesting that HDM-sIgE plays only a minor role in allergic reactions of the skin.
The colloquial term for AR, ‘hay fever’, has its origin in the fact that pollen is the most common cause for the allergic condition in western countries. Based on our results, however, ‘mite fever’ would be a more accurate term in the tropical setting. Reports from countries with similar tropical climates such as Hong Kong, Malaysia, Thailand, Indonesia and Vietnam have also identified HDM as a major allergen 9,13,14,20, but the cause for the shift in the trigger remains enigmatic. Pollen is present in both environments, although less seasonal in the tropics, and while Blomia tropicalis is indeed a species restricted to tropical regions, Dermatophagoides pteronyssinus is endemic worldwide. It is therefore still an open question why the allergic response in tropical Southeast Asia is so dominated by this allergen. High humidity and ambient temperatures are certainly ideal for HDM propagation 11,12. While a consistently high HDM-load causes the perennial allergic symptomology 11,12,21, the same climate factors should also propagate fungi. However, based on our study, IgE-mediated allergic reactions against this outdoor allergen class are almost completely absent in Singapore, leading to the conclusion that lifestyle is an important contributor to the apparent bias toward dust mite reactivity. Clearly, in Singapore, more time is spent indoors, especially in air-conditioned spaces, which may have marked health implications. Humidity as well as rate and mode of ventilation clearly affects allergic airway phenotypes 22. Additionally, HDM-sensitized asthma patients living in homes with mechanical ventilation rather than air-conditioning exhibited overall clinical improvement through reduced HDM exposure 23.
House dust mite allergens exert a substantial burden on direct healthcare and its related costs 24. The mono-specific trigger of the allergic airway response in tropical regions, however, offers a unique opportunity for effective prevention and treatment. In contrast to other parts of the world, where poly sensitization and seasonal variations complicate allergic disease management, interventions could focus here on a single allergen. In patients with AR, allergen-specific immunotherapy had long-term clinical benefits and also reduced the risk of developing asthma 25,26. Allergen avoidance, in turn, is also predictably effective in managing allergic phenotypes especially in tropical environments. By simply reducing the exposure to HDM, the prevalence of both asthma and AR can be reduced here 27–29.
In conclusion, the causative agents of allergy in the tropics significantly differ from the complex etiology observed in temperate countries. Although the prevalence of allergic airway diseases is substantially higher, their mono-specific cause also offers a unique opportunity to study, treat, and manage the allergic phenotypes by specifically targeting HDM allergens.
Acknowledgments
The authors would like to thank all the volunteers and their family members who participated in this study. We would also like to thank Ramani Anantharaman, Parate Pallavi Nilkanth, Bani Kaur Suri, Sri Anusha Matta, and other members of the functional genomics laboratory at National University of Singapore for helping in sample collection for the epidemiological cohort. We would like to thank Au Bijin Veonice and Tang Suisheng from the Singapore Immunology Network for sample preparation, and Lucy Robinson (Insight Editing London) for commercial assistance in editing of the manuscript.
Author contributions
WDY, OR, and AKA performed conception and design; AKA, BL, PKJ, OR, WDY, CFT, and MP carried out analysis and interpretation; AKA, JC, AN, CFT, OR, WDY, MP, and BL were involved in drafting the manuscript for important intellectual content.
Funding
This study was supported by grants from the Singapore Immunology Network (SIgN-06-006, SIgN-08-020 and SIgN-10-029); the National Medical Research Council (NMRC/1150/2008), Singapore; the Biomedical Research Council, Singapore; SIgN core funding from the Agency for Science, Technology and Research (A*STAR); and the National University of Singapore for the Graduate Research Scholarship for students involved in the study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Specific immunoglobulin E (IgE) levels for the house dust mite species Dermatophagoides pteronyssinus and Blomia tropicalis.
Influence of the time of exposure to tropical urban environment on the strength of sensitization to Dermatophagoides pteronyssinus and Blomia tropicalis in the discovery cohort (Table 1).
House dust mite sensitization and allergic symptoms in migrants from tropical Malaysia stratified by number of years of residence in Singapore.
House dust mite-specific immunoglobulin E (IgE) and total serum IgE are significantly higher in individuals with allergic phenotypes.
Association of house dust mite-specific skin prick test with allergic phenotypes.
Definitions used in the study.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 4.Kerkhof M, Dubois AE, Postma DS, Schouten JP, de Monchy JG. Role and interpretation of total serum IgE measurements in the diagnosis of allergic airway disease in adults. Allergy. 2003;58:905–911. doi: 10.1034/j.1398-9995.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 6.Burney P, Malmberg E, Chinn S, Jarvis D, Luczynska C, Lai E. The distribution of total and specific serum IgE in the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1997;99:314–322. doi: 10.1016/s0091-6749(97)70048-4. [DOI] [PubMed] [Google Scholar]
- 7.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 8.Pawankar R, Baena-Cagnani CE, Bousquet J, Canonica GW, Cruz AA, Kaliner MA, et al. State of world allergy report 2008: allergy and chronic respiratory diseases. World Allergy Organ J. 2008;1(Suppl 6):S4–S17. doi: 10.1097/1939-4551-1-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawankar R, Bunnag C, Chen Y, Fukuda T, Kim YY, Le LT, et al. Allergic rhinitis and its impact on asthma update (ARIA 2008)–western and Asian-Pacific perspective. Asian Pac J Allergy Immunol. 2009;27:237–243. [PubMed] [Google Scholar]
- 10.Beasley R, Ellwood P, Asher I. International patterns of the prevalence of pediatric asthma the ISAAC program. Pediatr Clin North Am. 2003;50:539–553. doi: 10.1016/s0031-3955(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 11.Chew FT, Zhang L, Ho TM, Lee BW. House dust mite fauna of tropical Singapore. Clin Exp Allergy. 1999;29:201–206. doi: 10.1046/j.1365-2222.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 12.Chew FT, Lim SH, Goh DY, Lee BW. Sensitization to local dust-mite fauna in Singapore. Allergy. 1999;54:1150–1159. doi: 10.1034/j.1398-9995.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- 13.Daengsuwan T, Lee BW, Visitsuntorn N, Charoenratanakul S, Ruangrak S, Jirapongsananuruk O, et al. Allergen sensitization to aeroallergens including Blomia tropicalis among adults and childhood asthmatics in Thailand. Asian Pac J Allergy Immunol. 2003;21:199–204. [PubMed] [Google Scholar]
- 14.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99:594–599. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 15.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 16.A.F. Coca RAC. On the classification of the phenomena of hypersensitivities. J Immunol. 1923;8:163–182. [Google Scholar]
- 17.Zhang L, Chew FT, Soh SY, Yi FC, Law SY, Goh DY, et al. Prevalence and distribution of indoor allergens in Singapore. Clin Exp Allergy. 1997;27:876–885. [PubMed] [Google Scholar]
- 18.Andiappan AK, Anantharaman R, Nilkanth PP, de Wang Y, Chew FT. Evaluating the transferability of Hapmap SNPs to a Singapore Chinese population. BMC Genet. 2010;11:36. doi: 10.1186/1471-2156-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 20.Baratawidjaja IR, Baratawidjaja PP, Darwis A, Soo-Hwee L, Fook-Tim C, Bee-Wah L, et al. Prevalence of allergic sensitization to regional inhalants among allergic patients in Jakarta, Indonesia. Asian Pac J Allergy Immunol. 1999;17:9–12. [PubMed] [Google Scholar]
- 21.Kidon MI, Chiang WC, Liew WK, Ong TC, Tiong YS, Wong KN, et al. Mite component-specific IgE repertoire and phenotypes of allergic disease in childhood: the tropical perspective. Pediatr Allergy Immunol. 2011;22:202–210. doi: 10.1111/j.1399-3038.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 22.Zuraimi MS, Tham KW, Chew FT, Ooi PL. The effect of ventilation strategies of child care centers on indoor air quality and respiratory health of children in Singapore. Indoor Air. 2007;17:317–327. doi: 10.1111/j.1600-0668.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- 23.Harving H, Korsgaard J, Dahl R. Clinical efficacy of reduction in house-dust mite exposure in specially designed, mechanically ventilated “healthy” homes. Allergy. 1994;49:866–870. doi: 10.1111/j.1398-9995.1994.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 24.Rod NH, Kristensen TS, Lange P, Prescott E, Diderichsen F. Perceived stress and risk of adult-onset asthma and other atopic disorders: a longitudinal cohort study. Allergy. 2012;67:1408–1414. doi: 10.1111/j.1398-9995.2012.02882.x.. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson H, Blaiss M, Nolte H, Würtz SØ, Andersen JS, Durham SR. Efficacy and safety of the SQ-standardized grass allergy immunotherapy tablet in mono- and polysensitized subjects. Allergy. 2013;68:252–255. doi: 10.1111/all.12074. [DOI] [PubMed] [Google Scholar]
- 27.Vervloet D, Penaud A, Razzouk H, Senft M, Arnaud A, Boutin C, et al. Altitude and house dust mites. J Allergy Clin Immunol. 1982;69:290–296. doi: 10.1016/s0091-6749(82)80006-7. [DOI] [PubMed] [Google Scholar]
- 28.Halken S. Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention. Pediatr Allergy Immunol. 2004;15(16):9–32. doi: 10.1111/j.1399-3038.2004.0148b.x. 4–5. [DOI] [PubMed] [Google Scholar]
- 29.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specific immunoglobulin E (IgE) levels for the house dust mite species Dermatophagoides pteronyssinus and Blomia tropicalis.
Influence of the time of exposure to tropical urban environment on the strength of sensitization to Dermatophagoides pteronyssinus and Blomia tropicalis in the discovery cohort (Table 1).
House dust mite sensitization and allergic symptoms in migrants from tropical Malaysia stratified by number of years of residence in Singapore.
House dust mite-specific immunoglobulin E (IgE) and total serum IgE are significantly higher in individuals with allergic phenotypes.
Association of house dust mite-specific skin prick test with allergic phenotypes.
Definitions used in the study.