Abstract
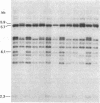
Susceptibility to multiple sclerosis (MS) has been linked to the immunoglobulin G (Gm) markers as well as HLA-DR genes. We have used a genomic Ig gamma 1 probe which detects polymorphisms in the gamma 1, gamma 2, gamma 3 and pseudogamma genes to identify restriction fragment length polymorphisms associated with MS. A negative association was found between a 5.9-kilobase (kb) Bst EII gamma 3 fragment and MS. Southern blot analysis of genomic DNA revealed the presence of this fragment in 84 of 140 (60.0%) controls, but in only 17 of 59 (28.8%) MS patients. The frequency of the fragment in 47 myasthenia gravis and 16 Graves' disease patients was similar to that in controls, 60.0 and 62.5%, respectively.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batchelor J. R., Compston A., McDonald W. I. The significance of the association between HLA and multiple sclerosis. Br Med Bull. 1978 Sep;34(3):279–284. doi: 10.1093/oxfordjournals.bmb.a071512. [DOI] [PubMed] [Google Scholar]
- Brautbar C., Alter M., Kahana E. HLA antigens in multiple sclerosis. Neurology. 1976 Jun;26(6 Pt 2):50–53. doi: 10.1212/wnl.26.6_part_2.50. [DOI] [PubMed] [Google Scholar]
- Farid N. R., Newton R. M., Noel E. P., Marshall W. H. Gm phenotypes in autoimmune thyroid disease. J Immunogenet. 1977 Dec;4(6):429–432. doi: 10.1111/j.1744-313x.1977.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Feder J., Yen L., Wijsman E., Wang L., Wilkins L., Schroder J., Spurr N., Cann H., Blumenberg M., Cavalli-Sforza L. L. A systematic approach for detecting high-frequency restriction fragment length polymorphisms using large genomic probes. Am J Hum Genet. 1985 Jul;37(4):635–649. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Haile R. W., Goldstein A., Field L., Marazita M. L. A linkage analysis of the Gm locus and multiple sclerosis. Genet Epidemiol. 1985;2(1):29–34. doi: 10.1002/gepi.1370020104. [DOI] [PubMed] [Google Scholar]
- Johnson M. J., de Lange G., Cavalli-Sforza L. L. Ig gamma restriction fragment length polymorphisms indicate an ancient separation of Caucasian haplotypes. Am J Hum Genet. 1986 May;38(5):617–640. [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Weiss J. B., Brown R. J., Lee T., Schanfield M. S. Immunoglobulin allotype markers in gluten-sensitive enteropathy. Lancet. 1983 Apr 30;1(8331):952–953. doi: 10.1016/s0140-6736(83)92080-9. [DOI] [PubMed] [Google Scholar]
- Koprowski H., DeFreitas E. C., Harper M. E., Sandberg-Wollheim M., Sheremata W. A., Robert-Guroff M., Saxinger C. W., Feinberg M. B., Wong-Staal F., Gallo R. C. Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature. 1985 Nov 14;318(6042):154–160. doi: 10.1038/318154a0. [DOI] [PubMed] [Google Scholar]
- Kurdi A., Ayesh I., Abdallat A., Maayta U. Different B lymphocyte alloantigens associated with multiple sclerosis in Arabs and North Europeans. Lancet. 1977 May 28;1(8022):1123–1125. doi: 10.1016/s0140-6736(77)92383-2. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F., Hyllested K. Multiple sclerosis in the Faroe Islands: I. Clinical and epidemiological features. Ann Neurol. 1979 Jan;5(1):6–21. doi: 10.1002/ana.410050104. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Einarson B. L., Lennon V. A., Seybold M. E. Pathological mechanisms in experimental autoimmune myasthenia gravis. I. Immunogenicity of syngeneic muscle acetylcholine receptor and quantitative extraction of receptor and antibody-receptor complexes from muscles of rats with experimental automimmune myasthenia gravis. J Exp Med. 1976 Sep 1;144(3):726–738. doi: 10.1084/jem.144.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone N., Feder J., Cann H., van West B., Hwang J., Takahashi N., Honjo T., Piazza A., Cavalli-Sforza L. L. Multiple DNA fragment polymorphisms associated with immunoglobulin mu chain switch-like regions in man. Proc Natl Acad Sci U S A. 1983 Jan;80(2):467–471. doi: 10.1073/pnas.80.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A., Scherz R., Käser H., Skvaril F. Evidence for an association between uncommon Gm phenotypes and neuroblastoma. Lancet. 1977 Jan 1;1(8001):23–24. doi: 10.1016/s0140-6736(77)91657-9. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Mizuno N., Arima N., Wakisaka A., Okimoto K., Akazawa Y., Tsuji K., Fujita T. IgG heavy-chain (Gm) allotypes and immune response to insulin in insulin-requiring diabetes mellitus. N Engl J Med. 1981 Feb 12;304(7):407–409. doi: 10.1056/NEJM198102123040706. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Nishitani H., Ota K., Fujita T., Tsuji K. Gm allotypes in myasthenia gravis. Lancet. 1980 Mar 29;1(8170):677–680. [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Nishitani H., Takatsuki K., Kasukawa R., Nakayama S., Izumi S., Fujita T., Tsuji K. IgG heavy chain allotypes (Gm) in autoimmune diseases. Clin Exp Immunol. 1980 Oct;42(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Ockhuizen T., Pandey J. P., Veltri R. W., Arlen M., Fudenberg H. H. Immunoglobulin allotypes in patients with squamous cell carcinoma of the head and neck. Cancer. 1982 May 15;49(10):2021–2024. doi: 10.1002/1097-0142(19820515)49:10<2021::aid-cncr2820491013>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Palmer D. L., Minard B. J., Cawley L. P. Letter: IgG subgroups in cerebrospinal fluid in multiple sclerosis. N Engl J Med. 1976 Feb 19;294(8):447–448. doi: 10.1056/nejm197602192940821. [DOI] [PubMed] [Google Scholar]
- Pandey J. P., Fudenberg H. H., Virella G., Kyong C. U., Loadholt C. B., Galbraith R. M., Gotschlich E. C., Parke J. C., Jr Association between immunoglobulin allotypes and immune responses to Haemophilus influenzae and Meningococcus polysaccharides. Lancet. 1979 Jan 27;1(8109):190–192. doi: 10.1016/s0140-6736(79)90584-1. [DOI] [PubMed] [Google Scholar]
- Pandey J. P., Goust J. M., Salier J. P., Fudenberg H. H. Immunoglobulin G heavy chain (Gm) allotypes in multiple sclerosis. J Clin Invest. 1981 Jun;67(6):1797–1800. doi: 10.1172/JCI110220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey J. P., Zollinger W. D., Fudenberg H. H., Loadholt C. B. Immunoglobulin allotypes and immune response to meningococcal group B polysaccharide. J Clin Invest. 1981 Nov;68(5):1378–1380. doi: 10.1172/JCI110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M. S., Safai B., Myskowski P. L., Gold J. W., Pandey J., Dupont B. Frequencies of HLA and Gm immunogenetic markers in Kaposi's sarcoma. Tissue Antigens. 1983 Jan;21(1):1–8. doi: 10.1111/j.1399-0039.1983.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ryder L. P., Svejgaard A., Dausset J. Genetics of HLA disease association. Annu Rev Genet. 1981;15:169–187. doi: 10.1146/annurev.ge.15.120181.001125. [DOI] [PubMed] [Google Scholar]
- Salier J. P., Glynn P., Goust J. M., Cuzner M. L. Distribution of nominal and latent IgG (Gm) allotypes in plaques of multiple sclerosis brain. Clin Exp Immunol. 1983 Dec;54(3):634–640. [PMC free article] [PubMed] [Google Scholar]
- Salier J. P., Goust J. M., Pandey J. P., Fudenberg H. H. Preferential synthesis of the G1m(1) allotype of IgG1 in the central nervous system of multiple sclerosis patients. Science. 1981 Sep 18;213(4514):1400–1402. doi: 10.1126/science.6973823. [DOI] [PubMed] [Google Scholar]
- Sanders P. A., de Lange G. G., Dyer P. A., Grennan D. M. Gm and Km allotypes in rheumatoid arthritis. Ann Rheum Dis. 1985 Aug;44(8):529–532. doi: 10.1136/ard.44.8.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanfield M. S., Wells J. V., Fudenberg H. H. Immunoglobulin allotypes and response to tetanus toxoid in Papua, New Guinea. J Immunogenet. 1979 Oct;6(5):311–315. doi: 10.1111/j.1744-313x.1979.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Schultheis W., Peter H. H., Deicher H. Gm(1) and Gm(2) immunoglobulin allotypes in patients with malignant melanoma. Humangenetik. 1975 Jul 23;28(3):177–181. doi: 10.1007/BF00278542. [DOI] [PubMed] [Google Scholar]
- Sheremata W. A., Poskanzer D. C., Withum D. G., MacLeod C. L., Whiteside M. E. Unusual occurrence on a tropical island of multiple sclerosis. Lancet. 1985 Sep 14;2(8455):618–618. doi: 10.1016/s0140-6736(85)90621-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Nathanson N. The genetics of susceptibility to multiple sclerosis. Epidemiol Rev. 1982;4:45–65. doi: 10.1093/oxfordjournals.epirev.a036251. [DOI] [PubMed] [Google Scholar]
- Svejgaard A., Platz P., Ryder L. P. HLA and disease 1982--a survey. Immunol Rev. 1983;70:193–218. doi: 10.1111/j.1600-065x.1983.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Uno H., Sasazuki T., Tamai H., Matsumoto H. Two major genes, linked to HLA and Gm, control susceptibility to Graves' disease. Nature. 1981 Aug 20;292(5825):768–770. doi: 10.1038/292768a0. [DOI] [PubMed] [Google Scholar]
- Vandvik B., Natvig J. B., Wiger D. IgG1 subclass restriction of oligoclonal IgG from cerebrospinal fluids and brain extracts in patients with multiple sclerosis and subacute encephalitides. Scand J Immunol. 1976;5(4):427–436. doi: 10.1111/j.1365-3083.1976.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Vartdal F., Vandvik B. Multiple sclerosis: subclasses of intrathecally synthesized IgG and measles and varicella zoster virus IgG antibodies. Clin Exp Immunol. 1983 Dec;54(3):641–647. [PMC free article] [PubMed] [Google Scholar]
- Visscher B. R., Sullivan C. B., Detels R., Madden D. L., Sever J. L., Terasaki P. I., Park M. S., Dudley J. P. Measles antibody titers in multiple sclerosis patients and HLA-matched and unmatched siblings. Neurology. 1981 Sep;31(9):1142–1145. doi: 10.1212/wnl.31.9.1142. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Ellison G. W. A working protocol to be used as a guideline for trials in multiple sclerosis. Arch Neurol. 1983 Oct 21;40(11):704–710. doi: 10.1001/archneur.1983.04050100044018. [DOI] [PubMed] [Google Scholar]
- Weitkamp L. R., Nee L., Keats B., Polinsky R. J., Guttormsen S. Alzheimer disease: evidence for susceptibility loci on chromosomes 6 and 14. Am J Hum Genet. 1983 May;35(3):443–453. [PMC free article] [PubMed] [Google Scholar]
- Wells J. V., Fudenberg H. H., MacKay I. R. RElation of the human antibody response to flagellin to GM genotype. J Immunol. 1971 Dec;107(6):1505–1511. [PubMed] [Google Scholar]
- Whittingham S., Mathews J. D., Schanfield M. S., Matthews J. V., Tait B. D., Morris P. J., Mackay I. R. Interactive effect of Gm allotypes and HLA-B locus antigens on the human antibody response to a bacterial antigen. Clin Exp Immunol. 1980 Apr;40(1):8–15. [PMC free article] [PubMed] [Google Scholar]
- Whittingham S., Mathews J. D., Schanfield M. S., Tait B. D., Mackay I. R. Interaction of HLA and Gm in autoimmune chronic active hepatitis. Clin Exp Immunol. 1981 Jan;43(1):80–86. [PMC free article] [PubMed] [Google Scholar]
- Willcox N., Demaine A. G., Newsom-Davis J., Welsh K. I., Robb S. A., Spiro S. G. Increased frequency of IgG heavy chain marker Glm(2) and of HLA-B8 in Lambert-Eaton myasthenic syndrome with and without associated lung carcinoma. Hum Immunol. 1985 Sep;14(1):29–36. doi: 10.1016/0198-8859(85)90062-x. [DOI] [PubMed] [Google Scholar]
- Zibetti A., Cazzullo C. L., Smeraldi E., Scorza-Smeraldi R. HLA typing on Italian multiple sclerosis population. Boll Ist Sieroter Milan. 1978 Jan 31;56(6):539–543. [PubMed] [Google Scholar]
- de Lange G., Wright P., van Eede P., van Leeuwen F., Hoang T. L., Nguyen T. D. Association between leprosy and immunoglobulin allotypes: Gm-A2m and Km frequencies in Vietnamese. J Immunogenet. 1984 Jun-Aug;11(3-4):173–180. doi: 10.1111/j.1744-313x.1984.tb01054.x. [DOI] [PubMed] [Google Scholar]