Abstract
Caregiving may be burdensome to caregivers, negatively affecting health and impacting decisions to institutionalize patients. It is unclear how caregiver depression changes over longer periods or whether heterogeneous trajectories for caregivers are apparent. The goals of this article are to characterize the course of depressive symptoms among caregivers over time and to examine the impact of baseline patient and caregiver characteristics on these trajectories. Patients with dementia and their caregivers were followed every 6 months for up to 6 years or until death (n = 133). Growth mixture modeling identified trajectories of caregiver depression over time. Most caregivers had stable trajectories of symptoms, with a smaller subset showing evidence of wear-and-tear. Patient clinical characteristics had no impact on symptom course for caregivers. Future work should utilize a longitudinal perspective and consider that there may be heterogeneous trajectories for caregivers. Those caregivers who follow a wear-and-tear trajectory may require targeted interventions to improve outcomes.
INTRODUCTION
The patient-caregiver relationship is extending as patients are living longer with chronic illnesses such as dementia. The average caregiver serves in their caregiving capacity for 4.6 years (National Alliance for Caregiving & AARP, 2009). Caregivers of people with Alzheimer disease (AD) and other dementias provide more hours of help, on average, than caregivers of other older people and serve in their caregiving role for longer periods of time. According to the 2012 report from the Alzheimer’s Association, 32% of caregivers serve in their role for 5 or more years (Alzheimer’s Association, 2012). However, the majority of studies on dementia caregiving are cross-sectional (Pinquart & Sorensen, 2003). Far fewer major caregiving studies to date (e.g., Alspaugh, Stephens, Townsend, Zarit, & Greene, 1999; Aneshensel, Pearlin, Mullan, Zarit, & Whitlach, 1995; Mittelman, Roth, Haley, & Zarit, 2004) adopt longitudinal designs to determine how caregivers respond to stress of caregiving over time.
The stress process model has been used to conceptualize caregiving as an exposure to multiple long-term stressors (Pearlin, Mullan, Semple, & Skaff, 1990). This model differentiates between the objective, more concrete stressors (e.g., behavior symptoms or functional dependence) and the caregiver’s subjective experience of those stressors, or the caregiver’s internal response to the stressors (e.g., role overload or role captivity). In this model, the subjective appraisal of care-related stress (how difficult the caregiver perceives the stressor to be) is critical to understanding the effects of potential stressors. This model provides a framework for conceptualizing the three possible ways a caregiver may respond to the objective stressors of caregiving over time.
First, caregivers may have increased “wear and tear” or stress over time with increasing negative repercussions for mental health (Townsend, Noelker, Deimling, & Bass, 1989). For example, the spouse of a patient with dementia may find that over time caring for her husband and managing the increased functional limitations and behavioral symptoms becomes more challenging, resulting in increased depression. An alternative trajectory is “adaptation” (Townsend et al., 1989), which proposes that over time caregivers adapt to stressful situations and become less negative in their stress appraisals. In other words, the caregiver adapts to the cognitive changes in the patient and may consequently change expectations for their relationship with the patient which mitigates negative effects of the stressor. Caregivers can acclimate to their circumstances, experiencing little change or even improvement over time. While the caregiver may initially find it difficult to accept that their spouse is prone to verbal outbursts and is easily agitated, over time they may become more tolerant of these behaviors and appreciate the limited positive interactions they have with the patient. For example, a spouse may fare worse in the beginning of their caregiving role, but after adjusting to their tasks and even experiencing positive rewards from their work, they adjust and their stress level declines and their mood improves, despite the presence of increasingly challenging behaviors in their spouse. Finally, caregivers may also maintain a constant or stable level of depressive symptoms or reported feelings of burden despite patient decline over time. In other words, despite patient cognitive and functional decline and potential increases in behavioral disturbances, the caregiver’s outcomes do not change.
Several longitudinal studies have examined change in mental health of caregivers over varied periods of time and have even sought to characterize the overall course of symptoms throughout the caregiving experience. While a handful of studies support the wear and tear hypothesis (i.e., evidence that for some individuals, stress or depression increases over time) (Mittelman et al., 2004; Pot, Deeg, & Van Dyck, 1997; Roth, Haley, Owen, Clay, & Goode, 2001), the majority of studies to date support the adaptation or stability hypotheses and suggest that distress and depression levels reach a plateau and may even decrease over time (Alspaugh et al., 1999; Aneshensel et al., 1995; Danhauer, McCann, Gilley, Beckett, Bienias, & Evans, 2004; Gaugler, Davey, Pearlin, & Zarit, 2000; Goode, Haley, Roth, & Ford, 1998; Li, Seltzer, & Greenberg, 1999; Powers, Gallagher-Thompson, & Kraemer, 2002; Schulz & Williamson, 1991; Townsend et al., 1989). The caregiver bereavement literature have identified multiple and distinct trajectories of caregiver depressive symptoms following a patient’s death (Aneshensel, Botticello, & Yamamoto-Mitani, 2004; Bonanno, Wortman, & Nesse, 2004; Zhang, Mitchell, Bambauer, Jones, & Prigerson, 2008). For example, Zhang and colleagues (2008) find three heterogeneous trajectories of depression among bereaved dementia caregivers and Bonanno et al. (2004) distinguished five unique trajectories of bereavement outcome among older adults losing spouses: common grief, chronic grief, chronic depression, depression followed by improvement, and resilience.
Only one study to date has distinguished between groups of non-bereaved caregivers of dementia to determine if there are varied symptom trajectories (Taylor, Ezell, Kuchibhatla, Ostbye, & Clipp, 2008). Taylor and colleagues found that among female spousal caregivers followed up to 3 years, there were four unique trajectories of depressive symptoms representing different levels of symptoms (high, moderate, low, and very low) that each remained stable. Further analyses are necessary over longer time periods in order to assess whether caregivers experience more problematic wear and tear trajectories that may require targeted intervention.
Study Aims
The overall aim of this study is to characterize the course of depressive symptoms among caregivers over time and determine if there are distinct symptom trajectories using latent curve analysis. Specifically, we are interested in determining if there is a distinct group of caregivers who have increased depressive symptoms over time. We will also explore whether baseline patient and caregiver characteristics are associated with emergent trajectories in order to identify those caregivers at risk of decline who may require long-term targeted interventions.
METHODS
Sample
The “Predictors 2” multi-site observational cohort study was initiated in 1997 to understand the natural course of dementia. The cohort consists of patients with probable AD and dementia with Lewy Bodies (DLB) who were followed prospectively from early stages of patient illness. Patients were recruited from memory disorder centers or private physician offices in three sites between 1997 and 2008: Columbia University College of Physicians and Surgeons; Johns Hopkins University School of Medicine; and Massachusetts General Hospital. Study inclusion and exclusion criteria and evaluation procedures have been fully described elsewhere (Stern, Folstein, Albert, Richards, Miller, Bylsma, et al., 1993). Briefly, all patients were diagnosed in a consensus conference with at least two faculty physicians specializing in dementia and one faculty neuropsychologist. All AD patients met NINCDS-ADRDA criteria for probable AD (McKhann, Drachman, Folstein, Katzman, Price, & Stadlan, 1984) and intellectual impairment was documented with neuropsychological testing. At entry into study, each AD participant was required to have relatively mild dementia operationalized as a modified Mini Mental State Examination (mMSE) (Stern, Sano, Paulson, & Mayeux, 1987) score > = 30, equivalent to a score of > = 16 on the Folstein Mini Mental State Examination (Hale, Cochran, & Hedgepeth, 1984). Patients with DLB were diagnosed according to the 1996 consensus guidelines for the disease (McKeith, Galasko, Kosaka, Perry, Dickson, Hansen, et al., 1996). Participants were also required to have at least one informant (family member or paid caregiver) available. Exclusion criteria were stroke, alcoholism, schizophrenia, schizoaffective disorder, and electroconvulsive treatments.
At baseline, the following data were collected about the patient via clinical assessment: patient demographic data, medical history, neurological evaluation, handedness, presenting features of cognitive impairment, functional status, family history of dementia, onset dating and features, and behavioral and psychological symptoms of dementia (BPSD). Follow-up data were collected at 6-month intervals via outpatient visit thereafter until dropout or death. If patients were unable to travel to the outpatient clinic for evaluation, they were visited at their homes, nursing homes, or healthcare facilities. Patients who did not respond at a particular visit could respond at a subsequent visit.
Beginning in 2004, we initiated the collection of detailed data on the demographics, mental health, and care activities provided by the family caregivers of the Predictors 2 patient cohort, regardless of patient date of entry into the study. This cohort of caregivers was called the “Caregiver Study.” Follow-up data on caregiver mental health status, level of care, and living situation were collected at 6 month intervals up to 6 years simultaneous to the collection of patient data. A total of 169 patients were active in the Predictors 2 cohort at the time of, or subsequent to, the onset of the Caregiver Study. Of these patients, six did not have an eligible informal caregiver (family member actively involved in day-to-day care for patients) to complete the study (3.6%). Of the 163 eligible patient-caregiver dyads, 98.2% have caregiver data available for at least one assessment; three caregivers refused to answer questions on their experiences as a caregiver. Caregiver depression data with at least two time points for analysis of longitudinal data were available for n = 133 patient-caregiver dyads (83% of total caregiver sample).
Measures
Caregiver depressive symptoms were measured at 6 month intervals by the six-item depression subsection of the brief symptom inventory (BSI) (Derogatis, 1993). Caregivers were asked how much during the past week they were bothered by the following: feeling lonely, feeling blue, feeling no interest in things, feeling hopeless about the future, feelings of worthlessness, and thoughts of ending your life using a 5-point Likert scale response for each item ranging from “not at all” to “extremely.” A higher score indicates higher depressive symptoms. The standardized Cronbach’s coefficient alpha was >.80, indicating acceptable reliability. Because data were highly skewed, we chose to dichotomize BSI scores as an outcome variable. There is no standard cutpoint for clinical depression for the BSI. Mean BSI scores in our sample were 1.4 and previously published mean BSI depression scores for female elderly caregivers is < 1.0 (Anthony-Bergstone, Zarit, & Gatz, 1988). Therefore, based on previous work in our group (Ornstein, Gaugler, Devanand, Scarmeas, Zhu, & Stern, 2012), we used conservative cutpoint BSI score of 2 in order to discern meaningful differences in depressive symptoms among our sample of dementia caregivers. By dichotomizing BSI scores as few to no depressive symptoms (< 2) and depressive symptoms (> = 2), caregivers categorized as having depressive symptoms were (a) one SD above the mean depressive symptom score and (b) indicated that on average each of the six symptoms bothered or impacted them from a minimal to extreme level.
Patient and Caregiver Characteristics
In addition to patient and caregiver demographic data, the following baseline patient and caregiver characteristics were examined as sources of variation among caregiving trajectories based on previous literature:
The Columbia University Scale for Psychopathology in Alzheimer’s Disease (CUSPAD) (Devanand, Miller, Richards, Marder, Bell, Mayeux, et al., 1992) was used to measure the presence of patient BPSD. The CUSPAD is a semistructured rating scale that a clinician or research assistant administers to the informant regarding the presence of 26 patient symptoms during the last month before each interview. Patient cognitive status was assessed using the mMSE (Stern et al., 1987) and functional status was assessed using parts I and II of the Blessed Dementia Rating Scale (BDRS) (Blessed, Tomlinson, & Roth, 1968). Patients’ medical histories were used to construct a modified version of the Charlson Index of Comorbidity (Charlson, Pompei, Ales, & MacKenzie, 1987). A modified Unified Parkinson’s Disease Rating Scale (Marcus, Marder, Bell, Dooneief, Mayeux, & Stern, 1991) was administered at each visit to measure the presence or absence of extrapyramidal signs (EPS) (e.g., tremors, rigidity). A dichotomous indicator was constructed for the use of EPS if any of the following 11 items were rated 2 or higher (0 being normal and 4 indicating maximum impairment): speech, facial expression, tremor at rest, neck rigidity, right arm rigidity, left arm rigidity, right leg rigidity, left leg rigidity, posture, gait, and bradykinesia). Duration of illness in years was estimated by a neurologist based on baseline interviews with the patient and caregiver. Whether the caregiver assisted with basic and instrumental activities of daily living, the amount of hours the patient spent per day with the caregiver, whether a home health aide/home attendant assisted with care, and caregiver’s employment status were reported by the caregiver.
Statistical Analysis
Descriptive Statistics
Change in caregiver depressive symptoms over time were examined by comparing the difference in first and last total BSI scores for each caregiver.
Trajectory Analysis
In order to determine whether there were distinct trajectories of change in caregiver depression over time, we used growth mixture modeling (GMM) (Andruff, Carraro, Thompson, Gaudreau, & Louvet, 2009). While conventional growth curve modeling treats data as part of a homogenous population with a common growth process, GMM hypothesizes that there are a fixed but unknown number of trajectory patterns observed within the population. Consequently, GMM enables us to test whether more than one distinct class can be used to describe the data. Each class possesses unique latent factors of growth that distinguishes subjects from those in a different subpopulation. For example, a sample may include one group who starts the study with a low level of a factor of interest and shows rapid increase while another group starts the study with a high level of the same factor and shows stability over time. GMM is a class of finite mixture models that assumes the population consists of a mixture of an unknown number of distributions and can accommodate different distributional forms (count data, binary, continuous). GMM makes use of all data despite attrition using the Full Information Maximum Likelihood method of estimation (FIML). GMM assumes all data are missing at random (MAR) and does not assume growth trajectories in a sample are homogenous (e.g., linear). We used the customized SAS procedure TRAJ to identify and describe distinct patterns of trajectories in caregiver depression (Jones, Nagin, & Roeder, 2001).
We first estimated a model with an intercept only and then added a linear and quadratic growth factor to determine the form of the growth model. We then proceeded to identify the number of trajectory classes. The number of trajectory classes is determined by sequentially increasing the number of classes, and examining the results and fit statistics. The optimal number of groups to form relatively homogenous clusters with similar trajectories is determined using Bayesian Information Criteria, with smaller values indicating better fit (Jones et al., 2001; Nagin & Tremblay, 2001). Because group membership is probabilistic (i.e., not observed), misclassification may occur and probabilities of group assignment must be evaluated. Posterior probabilities of belonging to each of the hypothetical groups defined by the trajectories were calculated from model parameter estimates, and the highest value was used to assign each caregiver to one group (Nagin, 1999).
After determining the number of trajectory classes, we examined the bivariate associations between the emerging caregiver depression classes and baseline patient demographic and clinical features, and caregivers’ demographic characteristics and level of care using chi-square tests for categorical variables, t-tests for normal continuous variables, and Kruskal Wallis test for non-normal continuous variables.
All analyses were conducted using SAS version 9.2.
RESULTS
The mean number of assessments available per caregiver was 4.84 (median = 4). Twenty-two percent of caregivers had two assessments (n = 29), 17% had three assessments (n = 22), 14% had four assessments (n = 18), 12% had five assessments (n = 16), 8% had six assessments (n = 10), 14% had seven assessments (n = 19), and 14% had between eight and twelve assessments completed (n = 19).
Overall we found that caregiver depression scores stayed the same or increased over time for most participants, with a much smaller percentage of caregivers showing a decrease. Only 21% had a decrease in depressive symptoms. The mean caregiver assessment score at baseline was 1.33 (SD = .53) and at last assessment was 1.51 (SD = .61). The average change score from first to last caregiver assessment was .18 (SD = .20) ranging from −2 to 1.83.
Trajectories
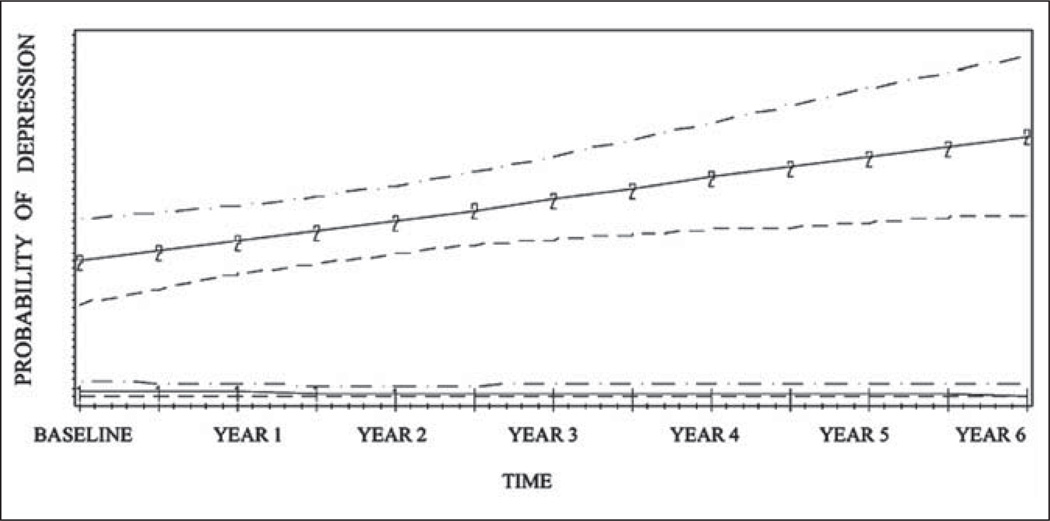
Using GMM, we identified two trajectories of caregiver depressive symptoms (Figure 1) which are depicted graphically with 95% confidence intervals. The most common trajectory represented 69% of all caregivers (n = 92) and was characterized by a consistently low probability of having depressive symptoms that remained stable over every time point (hereafter called “stable caregivers”). The remainder of the sample (n = 41; 31%) consisted of caregivers with a higher baseline risk of depression with a slight but steady increase over time in risk of depressive symptoms (hereafter called “wear-and-tear caregivers”). The three trajectory models examined included a small additional group (< 5% of caregivers) that had a steeper increase in symptoms before stabilizing over time in addition to the aforementioned groups of stable and wear-and-tear caregivers. However, standard fit statistics determined that the two trajectory class models best described the data (Table 1).
Figure 1.
Six-year trajectories of caregiver depression measured at 6 month time intervals and 95% confidence intervals (n = 133).
Note: 1-1-1-1: stable caregivers; 2-2-2-2: wear-and-tear caregivers.
Table 1.
Model Fit for Latent Class Analysis of Caregiver Depressive Symptoms
| No. of classes | AIC | BIC | SSABIC |
|---|---|---|---|
| 1 | −281.5 | −286 | −284.4 |
| 2 | −240.3 | −251.5 | −247.6 |
| 3 | −239.3 | −257.2 | −250.9 |
| 4 | −242.3 | −266.9 | −258.24 |
Note: AIC = Akaike information criteria; BIC = Bayesian information criteria; SSABIC = sample size adjusted Bayesian Information Criteria.
The average posterior probability of membership was 91% (stable caregivers) and 95% (wear-and-tear caregivers), suggesting a good level of correct group assignment. Minimum probabilities are all well above .50, suggesting that caregivers assigned to a group are more likely to belong to that group than not.
Characteristics of Groups Defined by Depressive Symptom Trajectories
As shown in Table 2, initial patient clinical characteristics such as cognitive status, functional status, presence of other medical comorbidities or EPS, presence of BPSD, and amount of time since dementia diagnosis did not differ for stable and wear-and-tear caregivers. The only differences between the groups were: gender, the relationship between the patients and caregivers, and the amount of time they spent together. Spouses of male patients and those who spent at least 12 hours a day with the patient at baseline were significantly more likely (p < .05) to be wear-and-tear caregivers. There was also a trend for caregivers who were less likely to work (part-time or full-time), older, live with the patient, and whose care-receiver was not in a nursing home to be more likely to experience a wear-and-tear trajectory.
Table 2.
Baseline Patient and Caregivers Characteristics by Caregiver Depression Trajectory Class
| Characteristic | Categories | Class 1: (n = 92) Stable caregivers |
Class 2: (n = 41) Wear-and-tear caregivers |
p-Value |
|---|---|---|---|---|
| Patient demographic and clinical characteristics | ||||
| Patient age | Mean ± SD | 75.2 | 76.78 | .25 |
| Patient gender |
Female Male |
63.04% 37.96% |
39.02% 60.98% |
.01 |
| Patient ethnicity | White | 90.22% | 90.44% | .99 |
| Site | Columbia University Johns Hopkins Mass General |
44.57% 23.91% 31.52% |
51.22% 19.51% 29.27% |
.76 |
| Diagnosis | Alzheimer’s disease dementia with Lewy Bodies |
85.87% 14.13% |
87.8% 12.2% |
.76 |
| Lives in nursing home | Yes | 31.9% | 17.1% | .09 |
| Neurologist estimation of duration of illness in years | Mean ± SD (range 1–18) | 7.57 (3.32) | 6.73 (2.7) | .17 |
| Modified Mini-mental state examination score at study baseline | Mean ± SD (range 9–30) | 17.59 | 19.06 | .34 |
| Blessed functional activity scale score at study baseline | Mean ± SD (range 0–13) | 6.95 | 7.41 | .53 |
| Presence of any behavioral symptom at baseline | Yes | 54.44% | 57.5% | .75 |
| Modified comorbidity index | 0 ≥ 1 | 51.14% 48.86 |
52.5% 47.5% |
.89 |
| Extrapyramidal signs | Yes | 14.44% | 12.5% | .77 |
| Caregiver characteristics and activities | ||||
| Caregiver age | Mean ± SD | 63.67 (13.35) |
68.65 (14.47) |
.06 |
| Caregiver gender | Female | 77.17% | 73.17% | .62 |
| Lives with patient | Yes | 54.35% | 70.73% | .08 |
| Relationship to patient | Spouse | 47.83% | 70.83% | .01 |
| Work at least part-time for pay | Yes | 51.25% | 33.33% | .07 |
| Home health aide in last 3 months | Yes | 23.46% | 31.58% | .35 |
| Time spent daily with patient |
None Up to 3 hours 3 to 5 hours 6 to 9 hours 9 to 12 hours More than 12 hours |
2.22% 35.56% 13.33% 12.22% 8.89% 27.78% |
2.44% 17.07% 9.76% 7.32% 4.88% 58.54% |
.04 |
| Assists patient with ADLs | Yes | 17.72% | 29.73% | .14 |
Note: SD = standard deviation; ADLs = Activities of daily living and include bathing, eating; IADLS = Instrumental activities of daily living and include shopping, housekeeping.
DISCUSSION
Similar to other studies, we found that caregiver level of depression was reasonably stable over time; however, we also found a distinct subset of caregivers who followed a wear-and-tear trajectory of decline. We did not find any evidence of a depressive symptom trajectory in which caregivers adapted over time. While most work on caregivers over long periods of time estimates one overall trajectory for caregiver symptoms, these findings suggest it may be more useful to identify disparate trajectories among caregivers. In longitudinal studies of caregivers, we would expect attrition biases to result in more caregivers who are better able to adjust to their roles. Because caregivers were followed beyond patient’s nursing home placement, the study design eliminates attrition biases noted in previous studies, in which only caregivers who can adjust to the challenges of daily patient care remain in follow-up studies (Gaugler, Kane, Kane, & Newcomer, 2005). At baseline, 27% of patients lived in nursing homes and over the course of follow up, 15 patients moved out of their home and into a nursing home.
Although depression levels were low overall in this sample of dementia caregivers, the finding that there is a group of caregivers who have worsening symptoms over time, suggests that this is an important area to continue studying. Because caregivers do not follow a uniform path over long periods of time, we may want to focus intervention efforts on the wear-and-tear caregivers. This is especially critical given limited community resources available to address caregiver mental health needs (Averting the Caregiving Crisis: Why we must act now, 2010; Zarit & Leitsch, 2001).
In this study, early disease behaviors and other clinical characteristics do not appear to determine the course of caregiver depression. Instead, caregivers who are the wives of patients, who are less likely to work, and who spend more time with the patients early in the course of illness, may be at greatest risk for decline. This finding is consistent with past research which finds that women experience greater psychological morbidity from caregiving than men (Pinquart & Sorensen, 2006) and that spouses may have a more negative response to dementia behaviors and caregiving responsibilities than adult children (Pinquart & Sorensen, 2003). Additionally, our finding that caregivers who live with patients are more likely to be wear and tear caregivers is consistent with research finding reduction in caregiver burden and stress following nursing home placement (Gaugler, Mittelman, Hepburn, & Newcomer, 2009, 2010). Future work should continue to discern distinct trajectories using extensive periods of follow-up to identify risk factors for long-term decline, especially among larger samples of caregivers. As we have learned from studies of caregiver bereavement (Aneshensel et al., 2004), caregivers may follow different trajectories over time.
The failure to consider that caregivers follow distinct paths while caring for patients may limit our ability to accurately conceptualize the long-term caregiving experience and to be able to appropriately provide for caregivers. While our findings support previous research that suggest that there may be distinct groups of caregivers in terms of depressive symptomatology over time (Taylor et al., 2008), this is the first study that examines multiple trajectories of caregiver depression for both male and female caregivers for up to 6 years of time. Moreover, it is the first study to our knowledge that uses latent curve analysis to suggest that there may be a distinct group of caregivers with increased depressive symptoms over time. There are some study limitations to note. Because the caregiver study was initiated after the inception of the Predictors 2 cohort, we did not have caregiver data concurrent to all measures of patient symptom behaviors resulting in a truncated view of the assessment of change in caregiver depression over time. A more comprehensive view of change in caregiving symptoms would begin at disease onset. On average, patients had been enrolled for 3 years (range 0–7.5 years) when caregiver data were initially collected for the study and many were no longer in mild stages of dementia. We did not, however, find any association between either cognitive status or neurological estimate of time with illness suggesting that this may not have impacted our findings. Another limitation is this study’s reliance on self-report data. While validated clinical assessments were used for measures of patient function, illness, and clinical characteristics, we relied on caregiver self-report of depressive symptoms. Validated clinical data on caregiver depression and depressive symptoms would have provided greater insight into how caregiver outcomes change over time. This sample had low levels of depression overall and at baseline using a conservative BSI depression cutpoint limiting ability to potentially discern caregivers with more varied courses of depressive symptoms. Future studies should continue to examine depression courses using alternate measures of depression Because GMM is a relatively new technique, determination of sample size and number of time points needs for good estimation and strong power is not definitive (Muthen, 2004), although Monte Carlo simulation studies have been used to determinate estimates (Lubke & Muthen, 2007). While GMM has been successfully applied to similar population samples (e.g., Aneshensel et al., 2004), confirmation of our trajectory patterns in other samples is necessary. We also did not measure caregiver appraisals or secondary stressors which may be important predictors of caregivers’ trajectories. Finally, our examination of baseline patient and caregiver characteristics associated with depression trajectories was exploratory and should be confirmed in larger data sets. Future work should examine patient clinical or behavioral characteristics (and their trajectories) and caregiver characteristics or life events that occur after the baseline assessment for a more complete understanding of what factors impact caregiver depressive trajectories.
Strengths of this study include a sample of dementia patients who were carefully diagnosed in a consensus conference and well-characterized early in their disease course. Furthermore, few longitudinal studies of caregiving consider multiple points of follow-up beyond 1–2 years, thereby compressing analysis of care provision and failing to capture the full spectrum of the prolonged dementia caregiving experience.
CONCLUSIONS
Despite the fact that patients with dementia often, and increasingly, live with their disease for many years such that caregiving is a long-term role, research has not focused on how caregivers respond or adapt to patient behaviors over time. This study finds that there is only a small subset of dementia caregivers who have increasing depressive symptoms over time, but fails to find any connection between patient clinical characteristics at baseline and the course of caregiver depression over time. Importantly, this study suggests that we should consider that the caregiving experience is hardly uniform and should be conceptualized as both heterogeneous and changing over time. Future work should focus on wives who are at high care levels as potentially requiring more intensive intervention to reduce depression.
Contributor Information
Katherine Ornstein, Mount Sinai School of Medicine, New York.
Joseph E. Gaugler, University of Minnesota, Minneapolis
Laura Zahodne, Columbia University Medical Center, New York
Yaakov Stern, Columbia University Medical Center, New York
REFERENCES
- Alspaugh ME, Stephens MA, Townsend AL, Zarit SH, Greene R. Longitudinal patterns of risk for depression in dementia caregivers: Objective and subjective primary stress as predictors. Psychology and Aging. 1999;14(1):34–43. doi: 10.1037//0882-7974.14.1.34. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: A tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- Aneshensel CS, Botticello AL, Yamamoto-Mitani N. When caregiving ends: The course of depressive symptoms after bereavement. Journal of Health and Social Behavior. 2004;45(4):422–440. doi: 10.1177/002214650404500405. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Pearlin LI, Mullan JT, Zarit SH, Whitlach CJ. Profiles in caregiving: The unexpected career. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Anthony-Bergstone CR, Zarit SH, Gatz M. Symptoms of psychological distress among caregivers of dementia patients. Psychology and Aging. 1988;3(3):245–248. doi: 10.1037//0882-7974.3.3.245. [DOI] [PubMed] [Google Scholar]
- Rosalynn Carter Institute for Caregiving. Averting the caregiving crisis: Why we must act now. 2010 [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Wortman CB, Nesse RM. Prospective patterns of resilience and maladjustment during widowhood. Psychology and Aging. 2004;19(2):260–271. doi: 10.1037/0882-7974.19.2.260. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Danhauer SC, McCann JJ, Gilley DW, Beckett LA, Bienias JL, Evans DA. Do behavioral disturbances in persons with Alzheimer’s disease predict caregiver depression over time? Psychology and Aging. 2004;19(1):198–202. doi: 10.1037/0882-7974.19.1.198. [DOI] [PubMed] [Google Scholar]
- Derogatis L. The Brief Symptom Inventory (BSI): Administration, scoring and procedures manual. Minneapolis, MN: NCS Pearson; 1993. [Google Scholar]
- Devanand DP, Miller L, Richards M, Marder K, Bell K, Mayeux R, et al. The Columbia University scale for psychopathology in Alzheimer’s disease. Archives of Neurology. 1992;49(4):371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Davey A, Pearlin LI, Zarit SH. Modeling caregiver adaptation over time: The longitudinal impact of behavior problems. Psychology and Aging. 2000;15(3):437–450. doi: 10.1037//0882-7974.15.3.437. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Kane RL, Kane RA, Newcomer R. The longitudinal effects of early behavior problems in the dementia caregiving career. Psychology and Aging. 2005;20(1):100–116. doi: 10.1037/0882-7974.20.1.100. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Predictors of change in caregiver burden and depressive symptoms following nursing home admission. Psychology and Aging. 2009;24(2):385–396. doi: 10.1037/a0016052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Clinically significant changes in burden and depression among dementia caregivers following nursing home admission. BMC Medicine. 2010;8:85. doi: 10.1186/1741-7015-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode KT, Haley WE, Roth DL, Ford GR. Predicting longitudinal changes in caregiver physical and mental health: A stress process model. Health and Psychology. 1998;17(2):190–198. doi: 10.1037//0278-6133.17.2.190. [DOI] [PubMed] [Google Scholar]
- Hale WD, Cochran CD, Hedgepeth BE. Norms for the elderly on the Brief Symptom Inventory. Journal of Consulting and Clinical Psychology. 1984;52(2):321–322. doi: 10.1037//0022-006x.52.2.321. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- Li LW, Seltzer MM, Greenberg JS. Change in depressive symptoms among daughter caregivers: An 18-month longitudinal study. Psychology and Aging. 1999;14(2):206–219. doi: 10.1037//0882-7974.14.2.206. [DOI] [PubMed] [Google Scholar]
- Lubke G, Muthén B. Performance of factor mixture moels as a function of model size, covariate effects, and class-specific parameters. Structural Equation Modeling. 2007;14(1):26–47. [Google Scholar]
- Marcus R, Marder K, Bell K, Dooneief G, Mayeux R, Stern Y. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Archives in Neurology. 1991;48(11):1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Roth DL, Haley WE, Zarit SH. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: Results of a randomized trial. Journal of Gerontology: Series B Psychological Science and Social Science. 2004;59(1):P27–P34. doi: 10.1093/geronb/59.1.p27. [DOI] [PubMed] [Google Scholar]
- Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage; 2004. pp. 345–368. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychology Methods. 1999;4(2):139. [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychology Methods. 2001;6(1):18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- National Alliance for Caregiving & AARP. Caregiving in the U.S. Washington, DC: National Alliance for Caregiving & AARP; 2009. [Google Scholar]
- Ornstein K, Gaugler JE, Devanand DP, Scarmeas N, Zhu C, Stern Y. The differential impact of unique behavioral and psychological symptoms for the dementia caregiver: How and why do patients’ individual symptom clusters impact caregiver depressive symptoms? American Journal of Geriatric Psychiatry. 2012 doi: 10.1016/j.jagp.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. Gerontologist. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta-analysis. Journal of Gerontology: Series B Psychological Science and Social Science. 2003;58(2):P112–P128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Gender differences in caregiver stressors, social resources, and health: An updated meta-analysis. Journal of Gerontology: Series B Psychological Science and Social Science. 2006;61(1):P33–P45. doi: 10.1093/geronb/61.1.p33. [DOI] [PubMed] [Google Scholar]
- Pot AM, Deeg DJH, Van Dyck R. Psychological well-being of informal caregivers of elderly people with dementia: Changes over time. Aging & Mental Health. 1997;1(3):261–268. [Google Scholar]
- Powers DV, Gallagher-Thompson D, Kraemer HC. Coping and depression in Alzheimer’s caregivers: Longitudinal evidence of stability. Journal of Gerontology: Series B Psychological Science and Social Science. 2002;57(3):P205–P211. doi: 10.1093/geronb/57.3.p205. [DOI] [PubMed] [Google Scholar]
- Roth DL, Haley WE, Owen JE, Clay OJ, Goode KT. Latent growth models of the longitudinal effects of dementia caregiving: A comparison of African American and White family caregivers. Psychology and Aging. 2001;16(3):427–436. [PubMed] [Google Scholar]
- Schulz R, Williamson GM. A 2-year longitudinal study of depression among Alzheimer’s caregivers. Psychology and Aging. 1991;6(4):569–578. doi: 10.1037//0882-7974.6.4.569. [DOI] [PubMed] [Google Scholar]
- Stern Y, Folstein M, Albert M, Richards M, Miller L, Bylsma F, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Disease and Associated Disorders. 1993;7(1):3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: Validity and reliability. Neurology. 1987;37(2) Suppl 1:179–179. [Google Scholar]
- Taylor DH, Jr, Ezell M, Kuchibhatla M, Ostbye T, Clipp EC. Identifying trajectories of depressive symptoms for women caring for their husbands with dementia. Journal of the American Geriatric Society. 2008;56(2):322–327. doi: 10.1111/j.1532-5415.2007.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A, Noelker L, Deimling G, Bass D. Longitudinal impact of interhousehold caregiving on adult children’s mental health. Psychology and Aging. 1989;4(4):393–401. doi: 10.1037//0882-7974.4.4.393. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Leitsch SA. Developing and evaluating community based intervention programs for Alzheimer’s patients and their caregivers. Aging Mental Health. 2001;5(Suppl 1):S84–S98. doi: 10.1080/13607860120044864. [DOI] [PubMed] [Google Scholar]
- Zhang B, Mitchell SL, Bambauer KZ, Jones R, Prigerson HG. Depressive symptom trajectories and associated risks among bereaved Alzheimer disease caregivers. American Journal of Geriatric Psychiatry. 2008;16(2):145–155. doi: 10.1097/JGP.0b013e318157caec. [DOI] [PubMed] [Google Scholar]