Abstract
Schwannomas and grade I meningiomas are non-metastatic neoplasms that share the common mutation of gene NF2. They usually appear in neurofibromatosis type 2 patients. Currently, there is no drug treatment available for both tumors, thus the use of wide expression technologies is crucial to identify therapeutic targets. Affymetrix Human Gene 1.0 ST was used to test global gene expression in 22 meningiomas, 31 schwannomas and, as non-tumoral controls, 3 healthy meningeal tissues, 8 non-tumoral nerves and 1 primary Schwann cell culture. A non-stringent P-value cut-off and fold change were used to establish deregulated genes. We identified a subset of genes that were upregulated in meningiomas and schwannomas when compared to their respectively healthy tissues, including PDGFD, CDH1 and SLIT2. Thus, these genes should be thoroughly studied as targets in a possible combined treatment.
Keywords: schwannoma, meningioma, microarray, comparative gene expression, NF2, neurofibromatosis 2
Introduction
Schwannomas are benign tumors that arise from Schwann cells. They typically appear in the vestibulocochlear nerve and are considered to be grade I tumors; approximately 95% are unilateral and present sporadically, whereas 5% are associated with neurofibromatosis type 2 syndrome (NF2). Patients with NF2 present with bilateral schwannomas and other tumors, frequently meningiomas, which originate from arachnoid cells, and account for 20% of all primary intracranial tumors. The current classification of meningiomas by the World Health Organization (WHO) includes three grades: 90% are classified as grade I tumors; ~8–9% are atypical grade II tumors; and 1–2% are anaplastic/malignant grade III tumors (1). Meningiomas have a recurrence rate of 18, 40 and 80% for grade I, II and III, respectively.
Preliminary cytogenetic studies have demonstrated the absence of one chromosome 22 in both neoplasms (2,3), thus suggesting a common genetic origin for at least some subgroups of these neurogenic tumors. Subsequently, NF2 gene (located at 22q12.2) inactivation was found to be due to several mechanisms, such as mutations or allelic loss due to monosomy or deletion of chromosome 22, accounting for up to 66% in schwannomas (4) and 18–50% in sporadic meningiomas, depending on the histopathological subtypes (5). In addition to the characteristic chromosome 22 loss, secondary alterations such as 1p deletions have been described in both tumor types, and these alterations appear to be related to tumor progression in meningiomas (6–8). Although DNA methylation studies on these neurogenic tumors have revealed the non-random involvement of this mechanism in the inactivation of some tumor-related genes (9–11), controversial data are available concerning the epigenetic (through CpG island aberrant methylation) NF2 inactivation in both neoplasms (12–16). Indeed, recent studies on genome-wide methylation suggest that this mechanism is associated with malignant transformation in meningiomas, and allows for the epigenetic subclassification of this tumor (17,18).
Global exome sequencing in meningiomas showed that, in grade I tumors, NF2 gene alteration (by mutation and/or loss of chromosome 22) is mutually exclusive with other gene mutations such as AKT1, TRAF7, KLF4 and SMO (19), but not with others such as NF1 and NEGR1 (20). In schwannomas, no alternative mutation has been found for those samples lacking hits over NF2 and, however, Merlin (the NF2 protein) does not seem to be present in the cases analyzed to date (21).
The expression analysis of tumor-related genes in meningiomas and schwannomas suggests a possible molecular subgroup classification in both tumors (22) with the involvement of differential regulatory pathways (23,24) related to the allelic losses at 1p and 14q in meningiomas (25). Whole genome expression analysis has been performed on schwannomas (26–28) and meningiomas (29–32). Whereas meningiomas have shown differential expression patterns based on progression and recurrence, but not strictly supported by grade (31), in schwannomas no distinctive pattern has been found using clinical correlations (28). However, a critical deregulation of microRNAs, including the upregulation of those located at the 14q32 chromosomal region, was a characteristic feature of vestibular tumors (33).
Intracranial non-recurrent WHO grade I meningiomas and schwannomas represent similar problems for patients, depending on the brain structures affected by their non-invasive growth. Currently, treatment options for patients with grade I meningiomas or schwannomas include surgery resection, radio-surgery and a ‘wait and see’ strategy. Thus, there is no available chemotherapeutic treatment for these tumors besides surgery, a situation especially traumatic for patients suffering bilateral vestibular schwannomas and several meningiomas such as those affected by NF2. Due to the common genetic origin of these tumors (NF2 inactivation), previous studies have attempted to identify targets with which to inhibit both schwannoma and meningioma progression. AR42, a histone deacetylase inhibitor, was found to suppress the proliferation of meningioma and schwannoma cell lines in vitro (34), and the same effect was shown by cucurbitacin D and goyazensolide in primary cultures (35).
In the present study, we used microarray technology to compare gene-expression patterns and identify genes and pathways of potential interest as key targets for the combined treatment of vestibular schwannomas and grade I meningiomas.
Materials and methods
Statementofethicsandsamples
The local Ethics Review Board of La Paz University Hospital approved the study protocol according to the principles of the Declaration of Helsinki. All patients received detailed information concerning the study and provided their written informed consent prior to their inclusion. In this study, we used RNA from 22 meningiomas, 31 schwannomas and, as non-tumoral controls, 3 healthy meningeal tissues, 8 non-tumoral nerves and 1 primary Schwann cell culture. The three control non-tumoral meningeal RNAs derived from two healthy males and one female and were purchased from BioChain® (cat. no. R1234043-10-D03; lot nos. B108134, A602330 and B501146).
RNA extraction and microarray experiments
The RNA was extracted with the RNeasy® Mini kit (Qiagen, Valencia, CA, USA) as indicated previously (28). For global gene expression, the Affymetrix Human Gene 1.0 ST was used. The expression profile of the meningiomas and the meninges samples can be accessed at the gene expression Omnibus (GEO) database GSE54934. The arrays of schwannomas and control nerves were previously published (28) and are available at the GEO database GSE39645. The arrays were processed at the Institute for Research in Biomedicine (IRB), Barcelona, Spain.
Statistical analysis
The normalization and summarization were performed using the robust multichip average (RMA). In order to reduce the batch effect among tumors (schwannomas and meningiomas) and controls (healthy nerves and meninges), a critical aspect for our analysis, we used ComBat (36). For data analysis, the genes were considered deregulated between groups when at least a 2-fold change in expression and a P<0.05 cut-off (ANOVA) was identified, as previously recommended by the MAQC consortium (37). For the comparison between schwannomas and meningiomas in order to obtain a list of genes with no changes among both tumor types, we used a more restrictive fold (<1.5) exclusively for this purpose, since the ComBat effect could have lowered these values and false-positives could appear. For comparative purposes, a list of differentially expressed genes and fold-change was obtained with the Geo2R web tool (http://www.ncbi.nlm.nih.gov/geo/geo2r/) in the series GSE43290, which includes 4 meninges as controls and 47 tumors (29). As our meningioma series mainly included grade I tumors, only the 33 WHO grade I meningiomas and the 4 controls included in this report were used for comparison.
DNA extraction
The DNA was extracted by standard methods, as previously described (22). The data regarding the NF2 status, including loss of heterozygosity of 22q (LOH), Multiplex ligation-dependent probe amplification (MLPA) of NF2 (SALSA P044) and sequence analysis by dHPLC were reported previously in detail (22,28), and were performed as described (22). Clinical and NF2 status data from the meningiomas correspond to cases M02, M04, M05, M07, M09, M10, M12, M14, M24, M25, M28, M29, M30, M31, M32, M33, M34, M38, M39, M40, M41 and M42, as previously reported (22). The complete case series of schwannomas from our previous report (28) was included.
Results and Discussion
Comparison with respect to previous analyses of meningiomas and the summary of results in schwannomas
Meningioma profiling was analyzed extensively in previous studies, and up to five expression subgroups were characterized (31), although this classification did not represent the actual WHO classification. Recurrence and progression appear to play a relevant role in the expression pattern of these tumors (32), and two meningioma groups were identified showing different clinical and pathological behaviors, more related to clinical outcome than to WHO grade per se. Furthermore, depending on the cytogenetic aberrations, differential expression patterns have been described (25,29). Tumors that presented monosomy of chromosome 22 and cases with multiple karyotype alterations had a differential expression pattern, whereas those cases with deletion of chromosome 1 alone showed random behavior (29). In summary, previous analyses of gene-expression patterns in meningiomas do not seem to accurately represent the current WHO classification, although recurrence and progression status might be reflected in these studies. We used 20 grade I meningiomas, 2 grade II meningiomas and 3 healthy meninges. Practically the same values were obtained when meningiomas grade II were removed from the study (data not shown). When we compared our results in meningiomas with those obtained from the dataset GSE43290 (29), we found a high consistency in our results, such as the downregulation of diverse genes such as SNAP25, MBP, TTR and VSNL1, and the upregulation of FBN2, FGF9 and SULF1 (full data available upon request). As in previous studies, our 2 cases of meningioma grade II did not show a different trend. The schwannoma expression profile was previously explained (28). In brief, the upregulation of SPP1, MET and associated genes or LATS2 was reported, whereas the downregulation of CAV1, AR and PAWR was found. In general, myelinization genes were overexpressed, suggesting that schwannoma cells could resemble a previous state of mature Schwann cells.
Gene co-overexpression in meningiomas and schwannomas
Using the ANOVA test at P<0.05 significance across the four groups (all meningiomas, schwannomas, control healthy meninges and control nerves), we obtained a list of 12,395 genes with differential expression among these four groups. Of those, 346 (data not shown; available upon request) did not meet the criteria established for accepting deregulation differences between both tumor groups, which was ≤1.5-fold of the differential expression between the schwannomas and the meningiomas, a limit value selected as deregulated between these two groups due to the correction effect of ComBat. Among those 346 genes with similar expression in tumors, 47 showed co-overexpression in schwannomas and meningiomas when compared with their respective controls at 2-fold (as ComBat correction would only be based on batch effect) (Table I). These genes included E-cadherin (CDH1), which is usually silenced by several mechanisms, such as the Wnt signaling pathway in human cancer, including meningiomas (38), and platelet-derived growth factor D (PDGFD), an activator for PDGFR-β (39). This pathway has been reported as overexpressed in multiple cancer types such as pancreatic cancer and brain tumors, including schwannomas (39). Another gene reported as expressed (and protein present) in meningiomas and schwannomas is tyrosine kinase receptor MET (40), which is responsible for cell migration, anchorage-independent growth and many other functions. High levels of this receptor have been found in a wide variety of tumors, such as breast cancer, renal cell carcinoma and head and neck tumors (41). Mechanisms such as point mutations, alternative splicing, genomic amplification and transcript amplification appear to participate in overexpression of c-MET (reviewed in ref. 42). Accordingly, we found MET upregulation in both neoplasms compared with their respective control tissues, and again, a similar level of expression between both tumor types was detected. SLIT2 is a member of the Slit family that modulates cell migration by binding with the Robo family. This gene has been found expressed in the development of several malignancies such as colorectal epithelial cell carcinogenesis (43). The findings in this report (43), suggest that Slit2-Robo1 causes E-cadherin degradation, and although our results show an upregulation of the E-cadherin gene, the former mechanism should not be ruled out in the tumors we studied. In other neoplasms, although expressed, SLIT2 does not seem to play any role (44). In agreement with the data from the study selected for validation (29), several genes, including CDH1, PDGFD, CX3CR1, CCND1 and SLIT2, were also upregulated, as shown in data obtained from meningioma dataset GSE43290 (29); in contrast, MET showed a trend of upregulation but did not reach 2-fold. Functional annotation using DAVID showed enrichment in inflammatory response, cell migration and defense response (data not shown; available upon request).
Table I.
Genes overexpressed in meningioma and schwannoma when compared with their respective control tissue.
| Gene | Database | Chromosome | C-M | N-S | M-S | P-value |
|---|---|---|---|---|---|---|
| CDH1 | NM_004360 | 16q22.1 | 5.4 | 5.8 | −1.0 | 8.35E-09 |
| PDGFD | NM_025208 | 11q22.3 | 4.4 | 6.2 | −1.1 | 7.48E-13 |
| SLIT2 | NM_004787 | 4p15.2 | 3.6 | 5.8 | −1.1 | 2.02E-13 |
| HLA-DPA1 | NM_033554 | 6p21.3 | 2.9 | 3.9 | −1.1 | 5.24E-07 |
| PAPPA | NM_002581 | 9q33.2 | 2.8 | 3.8 | −1.1 | 3.68E-06 |
| TREM2 | NM_018965 | 6p21.1 | 2.7 | 5.5 | −1.3 | 2.8E-12 |
| HLA-DPA1 | NM_033554 | 6p21.3 | 2.6 | 4.1 | −1.2 | 5.24E-07 |
| HPGDS | NM_014485 | 4q22.3 | 2.6 | 2.4 | −1.1 | 3.83E-07 |
| GPR34 | NM_001097579 | Xp11.4 | 2.6 | 12.0 | −1.5 | 9.9E-11 |
| CX3CR1 | NM_001337 | 3p21|3p21.3 | 2.5 | 5.7 | −1.2 | 2.15E-07 |
| ANKRD22 | NM_144590 | 10q23.31 | 2.5 | 7.3 | −1.4 | 1.04E-06 |
| C3 | NM_000064 | 19p13.3-p13.2 | 2.4 | 2.6 | −1.2 | 5.77E-05 |
| CYBB | NM_000397 | Xp21.1 | 2.4 | 4.1 | −1.2 | 1.11E-07 |
| LGALS3BP | NM_005567 | 17q25 | 2.4 | 2.9 | −1.1 | 4.67E-13 |
| WIPI1 | NM_017983 | 17q24.2 | 2.4 | 2.0 | −1.0 | 2.55E-12 |
| APOBEC3C | NM_014508 | 22q13.1 | 2.4 | 2.5 | −1.1 | 3.35E-08 |
| C3AR1 | NM_004054 | 12p13.31 | 2.4 | 4.8 | −1.2 | 4.43E-09 |
| FCGBP | NM_003890 | 19q13.1 | 2.3 | 9.7 | −1.3 | 1.03E-11 |
| FRAS1 | NM_025074 | 4q21.21 | 2.3 | 3.2 | −1.2 | 7.42E-08 |
| FLRT3 | NM_198391 | 20p11 | 2.3 | 3.8 | −1.2 | 1.76E-05 |
| FCGR1A | NM_000566 | 1q21.2–q21.3 | 2.3 | 4.3 | −1.3 | 1.66E-08 |
| MET | NM_001127500 | 7q31 | 2.3 | 4.9 | −1.3 | 1.22E-08 |
| ITPR3 | NM_002224 | 6p21 | 2.3 | 3.7 | −1.1 | 3.69E-12 |
| FCGR1B | NM_001017986 | 1p11.2 | 2.3 | 2.9 | −1.2 | 4.14E-08 |
| ALCAM | NM_001627 | 3q13.1 | 2.2 | 2.5 | −1.0 | 4.26E-09 |
| HLA-DPB1 | NM_002121 | 6p21.3 | 2.2 | 4.6 | −1.3 | 1.75E-07 |
| LAMB1 | NM_002291 | 7q22 | 2.2 | 2.0 | −1.2 | 4.57E-07 |
| C8orf84 | NM_153225 | 8q21.11 | 2.2 | 2.6 | −1.2 | 8.11E-06 |
| SLFN12 | NM_018042 | 17q12 | 2.2 | 2.3 | −1.2 | 4.14E-10 |
| FCGR1A | NM_000566 | 1q21.2–q21.3 | 2.2 | 3.1 | −1.2 | 1.66E-08 |
| LHFPL2 | NM_005779 | 5q14.1 | 2.1 | 2.3 | −1.1 | 8.13E-09 |
| MS4A6A | NM_152852 | 11q12.1 | 2.1 | 4.5 | −1.3 | 4.8E-08 |
| CD84 | NM_001184879 | 1q24 | 2.1 | 2.7 | −1.2 | 3.38E-09 |
| TRIM22 | NM_006074 | 11p15 | 2.1 | 2.2 | −1.1 | 2.09E-09 |
| CD4 | NM_000616 | 12pter-p12 | 2.1 | 2.5 | −1.1 | 1.69E-07 |
| CSF1R | NM_005211 | 5q32 | 2.1 | 3.8 | −1.2 | 5.21E-08 |
| GFRA1 | NM_005264 | 10q26.11 | 2.1 | 4.9 | −1.4 | 1.34E-07 |
| HLA-DPB1 | NM_002121 | 6p21.3 | 2.1 | 4.5 | −1.3 | 1.75E-07 |
| CD86 | NM_175862 | 3q21 | 2.1 | 2.9 | −1.3 | 1.03E-06 |
| C1QA | NM_015991 | 1p36.12 | 2.1 | 4.3 | −1.2 | 1.24E-07 |
| TLR7 | NM_016562 | Xp22.3 | 2.0 | 3.6 | −1.3 | 7E-08 |
| CCND1 | NM_053056 | 11q13 | 2.0 | 2.7 | −1.1 | 1.48E-11 |
| HLA-DQA1 | NM_002122 | 6p21.3 | 2.0 | 2.6 | −1.2 | 4.19E-05 |
| FAM105A | NM_019018 | 5p15.2 | 2.0 | 2.6 | −1.1 | 9.2E-08 |
| C6orf138 | NM_001013732 | 6p12.3 | 2.0 | 3.4 | −1.2 | 1.99E-10 |
| P2RY13 | NM_176894 | 3q24 | 2.0 | 2.4 | −1.2 | 3.08E-06 |
| PROS1 | NM_000313 | 3q11.2 | 2.0 | 5.0 | −1.4 | 9.1E-14 |
Official gene symbol is shown for every gene. C-M values correspond to the fold-change value of control healthy meninges (C) minus meningioma (M). In the case of N-S, N refers to nerve healthy tissue minus schwannoma (S). In the column M-S, meningioma (M) minus schwannoma (S) is performed. Only those genes with ≤1.5 fold-change between both tumors are shown.
Gene co-infraexpression in meningiomas and schwannomas
A total of 35 genes (Table II) with no difference in expression between schwannomas and meningiomas were underexpressed when compared with their respective controls in both neoplasms. Among them are selectin E (SELE) and Rho family GTPase 1 (RND1), which is linked to semaphorins (45,46) and cytoskeleton organization in axons. The chemokine (C-X-C motif) ligand 2 (CXCL2) was significantly downregulated in schwannomas and meningiomas, whereas the opposite trend has been shown in malignant neoplasms such as ovarian and endometrial cancer and oral squamous cell carcinoma (47). As schwannomas and meningiomas are usually non-invasive, this fact could explain the different trend in deregulation of CXCL2. Stathmin-like 2 (STMN2) showed the same pattern: upregulation in hepatoma cells but downregulation in schwannomas and meningiomas. Notably, STMN2 interacts with Rho family GTPase 1 (RND1) in axon extension (48), another gene that was downregulated in both tumors. Other downregulated genes in both tumors were E-selectin (SELE) and vascular adhesion protein 1 (AOC3), related to the tethering and rolling of leukocytes (49); thus, the non-invasive nature of grade I meningiomas and schwannomas could explain the downregulation of these genes. Validation with the dataset GSE43290 was performed, and included, among others, downregulation of AOC3, STMN2, SELE, RGS4, THBS4 and RND1. Functional analysis with DAVID included leukocyte and cell migration, heparin binding or membrane fraction (data available upon request).
Table II.
Genes infraexpressed in meningioma and schwannoma when compared with their respective control tissue.
| Gene | Database | Chromosome | C-M | N-S | M-S | P-value |
|---|---|---|---|---|---|---|
| SAA1 | NM_000331 | 11p15.1 | −2.0 | −2.7 | 1.1 | 0.000414 |
| INHBA | NM_002192 | 7p15-p13 | −2.0 | −4.1 | 1.2 | 2.42E-09 |
| PCDH18 | NM_019035 | 4q31 | −2.0 | −2.6 | 1.2 | 0.000183 |
| PTGIS | NM_000961 | 20q13.13 | −2.1 | −3.4 | 1.3 | 1.72E-06 |
| HHIP | NM_022475 | 4q28–q32 | −2.1 | −2.9 | 1.1 | 1.44E-06 |
| AQP9 | NM_020980 | 15q | −2.1 | −4.5 | 1.0 | 2.78E-08 |
| TCEAL2 | NM_080390 | Xq22.1–q22.3 | −2.1 | −2.4 | 1.4 | 0.000135 |
| S100A12 | NM_005621 | 1q21 | −2.2 | −5.6 | 1.1 | 9.51E-07 |
| PDE3A | NM_000921 | 12p12 | −2.2 | −2.0 | 1.2 | 2.03E-08 |
| S100A9 | NM_002965 | 1q21 | −2.2 | −4.6 | −1.0 | 2.63E-05 |
| PAK3 | NM_002578 | Xq23 | −2.3 | −4.1 | 1.3 | 5.36E-11 |
| SLC16A7 | NM_004731 | 12q13 | −2.3 | −2.2 | 1.1 | 4.89E-12 |
| PI16 | NM_153370 | 6p21.2 | −2.3 | −5.1 | 1.2 | 1.76E-09 |
| MGST1 | NM_145792 | 12p12.3-p12.1 | −2.3 | −3.6 | 1.1 | 0.001079 |
| FGFR2 | NM_000141 | 10q26 | −2.3 | −2.6 | 1.3 | 1.69E-09 |
| TRPM3 | NM_206946 | 9q21.12 | −2.4 | −2.0 | 1.1 | 3.65E-11 |
| PDZRN4 | NM_013377 | 12q12 | −2.5 | −3.0 | 1.1 | 1.14E-08 |
| THBS4 | NM_003248 | 5q13 | −2.5 | −3.5 | 1.0 | 1.71E-09 |
| STEAP4 | NM_024636 | 7q21.12 | −2.7 | −4.2 | 1.1 | 1.38E-07 |
| DCLK1 | NM_004734 | 13q13 | −2.8 | −2.4 | 1.1 | 2.22E-07 |
| ZNF385D | NM_024697 | 3p24.3 | −3.0 | −2.0 | 1.1 | 1.34E-05 |
| CXCL2 | NM_002089 | 4q21 | −3.1 | −3.3 | −1.0 | 7.52E-09 |
| FABP4 | NM_001442 | 8q21 | −3.1 | −13.5 | 1.2 | 1.29E-12 |
| IL6 | NM_000600 | 7p21 | −3.1 | −4.6 | −1.0 | 0.000397 |
| SELE | NM_000450 | 1q22–q25 | −3.3 | −6.6 | 1.0 | 2.29E-09 |
| SLC14A1 | NM_001128588 | 18q11–q12 | −4.0 | −3.3 | 1.0 | 2.7E-09 |
| APLNR | NM_005161 | 11q12 | −4.8 | −3.1 | −1.0 | 8.05E-11 |
| ADH1B | NM_000668 | 4q23 | −4.9 | −3.6 | −1.1 | 6.95E-07 |
| ADCYAP1R1 | NM_001118 | 7p14 | −5.1 | −3.3 | 1.0 | 6.98E-11 |
| RND1 | NM_014470 | 12q12 | −5.4 | −3.1 | −1.0 | 3.5E-09 |
| ADAMTS1 | NM_006988 | 21q21.2 | −6.0 | −2.4 | −1.1 | 4.64E-11 |
| HSPB8 | NM_014365 | 12q24.23 | −6.0 | −3.5 | 1.0 | 5.3E-07 |
| AOC3 | NM_003734 | 17q21 | −6.3 | −2.5 | −1.1 | 3.64E-11 |
| RGS4 | NM_001102445 | 1q23.3 | −6.7 | −2.1 | −1.1 | 2.55E-10 |
| STMN2 | NM_007029 | 8q21.13 | −9.2 | −2.9 | −1.1 | 4.92E-11 |
Official gene symbol is shown for every gene. C-M values correspond to the fold-change value of control healthy meninges (C) minus meningioma (M). In the case of N-S, N refers to nerve healthy tissue minus schwannoma (S). In the column M-S, meningioma (M) minus schwannoma (S) is performed. Only those genes with ≤1.5 fold-change between both tumors are shown.
Gene expression differences between meningiomas and schwannomas
The main goal of our study was to test gene expression profiles common to schwannomas and meningiomas in regard to their respective controls, and having taken into account their relative expression. However, we also studied the gene expression differences between both neurogenic neoplasms. As samples were processed in various batches, we used a Bayesian method to reduce the batch effect. Because of this effect, the differential expression of certain genes in schwannomas and meningiomas could have decreased. This issue, although it limits our information, is vital to our study since the batch effect was very marked; 192 genes were upregulated at 1.5-fold differences and P<0.05 (data available upon request) in schwannomas as compared to meningioma expression. Most of these genes are related to neuron migration and the myelin sheath, such as the following: peripheral myelin protein 2 (PMP2), expressed in the cytoplasmic side of myelin in the peripheral nervous system (50); myelin protein zero (MPZ), representing 50% of the total myelin protein in the peripheral nervous system (51); neurexin 1 (NRXN1), which mediates formation and maintenance of synaptic junctions (52); and neural cell adhesion molecule 2 (NCAM2), which is involved in axonal projection (53).
Upregulation in meningiomas compared with schwannomas gave us 88 genes (data available upon request) and included cellular retinoic acid binding protein 2 (CRABP2), a chaperon downregulated in high-grade gliomas (54), and secreted frizzled-related protein 2 gene (SFRP2), a gene identified as a tumor suppressor in a renal cell carcinoma cell line (55).
Another comparison concerned those genes that were upregulated in schwannomas with respect to nerves, and downregulated in meningiomas with respect to healthy meninges. These findings included genes such as hepatocyte cell adhesion molecule (HEPACAM), neuritin 1 (NRN1) and kinesin family member 1A (KIF1A).
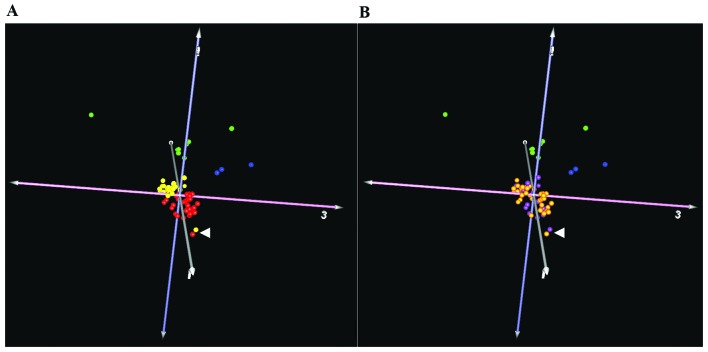
The NF2 mutation rate (determined by sequencing, MLPA and chromosome 22q LOH analyses) in this series was 74% for schwannomas and 68% for meningiomas. We compared the expression patterns in samples from both tumor types, and with respect to the presence or lack of any alteration in the NF2 gene (38 samples with alteration and 15 without any). Using these groups, we identified 2 genes with differential expression levels. The natriuretic peptide receptor C/guanylate cyclase C (atrionatriuretic peptide receptor C) (NPR3) was downregulated in those samples without NF2 alterations. This gene codes for a receptor coupled to various signaling transduction cascades in several tissues such as cardiac myocytes and fibroblasts (56). On the other hand, the G antigen 12J (GAGE12J) gene, transcribed in human fetal and tumoral tissues (57), was also downregulated, but on this occasion in tumors with NF2 alteration. As only 2 genes were detected, based on our microarray results in both neoplasms, it would seem that there is no differentiated subset of expression profiles of genes between samples with or without alteration over NF2 in grade I meningiomas and schwannomas (Fig. 1). Nevertheless, single genes could be altered in tumors with or without NF2 alteration, although such a reduced number could be due to outlier values.
Figure 1.
Principal component analysis (PCA) of all samples studied. Green dots represent non-tumoral healthy nerves and the blue dots non-tumoral meninges samples. (A) Red dots are all the schwannomas studied, while yellow dots are the meningioma samples. Both entities are clearly separated, except for two samples (white arrow), one corresponding to a schwannoma from an NF2 patient and the other to a grade I meningioma. The most remote green dot corresponds to the cultured Schwann cells used as controls. (B) Violet dots are samples without alteration in the NF2 gene, and gold dots are tumors carrying such an alteration. The same point of view is shown in both images A and B, and no clear distinction between those two groups was found.
At present, there is no chemotherapeutic treatment available for either meningiomas or schwannomas, thus research for a combined solution could be of great value to those patients affected with both tumor types, primarily patients with neurofibromatosis type 2. In this study, we found a set of genes with aberrant expression in both entities compared with their respective control tissue, but with similar expression levels between these tumors, including PDGF, c-Met or Slit2 pathways. Thus, these and the other genes identified in this study, and their regulatory pathways, might be of interest for further experiments in the search for common solutions for patients affected by schwannomas and meningiomas.
Acknowledgements
The authors would like to thank Carolina Peña-Granero for her excellent technical assistance. This study was supported by grants PI10/1972 and PI13/00055 from Fondo de Investigaciones Sanitarias, Ministerio de Ciencia e Innovación, Spain; and PI13/00800, from the Fundación Sociosanitaria de Castilla-La Mancha, Spain.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumors of the Central Nervous System. IARC Press; Lyon: 2007. [Google Scholar]
- 2.Zankl H, Zang KD. Cytological and cytogenetical studies on brain tumors. 4. Identification of the missing G chromosome in human meningiomas as no. 22 by fluorescence technique. Humangenetik. 1972;14:167–169. doi: 10.1007/BF00273305. [DOI] [PubMed] [Google Scholar]
- 3.Rey JA, Bello MJ, De Campos JM, Kusak ME, Moreno S. Cytogenetic analysis in human neurinomas. Cancer Genet Cytogenet. 1987;28:187–188. doi: 10.1016/0165-4608(87)90372-4. [DOI] [PubMed] [Google Scholar]
- 4.Hadfield KD, Smith MJ, Urquhart JE, Wallace AJ, Bowers NL, King AT, Rutherford SA, Trump D, Newman WG, Evans DG. Rates of loss of heterozygosity and mitotic recombination in NF2 schwannomas, sporadic vestibular schwannomas and schwanno-matosis schwannomas. Oncogene. 2010;29:6216–6221. doi: 10.1038/onc.2010.363. [DOI] [PubMed] [Google Scholar]
- 5.Hansson CM, Buckley PG, Grigelioniene G, Piotrowski A, Hellström AR, Mantripragada K, Jarbo C, Mathiesen T, Dumanski JP. Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus. BMC Genomics. 2007;8:16. doi: 10.1186/1471-2164-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone PE, Bello MJ, de Campos JM, Vaquero J, Sarasa JL, Pestaña A, Rey JA. NF2 gene mutations and allelic status of 1p, 14q and 22q in sporadic meningiomas. Oncogene. 1999;18:2231–2239. doi: 10.1038/sj.onc.1202531. [DOI] [PubMed] [Google Scholar]
- 7.Leone PE, Bello MJ, Mendiola M, Kusak ME, De Campos JM, Vaquero J, Sarasa JL, Pestana A, Rey JA. Allelic status of 1p, 14q and 22q and NF2 gene mutations in sporadic schwannomas. Int J Mol Med. 1998;1:889–892. doi: 10.3892/ijmm.1.5.889. [DOI] [PubMed] [Google Scholar]
- 8.Bello MJ, de Campos JM, Kusak ME, Vaquero J, Sarasa JL, Pestaña A, Rey JA. Allelic loss at 1p is associated with tumor progression of meningiomas. Genes Chromosomes Cancer. 1994;9:296–298. doi: 10.1002/gcc.2870090411. [DOI] [PubMed] [Google Scholar]
- 9.Bello MJ, Martinez-Glez V, Franco-Hernandez C, Pefla-Granero C, de Campos JM, Isla A, Lassaletta L, Vaquero J, Rey JA. DNA methylation pattern in 16 tumor-related genes in schwannomas. Cancer Genet Cytogenet. 2007;172:84–86. doi: 10.1016/j.cancergencyto.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Bello MJ, Amiñoso C, Lopez-Marin I, Arjona D, Gonzalez-Gomez P, Alonso ME, Lomas J, de Campos JM, Kusak ME, Vaquero J, Isla A, Gutierrez M, Sarasa JL, Rey JA. DNA methylation of multiple promoter-associated CpG islands in meningiomas: relationship with the allelic status at 1p and 22q. Acta Neuropathol. 2004;108:413–421. doi: 10.1007/s00401-004-0911-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Pang JC, Dong S, Mao B, Poon WS, Ng HK. Aberrant CpG island hypermethylation profile is associated with atypical and anaplastic meningiomas. Hum Pathol. 2005;36:416–425. doi: 10.1016/j.humpath.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Kino T, Takeshima H, Nakao M, Nishi T, Yamamoto K, Kimura T, Saito Y, Kochi M, Kuratsu J, Saya H, Ushio Y. Identification of the cis-acting region in the NF2 gene promoter as a potential target for mutation and methylation-dependent silencing in schwannoma. Genes Cell. 2001;6:441–454. doi: 10.1046/j.1365-2443.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Gomez P, Bello MJ, Alonso ME, Lomas J, Arjona D, de Campos JM, Vaquero J, Isla A, Lassaletta L, Gutierrez M, Sarasa JL, Rey JA. CpG island methylation in sporadic and neurofibromatis type 2-associated schwannomas. Clin Cancer Res. 2003;9:5601–5606. [PubMed] [Google Scholar]
- 14.Lomas J, Bello MJ, Arjona D, Alonso ME, Martinez-Glez V, Lopez-Marin I, Amiñoso C, de Campos JM, Isla A, Vaquero J, Rey JA. Genetic and epigenetic alteration of the NF2 gene in sporadic meningiomas. Genes Chromosomes Cancer. 2005;42:314–319. doi: 10.1002/gcc.20141. [DOI] [PubMed] [Google Scholar]
- 15.Kullar PJ, Pearson DM, Malley DS, Collins VP, Ichimura K. CpG island hypermethylation of the neurofibromatosis type 2 (NF2) gene is rare in sporadic vestibular schwannomas. Neuropathol Appl Neurobiol. 2010;36:505–514. doi: 10.1111/j.1365-2990.2010.01090.x. [DOI] [PubMed] [Google Scholar]
- 16.Koutsimpelas D, Ruerup G, Mann WJ, Brieger J. Lack of neurofibromatosis type 2 gene promoter methylation in sporadic vestibular schwannomas. ORL J Otorhinolaryngol Relat Spec. 2012;74:33–37. doi: 10.1159/000334968. [DOI] [PubMed] [Google Scholar]
- 17.Kishida Y, Natsume A, Kondo Y, Takeuchi I, An B, Okamoto Y, Shinjo K, Saito K, Ando H, Ohka F, Sekido Y, Wakabayashi T. Epigenetic subclassification of meningiomas based on genome-wide DNA methylation analyses. Carcinogenesis. 2012;33:436–441. doi: 10.1093/carcin/bgr260. [DOI] [PubMed] [Google Scholar]
- 18.Gao F, Shi L, Russin J, Zeng L, Chang X, He S, Chen TC, Giannotta SL, Weisenberger DJ, Zada G, Mack WJ, Wang K. DNA methylation in the malignant transformation of meningiomas. PloS One. 2013;8:e54114. doi: 10.1371/journal.pone.0054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stemmer-Rachamimov AO, Xu L, Gonzalez-Agosti C, Burwick JA, Pinney D, Beauchamp R, Jacoby LB, Gusella JF, Ramesh V, Louis DN. Universal absence of merlin, but not other ERM family members, in schwannomas. Am J Pathol. 1997;151:1649–1654. [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Glez V, Franco-Hernandez C, Alvarez L, De Campos JM, Isla A, Vaquero J, Lassaletta L, Casartelli C, Rey JA. Meningiomas and schwannomas: molecular subgroup classification found by expression arrays. Int J Oncol. 2009;34:493–504. [PubMed] [Google Scholar]
- 23.Torres-Martín M, Martinez-Glez V, Peña-Granero C, Isla A, Lassaletta L, De Campos JM, Pinto GR, Burbano RR, Meléndez B, Castresana JS, Rey JA. Gene expression analysis of aberrant signaling pathways in meningiomas. Oncol Lett. 2013;6:275–279. doi: 10.3892/ol.2013.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres-Martín M, Martinez-Glez V, Peña-Granero C, Lassaletta L, Isla A, de Campos JM, Pinto GR, Burbano RR, Meléndez B, Castresana JS, Rey JA. Expression analysis of tumor-related genes involved in critical regulatory pathways in schwannomas. Clin Transl Oncol. 2013;15:409–411. doi: 10.1007/s12094-012-0937-5. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Glez V, Alvarez L, Franco-Hernández C, Torres-Martin M, de Campos JM, Isla A, Vaquero J, Lassaletta L, Castresana JS, Casartelli C, Rey JA. Genomic deletions at 1p and 14q are associated with an abnormal cDNA microarray gene expression pattern in meningiomas but not in schwannomas. Cancer Genet Cytogenet. 2010;196:1–6. doi: 10.1016/j.cancergencyto.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Aarhus M, Bruland O, Sætran HA, Mork SJ, Lund-Johansen M, Knappskog PM. Global gene expression profiling and tissue microarray reveal novel candidate genes and downregulation of the tumor suppressor gene CAV1 in sporadic vestibular schwannomas. Neurosurgery. 2010;67:998–1019. doi: 10.1227/NEU.0b013e3181ec7b71. [DOI] [PubMed] [Google Scholar]
- 27.Cayé-Thomasen P, Borup R, Stangerup S-E, Thomsen J, Nielsen FC. Deregulated genes in sporadic vestibular schwannomas. Otol Neurotol. 2010;31:256–266. doi: 10.1097/MAO.0b013e3181be6478. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Martin M, Lassaletta L, San-Roman-Montero J, De Campos JM, Isla A, Gavilan J, Melendez B, Pinto GR, Burbano RR, Castresana JS, Rey JA. Microarray analysis of gene expression in vestibular schwannomas reveals SPP1/MET signaling pathway and androgen receptor deregulation. Int J Oncol. 2013;42:848–862. doi: 10.3892/ijo.2013.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabernero MD, Maillo A, Gil-Bellosta CJ, Castrillo A, Sousa P, Merino M, Orfao A. Gene expression profiles of meningiomas are associated with tumor cytogenetics and patient outcome. Brain Pathol. 2009;19:409–420. doi: 10.1111/j.1750-3639.2008.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller A, Ludwig N, Backes C, Romeike BFM, Comtesse N, Henn W, Steudel W-I, Mawrin C, Lenhof H-P, Meese E. Genome wide expression profiling identifies specific deregulated pathways in meningioma. Int J Cancer. 2009;124:346–351. doi: 10.1002/ijc.23942. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Liu J, Patel S, Cloughesy T, Lai A, Farooqi H, Seligson D, Dong J, Liau L, Becker D, Mischel P, Shams S, Nelson S. Genomic landscape of meningiomas. Brain Pathol. 2010;20:751–762. doi: 10.1111/j.1750-3639.2009.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Magán E, Campos-Martín Y, Mur P, Fiaño C, Ribalta T, García JF, Rey JA, Rodríguez de Lope A, Mollejo M, Meléndez B. Genetic alterations associated with progression and recurrence in meningiomas. J Neuropathol Exp Neurol. 2012;71:882–893. doi: 10.1097/NEN.0b013e31826bf704. [DOI] [PubMed] [Google Scholar]
- 33.Torres-Martin M, Lassaletta L, de Campos JM, Isla A, Gavilan J, Pinto GR, Burbano RR, Latif F, Melendez B, Castresana JS, Rey JA. Global profiling in vestibular schwannomas shows critical deregulation of microRNAs and upregulation in those included in chromosomal region 14q32. PloS One. 2013;8:e65868. doi: 10.1371/journal.pone.0065868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush ML, Oblinger J, Brendel V, Santarelli G, Huang J, Akhmametyeva EM, Burns SS, Wheeler J, Davis J, Yates CW, Chaudhury AR, Kulp S, Chen CS, Chang LS, Welling DB, Jacob A. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro Oncol. 2011;13:983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spear SA, Burns SS, Oblinger JL, Ren Y, Pan L, Kinghorn AD, Welling DB, Chang LS. Natural compounds as potential treatments of NF2-deficient schwannoma and meningioma: cucurbitacin D and goyazensolide. Otol Neurotol. 2013;34:1519–1527. doi: 10.1097/MAO.0b013e3182956169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 37.MAQC Consortium. Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville F, Kawasaki ES, Lee KY, Luo Y, Sun YA, Willey JC, Setterquist RA, Fischer GM, Tong W, Dragan YP, Dix DJ, Frueh FW, Goodsaid FM, Herman D, Jensen RV, Johnson CD, Lobenhofer EK, Puri RK, Schrf U, Thierry-Mieg J, Wang C, Wilson M, Wolber PK, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–1161. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou K, Wang G, Wang Y, Jin H, Yang S, Liu C. The potential involvement of E-cadherin and beta-catenins in meningioma. PloS One. 2010;5:e11231. doi: 10.1371/journal.pone.0011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Kong D, Li Y, Sarkar FH. PDGF-D signaling: a novel target in cancer therapy. Curr Drug Targets. 2009;10:38–41. doi: 10.2174/138945009787122914. [DOI] [PubMed] [Google Scholar]
- 40.Moriyama T, Kataoka H, Kawano H, Yokogami K, Nakano S, Goya T, Uchino H, Koono M, Wakisaka S. Comparative analysis of expression of hepatocyte growth factor and its receptor, c-met, in gliomas, meningiomas and schwannomas in humans. Cancer Lett. 1998;124:149–155. doi: 10.1016/s0304-3835(97)00469-2. [DOI] [PubMed] [Google Scholar]
- 41.Cipriani NA, Abidoye OO, Vokes E, Salgia R. MET as a target for treatment of chest tumors. Lung Cancer. 2009;63:169–179. doi: 10.1016/j.lungcan.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF, Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ, Geng JG. Slit-Robo signaling induces malignant transformation through Hakai-mediated E-cadherin degradation during colorectal epithelial cell carcinogenesis. Cell Res. 2011;21:609–626. doi: 10.1038/cr.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai CF, Jiang YZ, Li Y, Wang K, Liu PS, Patankar MS, Zheng J. Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol. 2011;135:475–485. doi: 10.1007/s00418-011-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanata SM, Hovatta I, Rohm B, Püschel AW. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse. J Neurosci. 2002;22:471–477. doi: 10.1523/JNEUROSCI.22-02-00471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hota PK, Buck M. Thermodynamic characterization of two homologous protein complexes: associations of the semaphorin receptor plexin-B1 RhoGTPase binding domain with Rnd1 and active Rac1. Protein Sci. 2009;18:1060–1071. doi: 10.1002/pro.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oue E, Lee JW, Sakamoto K, Iimura T, Aoki K, Kayamori K, Michi Y, Yamashiro M, Harada K, Amagasa T, Yamaguchi A. CXCL2 synthesized by oral squamous cell carcinoma is involved in cancer-associated bone destruction. Biochem Biophys Res Commun. 2012;424:456–461. doi: 10.1016/j.bbrc.2012.06.132. [DOI] [PubMed] [Google Scholar]
- 48.Li YH, Ghavampur S, Bondallaz P, Will L, Grenningloh G, Püschel AW. Rnd1 regulates axon extension by enhancing the microtubule destabilizing activity of SCG10. J Biol Chem. 2009;284:363–371. doi: 10.1074/jbc.A808126200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jalkanen S, Karikoski M, Mercier N, Koskinen K, Henttinen T, Elima K, Salmivirta K, Salmi M. The oxidase activity of vascular adhesion protein-1 (VAP-1) induces endothelial E- and P-selectins and leukocyte binding. Blood. 2007;110:1864–1870. doi: 10.1182/blood-2007-01-069674. [DOI] [PubMed] [Google Scholar]
- 50.Eylar EH, Szymanska I, Ishaque A, Ramwani J, Dubiski S. Localization of the P2 protein in peripheral nerve myelin. J Immunol. 1980;124:1086–1092. [PubMed] [Google Scholar]
- 51.Everly JL, Brady RO, Quarles RH. Evidence that the major protein in rat sciatic nerve myelin is a glycoprotein. J Neurochem. 1973;21:329–334. doi: 10.1111/j.1471-4159.1973.tb04253.x. [DOI] [PubMed] [Google Scholar]
- 52.Bottos A, Rissone A, Bussolino F, Arese M. Neurexins and neuroligins: synapses look out of the nervous system. Cell Mol Life Sci. 2011;68:2655–2666. doi: 10.1007/s00018-011-0664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alenius M, Bohm S. Identification of a novel neural cell adhesion molecule-related gene with a potential role in selective axonal projection. J Biol Chem. 1997;272:26083–26086. doi: 10.1074/jbc.272.42.26083. [DOI] [PubMed] [Google Scholar]
- 54.Campos B, Warta R, Chaisaingmongkol J, Geiselhart L, Popanda O, Hartmann C, von Deimling A, Unterberg A, Plass C, Schmezer P, Herold-Mende C. Epigenetically mediated downregulation of the differentiation-promoting chaperon protein CRABP2 in astrocytic gliomas. Int J Cancer. 2012;131:1963–1968. doi: 10.1002/ijc.27446. [DOI] [PubMed] [Google Scholar]
- 55.Konac E, Varol N, Yilmaz A, Menevse S, Sozen S. DNA methyltransferase inhibitor-mediated apoptosis in the Wnt/β-catenin signal pathway in a renal cell carcinoma cell line. Exp Biol Med. 2013;238:1009–1016. doi: 10.1177/1535370213498984. [DOI] [PubMed] [Google Scholar]
- 56.Rose RA, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol. 2008;586:353–366. doi: 10.1113/jphysiol.2007.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gjerstorff MF, Ditzel HJ. An overview of the GAGE cancer/ testis antigen family with the inclusion of newly identified members. Tissue Antigens. 2008;71:187–192. doi: 10.1111/j.1399-0039.2007.00997.x. [DOI] [PubMed] [Google Scholar]