Abstract
Despite evidence suggesting a role for cerebellar abnormalities in the pathogenesis of psychosis, the structure has yet to receive attention in individuals at ultrahigh risk for psychosis (UHR). Accumulating research has suggested that the cerebellum helps modulate cognition and movement, domains in which UHR individuals show impairment; understanding putative markers of risk, such as structural abnormalities and behavioral correlates, is essential. In this study, participants underwent a high-resolution structural brain scan and participated in a pursuit rotor experiment. Cerebellar regions associated with movement (anterior cerebellum) and cognition (crus I) were subsequently analyzed. UHR participants showed impaired performance on the pursuit rotor task, learned at a slower rate, and showed smaller cerebellar volumes compared with control participants. Left crus I volume was significantly associated with poor rate of learning. The present results suggest that cerebellar abnormalities and their behavioral correlates (poor learning and motor control) precede the onset of psychosis.
Keywords: procedural learning, cerebellum, MRI, prodrome, ultrahigh risk, psychosis
A growing number of studies have challenged the long-held assumption that the cerebellum is primarily involved in the control of movement. A body of literature has suggested that it may also be important for a number of cognitive functions, including working memory, language, attention, and affect regulation (Leiner, Leiner, & Dow, 1993; Schmahmann, 1996; Strick, Dum, & Fiez, 2009; Thach, Goodkin, & Keating, 1992). Because many of these functions are impaired in psychotic disorders, such as schizophrenia, the role of the cerebellum has garnered increasing interest and importance for innovative etiological theories, including cognitive dysmetria (Andreasen & Pierson, 2008; Picard, Amado, Mouchet-Mages, Olie, & Krebs, 2008).
The period preceding the onset of psychosis is marked by attenuated psychotic symptoms and a decline in functioning (Yung, Phillips, Yuen, & McGorry, 2004). During this period, individuals at ultrahigh risk for psychosis (UHR) show widespread gray and white matter abnormalities (Jung, Borgwardt, Fusar-Poli, & Kwon, 2012). Despite the evidence suggesting a range of structural abnormalities in UHR and a body of research focusing on the cerebellum in patients with schizophrenia, it is surprising how little focus the cerebellum has received in the UHR literature. Understanding structural brain information can be valuable for improving etiological conceptions, especially in a period in which many of the third variable confounds inherent in studies of formally psychotic patients are not yet as prevalent. UHR research can bolster early identification and intervention efforts. Indeed, as much as 36% of UHR individuals transition to full-blown psychosis within 3 years (Fusar-Poli et al., 2012). Yet only a few studies have mentioned cerebellar gray matter included among other imaging findings (Smieskova et al., 2010), and to our knowledge, there have been no studies that expressly focus on cerebellar gray matter morphology in UHR participants.
The cerebellum plays a large role in both the cognitive and the motor aspects of procedural learning. Procedural learning, also referred to as implicit learning, is learning by doing. Evidence of cerebellar involvement in procedural learning comes from a number of sources, including patients with focal lesions or atrophy of the cerebellum, functional imaging, and positron emission tomography (Flament, Ellermann, Kim, Ugurbil, & Ebner, 1996; Grafton et al., 1992; Jenkins, Brooks, Nixon, Frackowiak, & Passingham, 1994; Molinari et al., 1997; Pascual-Leone et al., 1993). The pursuit rotor task, a gold standard task of procedural learning, is ideal for examining cerebellar-behavior relationships because it relies on both motor and cognitive resources (Raz, Williamson, Gunning-Dixon, Head, & Acker, 2000). Specifically, the time-on-target variable, in which the participant matches a cursor or wand to a moving target traveling at a constant speed, gauges motor control. The change in time-on-target performance during the course of several blocks of trials provides an index of learning.
Although there is evidence of cerebellar involvement in the pursuit rotor task, to our knowledge, ours is the first study to examine regional cerebellar gray matter that may be associated with the task. In a study that examined shrinkage of the cerebellum due to aging, Raz et al. (2000) showed that reduced cerebellar volume was related to less time on target during the pursuit rotor task. Another study of motor learning, similar to the computerized pursuit rotor task, has suggested that patients with cerebellar degeneration have more difficulty controlling the force of their reach to a cursor on a computer monitor (Smith & Shadmehr, 2005).
Research on the topographical organization of the cerebellum has suggested that the anterior cerebellum (e.g., lobules I–V) is responsible for motor control, and the superior posterior areas, such as crus I of lobule VII (see Fig. 1 for an anatomical map of these regions), may be important for the storage of internal models related to motor function and, thus, responsible for learning (Manni & Petrosini, 2004; Marr, 1969; Schlerf, Verstynen, Ivry, & Spencer, 2010; Stoodley & Schmahmann, 2009, 2010). There is evidence to suggest procedural learning impairment in UHR individuals: Recent work has demonstrated deficits in pursuit rotor performance in individuals exhibiting nonclinical psychosis (otherwise healthy individuals who report at least one fleeting subclinical psychotic-like experience per year), and several studies have indicated similar deficits in patients with formal psychosis (Gomar et al., 2011; Huston & Shakow, 1948; Kern, Hartzell, Izaguirre, & Hamilton, 2010; Mittal, Dean, & Pelletier, 2012; Scherer, Stip, Paquet, & Bedard, 2003; Schwartz, Rosse, Veazey, & Deutsch, 1996).
Fig. 1.
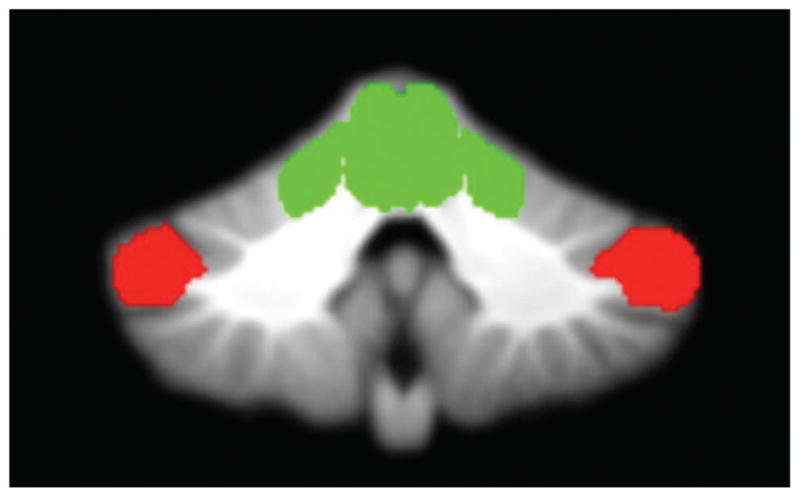
Cerebellar morphology. A priori regions of interest in the cerebellum included the anterior cerebellum (green) and crus I (red).
In the present study, we sought to examine cerebellar morphology and procedural learning, measured using the pursuit rotor in UHR participants. The pursuit rotor is a beneficial tool to assess cerebellar impairment in those at risk for psychosis because it is reliant on both motor control and cognitive processes. Furthermore, because of technological advances in MRI analysis, it is now possible to explore regional differences in the cerebellum on a lobule-by-lobule basis (Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009). Twenty-six UHR and 29 healthy control participants were recruited to test the hypotheses that compared with healthy control participants, UHR participants would perform more poorly on a pursuit rotor task and would show smaller cerebellar volume in areas related to motor control (lobules I–V) and cognitive function (crus I) and that those areas would be related to learning rate on the pursuit rotor task.
Method
Participants
Participants were recruited at the University of Colorado Boulder’s Adolescent Development and Preventive Treatment research program. Adolescent and young adult control and UHR participants (mean age = 18.10) were recruited by Craigslist and e-mail postings, newspaper advertisements, and community professional referrals. Exclusion criteria consisted of head injury, the presence of a neurological disorder, lifetime substance dependence, and the presence of any contraindication to the MRI environment (e.g., pregnant or metal in the body). The presence of an Axis I psychotic disorder (e.g., schizophrenia, schizoaffective disorder, schizophreniform) and the current/past use of any antipsychotic medication were exclusion criteria for UHR participants. The presence of any category of Axis I disorder or a psychotic disorder in a first-degree relative was an exclusion criterion for control participants. Healthy control participants were recruited through flyers and newspaper announcements (advertised as a study of neuroimaging and healthy development for volunteers with no family history of psychosis and no psychiatric symptoms). The protocol and informed consent procedures were approved by the university institutional review board. See Table 1 for the demographic characteristics of our sample.
Table 1.
Demographic Characteristics of the Sample
| Characteristic | Group
|
||
|---|---|---|---|
| UHR (n = 26) | Control (n = 29) | Total (N = 55) | |
| Male (n) | 16 | 12 | 28 |
| Female (n) | 10 | 17 | 27 |
| Age (years) | 18.52 (1.97) | 17.72 (2.64) | 18.10 (2.32) |
| Parental education (years) | 15.96 (2.58) | 15.90 (2.31) | 16.02 (2.32) |
| Handedness (n) | |||
| Right | 22 | 28 | 50 |
| Left | 4 | 1 | 5 |
| Wide Range Achievement Test | 108.65 (13.63) | 105.90 (11.43) | 107.34 (12.56) |
| Structured Interview for Prodromal Syndromes | |||
| Positive | 11.33 (5.20) | 0.83 (1.50) | 5.84 (6.50) |
| Negative | 11.1 (7.48) | 0.91 (1.47) | 5.9 (7.40) |
| Disorganized | 5.7 (3.66) | 0.48 (0.95) | 3.02 (3.71) |
| General | 6.76 (4.35) | 0.65 (1.53) | 3.57 (4.42) |
Note: Data are mean (SD) unless noted otherwise. Groups did not differ on variables of gender, age, handedness, parent education, or general intelligence. Ultrahigh risk for psychosis (UHR) participants were rated significantly higher than were control participants on all Structured Interview for Prodromal Syndromes domains (p ≤ .01).
Clinical interviews
The Structured Interview for Prodromal Syndromes (SIPS; Miller et al., 1999) was administered to diagnose a prodromal syndrome. UHR participants in the present study met SIPS criteria for a prodromal or high-risk syndrome, defined by moderate-to-severe but not psychotic levels of positive symptoms (rated from 3 to 5 on a 6-point scale) or a decline in global functioning accompanying the presence of schizotypal personality disorder or a family history of schizophrenia (Miller et al., 1999). The SIPS gauges several distinct categories of prodromal symptom domains, including positive, negative, and disorganized dimensions. A mean score for each category is used as an indicator of the respective dimensions of symptomatology. Training of advanced doctoral student interviewers was conducted during a 2-month period, and interrater reliabilities exceeded the minimum study criterion (κ ≥ 80).
The Structured Clinical Interview for the DSM-IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1995) was also administered to rule out a psychotic disorder diagnosis. This measure has been demonstrated to have excellent interrater reliability in adolescent populations (Martin, Pollock, Bukstein, & Lynch, 2000) and has been used in several previous studies focusing on adolescent populations with schizophrenia spectrum disorders (Howes et al., 2009).
Wide Range Achievement Test–Word Reading subtest
A subsection of 44 participants (UHR n = 23, control n = 21) were administered the Word Reading subtest of the fourth edition of the Wide Range Achievement Test (WRAT) as a measure of general intelligence. The WRAT is a well-validated and broadly used measure of achievement and broad learning ability for adolescents and young adults (Wilkinson & Robertson, 2006). Participants were asked to read 15 letters and 55 words. The total number of letters and words read correctly is transformed into a standard score normed for each age group. This measure was employed to test for the specificity of any detected relationships between procedural learning and cerebellar anomalies.
Procedural learning
We used a computerized version of the pursuit rotor task (Life Science Associates, New York, NY), which has been validated and used in other studies with schizophrenia samples as well as with young adult populations (Gomar et al., 2011; Mittal, Dean, et al., 2012). Participants were instructed to follow a moving target around a rectangular track with a mouse held in their preferred hand. Each trial lasted 20 s with a 5-s interval between trials. Participants were given four blocks of three trials each block, interspersed with 45-min rest periods after each block. Employing a widely used titration strategy (Gomar et al., 2011), the initial level of proficiency for each participant was equated during practice trials, and speed of the target stimulus was subsequently adjusted so that each participant reached a criterion of being able to maintain contact with the target from 20% to 25% of the time. Once the participant reached a level of proficiency, the speed of the moving target was kept constant at that level of proficiency for the four test blocks. To assess overall rate of procedural learning, we subtracted the mean percent time on target for Block 1 from the mean percent time on target for Block 4, creating a learning rate index.
Structural imaging
MRI of the brain was acquired on each participant using a 3-Tesla TIM Trio Siemens MRI scanner with 12-channel parallel imaging. A T1-weighted three-dimensional magnetization prepared rapid gradient multi-echo sequence (sagittal plane; repetition time = 2,530 ms; echo times = 1.64, 3.5, 5.36, 7.22, and 9.08 ms; generalized autocalibrating partially parallel acquisition, GRAPPA, parallel imaging factor 2; 1-mm3 isomorphic voxels; field of view = 256 mm; flip angle = 7°; 192 interleaved slices; time = 6.03 min) covering the whole brain was acquired for anatomic segmentation. A turbo spin echo proton density/T2-weighted acquisition (axial oblique aligned with anterior commissure-posterior commissure line; repetition time = 3,720 ms; echo time = 89 ms; GRAPPA parallel imaging factor 2; 0.9 × 0.9 mm voxels; field of view = 240 mm; flip angle = 120°; 77 interleaved 1.5-mm slices; time = 5.14 min) was acquired to check for incidental pathology.
Cerebellar morphology
Lobular volume was calculated using the lobular regions described in the spatially unbiased infratentorial template (SUIT) atlas (Diedrichsen, 2006; Diedrichsen et al., 2009) with the method employed by Bernard and Seidler (in press). Individual lobular volumes of each participant were determined for all lobules in the right and left hemispheres. First, we created 26 masks of each lobule and a mask for the whole cerebellum using the probabilistic SUIT atlas. Second, the cerebellum was extracted from the whole brain using the isolate function in the SUIT toolbox (Diedrichsen, 2006; Diedrichsen et al., 2009), implemented in SPM 8 (Welcome Department of Cognitive Neurology, London, England; http://www.fil.ion.ucl.ac.uk). We masked the extracted anatomical image with the threshold classification map that was produced during the isolation process. This procedure resulted in a high-resolution image of the cerebellum, excluding all surrounding cortical matter. Third, the SUIT cerebellum template was normalized to each individual participant’s cerebellar anatomical image (in native space) using Advanced Normalization Tools (Avants, Epstein, Grossman, & Gee, 2008). The transformation was first applied to the SUIT cerebellum, and then the resulting warp vectors were applied to the individual lobular masks. The result was a mask of each lobule normalized to individual subject space for each participant.
Finally, these masks were loaded into MRICron (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) and converted to volumes of interest. Volumes of interest were then overlaid onto each individual participant’s structural scan and inspected to ensure accurate registration. We then used MRICron to calculate the descriptive statistics for each lobule, providing us with the gray matter volume of each lobule in cubic centimeters. The volume of the anterior cerebellum was calculated by adding the gray matter volumes for lobules I–IV and V of both hemispheres. We were unable to create a mask for vermis crus I/II, so we created a sum of all lobular volumes and subtracted this sum from the whole cerebellar volume to calculate the volume of that region. For exploratory analysis, we calculated the volumes of other areas of the cerebellum that we did not hypothesize to be involved in the pursuit rotor task, including the left and right posterior cerebellum, the vermis, and lobule X. For the left and right posterior cerebellum, we combined the volumes of crus II and lobules VIIb–IX for each hemisphere. The volume of the vermis was calculated by adding vermis crus I/II and vermis lobules VI–X. Finally, we added the left and right lobule X volumes for a total volume of lobule X. This procedure was repeated for each individual participant.
In addition, Freesurfer (https://surfer.nmr.mgh.harvard.edu/fswiki) was used to automatically segment the magnetization prepared rapid gradient multi-echo sequence image and to calculate each participant’s total intracranial volume (TICV; i.e., the sum of whole-brain gray matter + white matter + cerebrospinal fluid). Normalized lobular volumes were calculated by dividing the total lobular volume by the TICV (Whitwell, Crum, Watt, & Fox, 2001). Subsequent analyses were performed using the normalized values for the anterior, right and left crus I, left and right posterior cerebellum, vermis, and lobule X.
Statistical approach
The present study included a total of 55 participants, and of those, 9 participants (4 UHR, 5 control) completed only the first three blocks of the pursuit rotor task. The remaining 46 participants (22 UHR, 24 control) completed all four blocks of the pursuit rotor task. An additional 3 participants were unable to participate in the imaging component because of contraindications to the MRI environment. Thus, 52 participants (25 UHR, 27 control) completed the imaging portion of the study. The correlation analysis between imaging and pursuit rotor variables therefore includes only 43 participants (21 UHR, 22 control).
Independent t tests and chi-square tests were employed to examine differences between groups in continuous and categorical demographic variables, respectfully. If necessary, results were adjusted for inequalities of variance as tested by Levene’s test. To test for procedural learning differences between groups, we conducted a 4 (Trial Block) × 2 (Diagnostic Group) repeated measures analysis of variance (ANOVA). Bivariate correlations were used to investigate associations between the cerebellar regions, WRAT scores, and learning rate index. Comparisons of correlations between groups were performed using Fisher’s r to z transformation. One-tailed tests were employed for directional hypotheses regarding group differences in hypothesized cerebellar regions of interest (i.e., anterior and left and right crus I) and procedural learning rate. Exploratory analysis of other regions (i.e., posterior, vermis, and lobule X) in the cerebellum involved two-tailed tests. Comparisons of demographic variables between groups, comparisons between groups on rotation speed for the pursuit rotor task, and achievement as measured by the WRAT employed two-tailed tests.
Results
There were no significant differences between groups on demographic characteristics, including gender, age, handedness, and parental education (see Table 1). The UHR group did not differ from the control group in terms of general intelligence as measured by the WRAT, t(42) = 0.72, p ≥ .4. UHR participants were rated significantly higher than were control participants on all SIPS symptom domains—positive: t(53) = 11.15, p ≤ .01; negative: t(53) = 7.59, p ≤ .01; disorganized: t(53) = 8.55, p ≤ .01; general t(53) = 6.97, p ≤ .01. Within the UHR group, 6 participants had a first-degree relative with schizophrenia or other psychotic disorder. In addition, 4 UHR participants had a diagnosis of schizotypal personality disorder. Axis I disorders in the UHR group included a history of mood (50%), anxiety (46%), attention-deficit/hyperactivity (19%), and somatoform (4%) disorders. Kolmogorov-Smirnov tests revealed that distributions of target cerebellar morphology, procedural learning, and WRAT target variables were normal, and as a result, analyses were conducted with parametric statistics.
Procedural learning: Pursuit rotor
As noted, the participants were titrated so that each individual reached a criterion of proficiency defined as being able to maintain contact with the target approximately 20% to 25% of the time. All participants met this criterion, and analyses of the participants’ proficient target rotation speed revealed no significant difference between the UHR group and the control group in rate of rotation. Following the titration process, participants began four trial blocks at 0, 45, 90, and 135 min consisting of three 20-s trials separated by a 5-s rest interval at their proficient speed. A single index of performance was computed by calculating the mean of the three trials for each of the four blocks.
A 2 (Group) × 4 (Block Performance) repeated measures ANOVA with percentage of time on target as the dependent variable was conducted. Results revealed a significant interaction, F(3, 132) = 2.66, p = .051, a main effect of group, F(1, 44) = 7.86, p ≤ .01, and a significant main effect of trial block, F(3, 132) = 66.71, p ≤ .01. The Group × Block Performance repeated measures ANOVA was also conducted in the subgroup of participants who completed the WRAT. Results were consistent with the whole study sample and revealed a significant interaction, F(3, 111) = 3.365, p ≤ .05, a main effect of group, F(1, 37) = 6.182, p ≤ .05, and a main effect of trial block, F(3, 111) = 57.22, p ≤ .01. These results strongly support the hypothesis that UHR individuals would perform more poorly on the pursuit rotor task.
Furthermore, these results indicate that the UHR group learned at a slower rate than did healthy control participants on the pursuit rotor task. Follow-up analysis using the learning rate index confirmed the results of the repeated measures ANOVA interaction in that the UHR group showed a significantly lower rate of learning compared with the control group, t(44) = −2.72, p ≤ .01, UHR mean (SE) = 9.01 (1.06), control mean (SE) = 13.26 (1.14). These results indicate that although both groups did indeed learn during the course of the task, the UHR participants performed at a lower level and learned at a slower rate compared with the control participants.
Cerebellar morphology
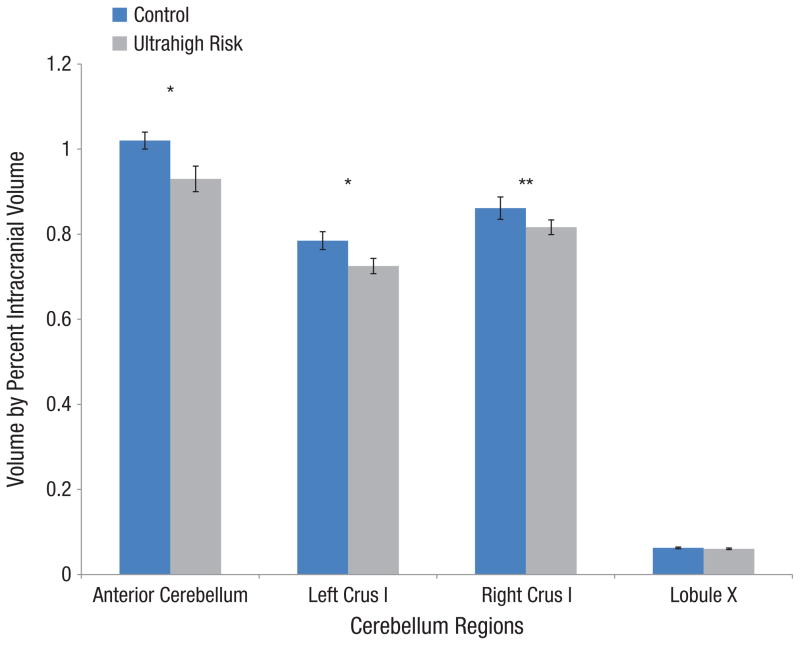
We initially assessed whether there were group differences in total cerebellar gray matter volume. Results revealed that compared with the control group, cerebellar volume was significantly smaller in the UHR group, t(41.27) = −2.785, p ≤ .01. Hypothesized regions of interest (anterior cerebellum, left and right crus I) were analyzed using a repeated measures ANOVA. There was a main effect of group, F(1, 50) = 4.76, p ≤ .05, and a significant region by group interaction, F(2, 50) = 5.142, p ≤ .01, showing that the groups varied across regions of interest. Follow-up t tests of the hypothesized regions revealed that compared with the control group, the UHR group had significantly smaller volume in the anterior cerebellum, t(44.84) = −2.29, p ≤ .05, and left crus I, t(50) = −2.15, p ≤ .05. There was a trend toward smaller right crus I volume in the UHR group, t(44.23) = −1.42, p ≤ .1 (see Fig. 2 for group differences in cerebellar morphology).
Fig. 2.
Group differences in cerebellar morphology. Error bars represent standard errors. *p ≤ .05, **p ≤ 0.1.
Exploratory analyses were conducted on several other regions of the cerebellum, including the left and right posterior cerebellum, vermis, and lobule X. The UHR group had smaller cerebellar volumes than did the control group in the right and left posterior cerebellum, t(41.97) = −3.11, p ≤ .01, and t(44.094) = −2.62, p ≤ .01, respectively, and the vermis t(43.14) = −3.09, p ≤ .01. The UHR group did not show any difference from the control group in volume for lobule X, t(50) = −0.756, p = .45. These results suggest that there are region-specific cerebellar anomalies in UHR individuals.
Correlations with general intelligence and cerebellar morphology
Bivariate correlations were used to examine potential relationships between general intelligence, as measured by the WRAT, and cerebellar morphology. The volume of the anterior cerebellum was not associated with WRAT score in either the UHR group, r(20) =−.052, p ≥ .5, or the control group, r(19) =−.67, p ≥ .5. In addition, the volume of the left crus I was not related to WRAT score in either the UHR group, r(20) =−.24, p ≥ .2, or the control group, r(19) =−.05, p ≥ .5. Right crus I volume showed a trend toward significance in the UHR group, r(20) =−.37, p ≤ .1, but was not significant in the control group, r(19) =−.05, p ≥ .5. These results showed that there were no significant correlations between any of the cerebellar regions hypothesized to be involved with procedural learning and general intelligence in either group, indicating that cerebellar morphology is not associated with general intelligence. Comparisons of correlations between groups using Fisher’s r to z transformation showed no significant differences, all zs ≤ 1.5, all ps ≥ .25.
Correlations with procedural learning and cerebellar morphology
Bivariate correlations were conducted to examine potential relationships between procedural learning, using the learning rate index, and cerebellar morphology within each group. Left crus I volume was significantly associated with procedural learning in the UHR group, r(19) = .38, p ≤ .05, but not in the control group, r(20) = .03, p ≥ .4. Right crus I and procedural learning correlations showed a trend toward significance in the UHR group, r(19) = .32, p ≤ .1, but not in the control group, r(20) = .11, p ≥ .3. There were no relationships with any of the other cerebellar regions, all rs ≤ .23, all ps ≤ .15. Comparisons of correlations between groups using a Fisher’s r to z transformation showed no significant differences, all zs ≤ 1.5, all ps ≥ .25. The within-group correlations suggest that crus I may be related specifically to impaired procedural learning and risk for psychosis.
Discussion
The results indicate impaired procedural learning in UHR individuals; compared with the control group, they performed at a lower level (i.e., less time on target) on the pursuit rotor task and did not learn as quickly, despite being titrated to a proficient speed on the task. This poor performance may be explained in part by cerebellar abnormalities because the structure is integral to procedural learning (Molinari et al., 1997). To this end, we have used advanced anatomical MRI analysis to examine cerebellar morphology and found that compared with healthy control individuals, UHR individuals have smaller volumes in specific areas of the cerebellum that are responsible for both motor control and cognition but not other regions. The finding of a relationship between a cognitive region of the cerebellum (crus I) and the overall rate of learning on the pursuit rotor task within the UHR group alone provides further support for the notion that cerebellar abnormalities affect crucial functions, such as procedural learning.
Although to date the cerebellum has not been a center of focus in the UHR literature, a number of studies that have focused on the entire brain help to provide a context for interpreting the present results. Pantelis et al. (2003) used voxel-based morphometry to show longitudinal trend level reductions of gray matter in the left posterior cerebellum in both participants who did and participants who did not go on to develop psychosis. Borgwardt, Riecher-Rossler, et al. (2007) found bilateral reductions in the volume of posterior cerebellar gray matter in at-risk individuals and those experiencing their first episode of schizophrenia, relative to control individuals, although recent work has not yielded differences between at-risk and control groups (Ziermans et al., 2012). Genetic high-risk individuals (i.e., a first-degree relative with psychosis) also show differences in the cerebellum compared with healthy control individuals. Specifically, researchers have found gray matter reductions in the right cerebellum and reduced activation in the left cerebellum during a functional MRI task in individuals at genetic risk who later developed schizophrenia (Job, Whalley, Johnstone, & Lawrie, 2005; Whalley et al., 2004). Taken together, previous research and the present findings suggest that cerebellar gray matter abnormalities may be an important part of the aberrant neurodevelopment in at-risk groups prior to the onset of psychosis.
Within the cerebellum, a number of regions contribute to procedural learning. Specific areas related to motor function are primarily located in the anterior cerebellum (i.e., lobules I–VI), and areas related to cognitive aspects of the pursuit rotor task are located in the posterior cerebellum (e.g., crus I; Grodd, Hulsmann, Lotze, Wildgruber, & Erb, 2001; Schlerf et al., 2010; Schmahmann, 1996; Schmahmann & Sherman, 1998; Stoodley & Schmahmann, 2009, 2010; Strick et al., 2009). In our study, UHR individuals showed smaller cerebellar gray matter volume in areas related to motor function and cognition compared with control individuals. The pursuit rotor requires motor control while reaching for the computer mouse and for arm-movement control. Deficits in anterior cerebellar volume may contribute to the differences in performance between control and UHR participants (Seidler & Noll, 2008; Stoodley & Schmahmann, 2010; Stoodley, Valera, & Schmahmann, 2012).
Crus I has been implicated in the performance of cognitive tasks but is also important in the performance of complex motor tasks (Schlerf et al., 2010; Stoodley et al., 2012; Stoodley & Schmahmann, 2009, 2010). We found differences in this area such that UHR participants have significantly smaller volume in left but not right crus I. The left cerebellar hemisphere is thought to control spatial processing (Bernard & Seidler, 2013; Stoodley & Schmahmann, 2009), which is important for successful performance on the pursuit rotor task. Our results suggest that smaller gray matter volume in the cerebellum related to motor and cognitive function contribute to the poorer performance and impaired learning of UHR individuals, compared with healthy control individuals, during procedural learning. In addition to its role in pursuit rotor, converging evidence suggests that crus I may be an area of shared impairment between schizophrenia and at-risk individuals (Kuhn, Romanowski, Schubert, & Gallinat, 2012).
Other areas of the cerebellum, including the posterior cerebellum, vermis, and lobule X, serve a variety of other cognitive, postural, and vestibular functions. The posterior regions have been shown to contribute to a number of cognitive functions, including working memory and executive function (Stoodley & Schmahmann, 2009, 2010; Strick et al., 2009). The vermis is associated with spinal control of movement, balance, and posture (Coffman, Dum, & Strick, 2011). Lobule X is considered important for vestibular functions, including the ocular reflexes and posture (Stoodley & Schmahmann, 2010). In the present study, exploratory analyses revealed smaller volume in the anterior, posterior, and medial cerebellum (vermis) but not lobule X in UHR participants compared with control participants. The results show that the hypothesized regions that contribute to the cognitive aspects of the pursuit rotor (i.e., crus I) were involved in the task. The differences in volume in other areas of the cerebellum may be related to other areas of cognition, balance, and motor function not associated with the pursuit rotor task. More work is clearly needed to explore possible associations within areas of the cerebellum related to cognitive performance on tasks such as working memory and executive function, as well as affective processes and postural impairment.
Theories of cortico-cerebellar involvement in procedural learning suggest a nuanced relationship through which the cerebellum participates in procedural learning by both feedback and feed-forward control processes (Marr, 1969; Seidler, Noll, & Thiers, 2004). Feedback control uses on-line detection of sensory errors (e.g., arm movement) and compares the predicted movement with the incoming sensory errors to adjust the movement. Feed-forward control uses the feedback process to update an internal model of the movement so that over time, the movement becomes more efficient (Imamizu et al., 2000; Seidler et al., 2004). The rate of learning is a measure of how quickly and efficiently a participant learns the task and may be a beneficial measure of cortico-cerebellar control processes. In the present study, the learning rate index was correlated with larger volume of left crus I. This finding is consistent with the results of Imamizu et al. (2000) and other researchers (Roland, Eriksson, Widen, & Stone-Elander, 1989; Seidler & Noll, 2008) who suggested that this area is important for internal models of motor skill. Taken together, cerebellar dysfunction in UHR individuals may contribute to widespread motor and cognitive dysfunction.
One leading theory implicating cortico-cerebellar impairment in schizophrenia, cognitive dysmetria, posits several models of cerebellar function (Andreasen & Pierson, 2008). In these models, the cerebellum acts as an all-purpose modulator of movement as well as thought (Andreasen & Pierson, 2008). Through the cortical-cerebellar-thalamo-cortical circuit, the cerebellum not only detects patterns and errors but also keeps time so that movement and thoughts are properly coordinated (Leiner, Leiner, & Dow, 1986, 1991; Schmahmann, 1996; Seidler, Kwak, Fling, & Bernard, 2013; Strick et al., 2009). In such a way, the cerebellum provides adaptive control for the central nervous system (Andreasen & Pierson, 2008; Thach et al., 1992). Because of its many functions and control over thoughts and movement, impairment in the cerebellum may explain the heterogeneous expression of psychotic disorders (Andreasen, 1999). The prodromal syndrome prior to the onset of illness is also characterized by heterogeneous symptoms of aberrant thought, impaired cognition, and movement abnormalities (Cornblatt et al., 2003; Cornblatt, Obuchowski, Roberts, Pollack, & Erlenmeyer-Kimling, 1999; Mittal, Dean, Pelletier, & Caligiuri, 2011; Mittal, Neumann, Saczawa, & Walker, 2008; Mittal, Smolen, et al., 2012).
In addition to the present results showing cognitive and movement impairment linked to regional volumetric deficits within the cerebellum, recent reports of altered cortico-cerebellar connectivity during a working memory task in relatives of schizophrenia patients (i.e., another high-risk group) have strengthened the implication of cerebellar involvement in the vulnerability for psychosis (Repovs & Barch, 2012; Repovs, Csernansky, & Barch, 2011). These findings are significant in that they also describe cognitive dysmetria prior to the onset of psychosis.
Schizophrenia patients and individuals who report psychotic-like experiences, such as fleeting auditory hallucinations, but are otherwise healthy show poorer performance on the pursuit rotor task (Gomar et al., 2011; Mittal, Dean, et al., 2012). Consistent with results from both the schizophrenia and the nonclinical psychosis literature, our results showed that compared with control individuals, UHR individuals performed more poorly on the pursuit rotor task (Gomar et al., 2011; Granholm, Bartzokis, Asarnow, & Marder, 1993; Huston & Shakow, 1948; Kern et al., 2010; Mittal, Dean, et al., 2012; Picard et al., 2008; Schwartz et al., 1996). In addition to poorer performance on the pursuit rotor task, UHR individuals in our study showed a slower rate of learning than did control individuals. These results should be approached with caution because the schizophrenia literature paints a more nuanced view of procedural learning. In a review of preserved cognitive function in schizophrenia, Gold, Hahn, Strauss, and Waltz (2009) acknowledged that although individuals with schizophrenia perform procedural learning tasks at a lower level than do healthy individuals, learning is preserved. Our results indicating that general intelligence was not affected in UHR individuals, and that it was not associated with any of the hypothesized cerebellar regions related to procedural learning, suggest that cerebellar abnormalities confer domain-specific dysfunction rather than broad impairment. Future investigations focusing on other respective structures as well as white matter tracts should seek to use more specific learning tasks (e.g., verbal and spatial learning paradigms) to clarify distinct and overlapping neural correlates of learning impairment in UHR individuals.
To our knowledge, ours is the first report to examine procedural learning in a UHR sample or focus specifically on cerebellar morphology in UHR participants. The use of the SUIT probabilistic cerebellar atlas (Diedrichsen et al., 2009) to examine specific lobules is also an important point of innovation because it allows the ability to distinguish between cognitive and motor areas within the cerebellum. The probabilistic atlas permits a more nuanced analysis of lobular gray matter within the cerebellum. Our study represents one of few studies to use this program to measure cerebellar gray matter volumes (Bernard & Seidler, in press) and is the first to explore different lobular regions within the cerebellum in a UHR sample.
Several limitations within this study are important to consider. First, research has suggested that there are alternatives to using TICV to normalize brain regions. One such alternative is a measure for total brain tissue volume (TBV; sum of total gray and white matter). In support of this method, Bigler and Tate (2001) argued that normalizing by TBV is more sensitive to global grey and white matter loss. This is an important measure to consider because UHR individuals show widespread grey matter loss prior to the onset of psychosis (Borgwardt, McGuire, et al., 2007; Borgwardt, Riecher-Rossler, et al., 2007; Fusar-Poli, Borgwardt, et al., 2011). Other researchers have argued that TICV is preferable because it shows that regions such as the cerebellum are reduced in volume independently of any other changes in the brain and that TICV accounts for the concomitant increase in cerebrospinal fluid with the decrease in gray and white matter (Bernard & Seidler, in press; Hannan et al., 2010).
It is important to note that our use of TICV allows this study to be compared with the existing literature because this method has been widely used in volumetric studies of UHR and schizophrenia patients (Hannan et al., 2010; Ho, Andreasen, Dawson, & Wassink, 2007; Ho, Andreasen, Ziebell, Pierson, & Magnotta, 2011; Mittal et al., 2010; Sullivan et al., 2000; Velakoulis et al., 2006; Ziermans et al., 2012). However, as alternative techniques become available, researchers in the UHR field will need to take steps to adopt them as well (e.g., a study designed to compare the TICV and TBV methods across brain structures in a large UHR sample).
Second, although our sample size is comparable to that used in many other high-impact UHR structural imaging investigations (Allen et al., 2011; Borgwardt, McGuire, et al., 2007; Borgwardt, Riecher-Rossler, et al., 2007; Broome et al., 2010; Fusar-Poli, Borgwardt, et al., 2011; Fusar-Poli, Broome, et al., 2011; Fusar-Poli, Stone, et al., 2011; Mittal et al., 2010; Morey et al., 2005; Niendam et al., 2006; Sabb et al., 2010; Stone et al., 2009), future studies with larger samples would be beneficial because increased power may allow for detection and control of potential confounding factors, which can threaten internal validity. A third, and related, limitation is the use of only one task to examine procedural learning. Although our results provide key insight into the deficits of UHR populations and extend our knowledge to include the domain of procedural learning, the specificity of impairment within the procedural learning domain remains unknown. Understanding the extent of this deficit necessitates comparison across procedural learning paradigms in future studies.
Fourth, to reduce the chance of Type I error in this report, we did not expand the exploratory analysis to all of the lobules defined by the SUIT template. We have focused on regions of the cerebellum that we hypothesized to contribute to the motor and cognitive function related to the pursuit rotor as well as reported exploratory results of larger regions in the cerebellum. Given that these exploratory results suggest widespread reduction of cerebellar grey matter volume, future studies that explore the individual cerebellar lobules stand to inform our understanding of impairment related to cerebellar functions in UHR individuals.
Although the present findings significantly advance our understanding of the role of cerebellar abnormalities in a UHR group, and speak to the complexity of the cerebellum’s role in coordinating higher-order activity, additional studies with multiple time points and clinical outcome data will be useful to determine whether signs of cerebellar dysfunction predict eventual conversion or course of illness. Larger samples obtained from multiple sites will also improve external validity. Furthermore, it is important to note that although the cerebellum is strongly related to motor control, research has suggested that it is also involved in non–motor related cognitive tasks that may also be impaired in schizophrenia (Leiner et al., 1993; Picard et al., 2008; Strick et al., 2009). The pursuit rotor requires a distributed network of brain areas to perform well, and includes the prefrontal cortex and basal ganglia as well as the cerebellum (Doyon & Benali, 2005; Doyon, Penhune, & Ungerleider, 2003; Grafton et al., 1992; Hatakenaka, Miyai, Mihara, Sakoda, & Kubota, 2007; Leiner et al., 1991). Doyon et al. (2003) have argued that cortico-striatal and cortico-cerebellar networks act together during procedural learning tasks. Future research should seek to expand our knowledge of impaired brain regions involved in procedural learning and use other cerebellar-specific paradigms in UHR samples (e.g., eye-blink conditioning, self-paced finger tapping, posture) to further elucidate the role of the cerebellum and procedural learning in the etiology of psychosis.
Acknowledgments
Funding
This work was supported by National Institutes of Health (NIH) Grant R01MH094650 to V. A. Mittal. J. A. Bernard was supported by NIH Grant T32AG000279 (R. Schwartz, principal investigator).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Reprints and permissions: sagepub.com/journalsPermissions.nav
Author Contributions
V. A. Mittal conceptualized the overall study and attained funding. D. J. Dean, J. A. Bernard, and V. A. Mittal developed the study concept. All authors contributed to the study design. Testing and data collection were performed by D. J. Dean, J. A. Bernard, J. M. Orr, A. L. Pelletier, T. Gupta, and E. E. Carol. D. J. Dean and J. A. Bernard performed the data analysis and interpretation under the supervision of V. A. Mittal. D. J. Dean drafted the manuscript, and J. A. Bernard, J. M. Orr, A. L. Pelletier, T. Gupta, E. E. Carol, and V. A. Mittal provided critical revisions. All authors approved the final version of the manuscript for submission.
References
- Allen P, Seal ML, Valli I, Fusar-Poli P, Perlini C, Day F, McGuire PK. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophrenia Bulletin. 2011;37:746–756. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Cerebellar contributions to visuomotor adaptation and motor sequence learning: An ALE meta-analysis. Frontiers in Human Neuroscience. 2013;7:27. doi: 10.3389/fnhum.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum. doi: 10.1007/s12311-013-0481-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Tate DF. Brain volume, intracranial volume, and dementia. Investigative Radiology. 2001;36:539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, Riecher-Rossler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. British Journal of Psychiatry. 2007;191(51):S69–S75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, McGuire PK. Regional gray matter volume abnormalities in the at risk mental state. Biological Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Broome MR, Fusar-Poli P, Matthiasson P, Woolley JB, Valmaggia L, Johns LC, McGuire PK. Neural correlates of visuospatial working memory in the “at-risk mental state. Psychological Medicine. 2010;40:1987–1999. doi: 10.1017/S0033291710000280. [DOI] [PubMed] [Google Scholar]
- Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proceedings of the National Academy of Sciences, USA. 2011;108:16068–16073. doi: 10.1073/pnas.1107904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: A neurodevelopmental perspective. Schizophrenia Bulletin. 2003;29:633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Development and Psychopathology. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I), Patient Edition. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Flament D, Ellermann JM, Kim SG, Ugurbil K, Ebner TJ. Functional magnetic resonance imaging of cerebellar activation during the learning of a visuomotor dissociation task. Human Brain Mapping. 1996;4:210–226. doi: 10.1002/hbm.460040302. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, McGuire P. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, Sacchetti E. Neuroanatomy of vulnerability to psychosis: A voxel-based meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Matthiasson P, Woolley JB, Mechelli A, Johns LC, McGuire P. Prefrontal function at presentation directly related to clinical outcome in people at ultrahigh risk of psychosis. Schizophrenia Bulletin. 2011;37:189–198. doi: 10.1093/schbul/sbp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Stone JM, Broome MR, Valli I, Mechelli A, McLean MA, McGuire PK. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Archives of General Psychiatry. 2011;68:881–890. doi: 10.1001/archgenpsychiatry.2011.46. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: Areas of preserved cognitive function in schizophrenia. Neuropsychology Review. 2009;19:294–311. doi: 10.1007/s11065-009-9098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar JJ, Pomarol-Clotet E, Sarro S, Salvador R, Myers CE, McKenna PJ. Procedural learning in schizophrenia: Reconciling the discrepant findings. Biological Psychiatry. 2011;69:49–54. doi: 10.1016/j.biopsych.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. Journal of Neuroscience. 1992;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Bartzokis G, Asarnow RF, Marder SR. Preliminary associations between motor procedural learning, basal ganglia T2 relaxation times, and tardive dyskinesia in schizophrenia. Psychiatry Research. 1993;50:33–44. doi: 10.1016/0925-4927(93)90022-a. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Human Brain Mapping. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan KL, Wood SJ, Yung AR, Velakoulis D, Phillips LJ, Soulsby B, Pantelis C. Caudate nucleus volume in individuals at ultra-high risk of psychosis: A cross-sectional magnetic resonance imaging study. Psychiatry Research. 2010;182:223–230. doi: 10.1016/j.pscychresns.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Hatakenaka M, Miyai I, Mihara M, Sakoda S, Kubota K. Frontal regions involved in learning of motor skill—A functional NIRS study. NeuroImage. 2007;34:109–116. doi: 10.1016/j.neuroimage.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. American Journal of Psychiatry. 2007;164:1890–1899. doi: 10.1176/appi.ajp.2007.05111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Archives of General Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of Geneneral Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Huston PE, Shakow D. Learning in schizophrenia: I. Pursuit learning. Journal of Personality. 1948;17:52–74. doi: 10.1111/j.1467-6494.1948.tb01194.x. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: A study with positron emission tomography. Journal of Neuroscience. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. NeuroImage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Jung WH, Borgwardt S, Fusar-Poli P, Kwon JS. Gray matter volumetric abnormalities associated with the onset of psychosis. Frontiers in Psychiatry. 2012;3:101. doi: 10.3389/fpsyt.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Hartzell AM, Izaguirre B, Hamilton AH. Declarative and nondeclarative memory in schizophrenia: What is impaired? What is spared? Journal of Clinical and Experimental Neuropsychology. 2010;32:1017–1027. doi: 10.1080/13803391003671166. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Romanowski A, Schubert F, Gallinat J. Reduction of cerebellar grey matter in Crus I and II in schizophrenia. Brain Structure and Function. 2012;217:523–529. doi: 10.1007/s00429-011-0365-2. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behavioral Neuroscience. 1986;100:443–454. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: Its computing, cognitive, and language skills. Behavioural Brain Research. 1991;44:113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends in Neurosciences. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Manni E, Petrosini L. A century of cerebellar somatotopy: A debated representation. Nature Reviews Neuroscience. 2004;5:241–249. doi: 10.1038/nrn1347. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. Journal of Physiology. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Pollock N, Bukstein O, Lynch K. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug and Alcohol Dependence. 2000;59:173–176. doi: 10.1016/s0376-8716(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatric Quarterly. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Daley M, Shiode MF, Bearden CE, O’Neill J, Cannon TD. Striatal volumes and dys-kinetic movements in youth at high-risk for psychosis. Schizophrenia Research. 2010;123:68–70. doi: 10.1016/j.schres.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A. Dermatoglyphic asymmetries and fronto-striatal dysfunction in young adults reporting non-clinical psychosis. Acta Psychiatrica Scandinavica. 2012;126:290–297. doi: 10.1111/j.1600-0447.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophrenia Research. 2011;132:194–196. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Archives of General Psychiatry. 2008;65:165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Smolen A, Dean DJ, Pelletier AL, Lunsford-Avery J, Smith A. BDNF Val66Met and spontaneous dyskinesias in non-clinical psychosis. Schizophrenia Research. 2012;140:65–70. doi: 10.1016/j.schres.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, Petrosini L. Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain. 1997;120(Pt. 10):1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Archives of General Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O’Brien M, Cannon TD. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophrenia Research. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: A cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural learning in Parkinson’s disease and cerebellar degeneration. Annals of Neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO. The role of the cerebellum in schizophrenia: An update of clinical, cognitive, and functional evidences. Schizophrenia Bulletin. 2008;34:155–172. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microscopy Research and Technique. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Frontiers in Human Neuroscience. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biological Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Eriksson L, Widen L, Stone-Elander S. Changes in regional cerebral oxidative metabolism induced by tactile learning and recognition in man. European Journal of Neuroscience. 1989;1(1):3–18. doi: 10.1111/j.1460-9568.1989.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Sabb FW, van Erp TG, Hardt ME, Dapretto M, Caplan R, Cannon TD, Bearden CE. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophrenia Research. 2010;116:173–183. doi: 10.1016/j.schres.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer H, Stip E, Paquet F, Bedard MA. Mild procedural learning disturbances in neuroleptic-naive patients with schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(1):58–63. doi: 10.1176/jnp.15.1.58. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Verstynen TD, Ivry RB, Spencer RM. Evidence of a novel somatopic map in the human neocerebellum during complex actions. Journal of Neurophysiology. 2010;103:3330–3336. doi: 10.1152/jn.01117.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt. 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Rosse RB, Veazey C, Deutsch SI. Impaired motor skill learning in schizophrenia: Implications for corticostriatal dysfunction. Biological Psychiatry. 1996;39:241–248. doi: 10.1016/0006-3223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Kwak Y, Fling BW, Bernard JA. Neurocognitive mechanisms of error-based motor learning. Advances in Experimental Medicine and Biology. 2013;782:39–60. doi: 10.1007/978-1-4614-5465-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. Journal of Neurophysiology. 2008;99:1836–1845. doi: 10.1152/jn.01187.2007. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. NeuroImage. 2004;22:1775–1783. doi: 10.1016/j.neuroimage.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Borgwardt SJ. Neuroimaging predictors of transition to psychosis—A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. Journal of Neurophysiology. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, McGuire PK. Glutamate dysfunction in people with prodromal symptoms of psychosis: Relationship to gray matter volume. Biological Psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Archives of General Psychiatry. 2000;57:894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annual Review of Neuroscience. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: A magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of General Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127(Pt. 3):478–490. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT4) [Software and program kit] Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: Implications for longitudinal quantitative MR imaging. American Journal of Neuroradiology. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: Psychopathology and clinical features. Schizophrenia Research. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, Durston S. Progressive structural brain changes during development of psychosis. Schizophrenia Bulletin. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]