Abstract
Continuous-flow left ventricular assist devices (LVADs) subject elements of the blood to significant stress, resulting in clinically significant and subclinical hemolysis. We sought to prospectively determine if baseline red cell osmotic fragility of an advanced heart failure patient - influences the hemolytic response to LVAD support. Osmotic fragility assesses the degree of red blood cell hemolysis under varying degrees of osmotic stress. Assays were prospectively obtained on 50 consecutive patients prior to placement of continuous flow LVADs: HeartMate II (n=34), Jarvik 2000 (n=5), HeartWare (n=6). The mean age was 60.2 years, 87% were male, and 47% were nonischemic. The overall median post-LVAD LDH was 583 (427–965) and there was no difference among devices. Mean hemolysis was 15.68 ± 12.96% at 0.45%NaCL (the inflection point of the osmotic fragility hemolysis curve). A scatter plot did not reveal any relationship between pre-op osmotic fragility and post-op LDH. Linear regression confirmed no predictive relationship (p=0.71). In conclusion, preoperative variations in osmotic fragility do not appear to account for differences in hemolysis following VAD placement. Mechanical forces generated by existing LVADs result in similar levels of biochemical hemolysis, as assessed by LDH, despite baseline differences in a patient’s osmotic red cell fragility.
Keywords: Hemolysis, LVAD, Left Ventricular Assist Device, Osmotic Fragility, Mechanical Circulatory Device, VAD, Ventricular Assist Device
Ventricular assist devices (VADs), have markedly improved survival and quality of life for patients with advanced heart failure, but these devices carry significant risks including pump thrombosis, stroke, and life-threatening bleeding.1,2 The inherent need for anticoagulation in the face of an environment that is conducive to bleeding challenges all clinicians caring for these patients. Major bleeding events have ranged from 1.13 to 1.66 per patient-year for continuous flow devices. Conversely, thromboembolic events can result in ischemic stroke, peripheral arterial thrombosis or pump thrombosis.1–3 Pump thrombosisis relatively uncommon (0.02–0.03 events per patient-year), but remains one of the most feared complications of VAD therapy and its incidence appears to be rising.1,2,4,5
A recent analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) noted that pump thrombosis and pump-related hemolysis were identified in 54% of patients who underwent pump exchange.6 Outside of those that actually develop pump thrombosis, all current generation LVADs result in some degree of increased lactate dehydrogenase (LDH). Indeed, the elevated levels of lactate dehydrogenase (LDH) and plasma free hemoglobin, markers for hemolysis, are frequently seen at the time of VAD complications as well as during support with normally functioning devices.7,8
INTERMACS defines hemolysis as a plasma-free hemoglobin value of greater than 40mg/dl in association with clinical signs of hemolysis. This level of plasma free hemoglobin is much higher than the <5 mg/dl defined as normal in patients without mechanical circulatory support. This is reflective of the supra-physiologic levels of hemolysis generated by all current generation VADs.6,9,10 The implications of lesser, subclinical elevations in hemolysis remain clinically unknown, although higher degrees of hemolysis as measured by elevation in LDH have clearly been linked to device complications.11 The recognition of this relationship prompted many centers to routinely screen for hemolysis following LVAD placement.12,13
To date, scant information exists defining which advanced heart failure patients may be more or less prone to develop post-LVAD hemolysis and the clinically significant effects of lesser degrees of hemolysis. As such, we sought to determine if the baseline osmotic stability of a red blood cell (RBC) influenced the ability of the RBC to withstand the mechanical forces of continuous flow VADs. The osmotic fragility assay has been traditionally utilized to evaluate patients with certain RBC membrane defects, such as hereditary spherocytosis. Erythrocyte osmotic fragility can be directly assayed and does correlate to the severity of disease and degree of clinically significant hemolysis seen in these patients.14,15 While patients with certain RBC membrane defects represent one end of the osmotic fragility spectrum, patients without such membrane cell defects will have RBCs that are relatively more or less resistant to hemolysis under osmotic stress. With the potential for differential RBC osmotic fragility, we prospectively investigated the degree to which the patient’s baseline red cell fragility, as measured by osmotic fragility, contributes to the degree of post-VAD hemolysis.
Methods
Study Patients
Osmotic Fragility assays were prospectively obtained prior to LVAD placement on 50consecutive patients from February 2011 to September 2012. Five patients were excluded from analysis due to the presence of a mechanical circulatory support at the time of VAD placement (extracorporeal membrane oxygenation orpercutaneous support). Furthermore, no patients with biventricular devices were included. The remaining 45 patients were included in the analysis. The primary hypothesis was that pre-implant red blood osmotic cell fragility would impact post-implant hemolysis (as measured by LDH) in patients undergoing LVAD placement.
Two groups were defined, high osmotic fragility and low osmotic fragility, on the basis of whether the percent hemolysis was above or below the median. Baseline characteristics including medications and hemodynamic parameters were recorded as the most recent values available prior to LVAD placement. Length of stay was defined as starting with the date of LVAD implantation until discharge. The study was approved by the University of Utah institutional review board.
Laboratory analysis
Erythrocyte osmotic fragility assay was performed using spectrophotometry to quantify the percent erythrocyte hemolysis under varying osmotic stresses ranging from 0.9%sodium chloride (NaCl) to0% NaCl (water). Percentage hemolysis in 0.45% NaCl (the approximate inflection point of the osmotic fragility inflection curve) was used to define two groups (Low Fragility & High Fragility) with osmotic fragility below and above the median respectively. LDH was measured as total serum LDH using a quantitative enzymatic assay, normal range 100–253 U/L. This LDH level was obtained as close to 4 weeks postoperatively as possible or at discharge to attempt to diminish the effect of perioperative LDH elevations. LDH results are reported as median and interquartile range. All laboratory analysis was performed at the University of Utah ARUP National Reference Laboratory (Salt Lake City, Utah, USA).
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation, median with interquartile range (IQR) or percentages. Length of stay, follow-up time, and survival were described using median with IQR. Categorical variables were analyzed using the chi-square test or Fisher’s exact test when expected cell counts were less than 5. Student’s t-test (two-tailed) was used to compare continuous variables. Linear regression was used to test the relationship between pre-VAD osmotic fragility and post-VAD LDH. Survival Analysis was performed using the Kaplan-Meier method with log-rank test to compare survival curves. A p<0.05 was considered significant. All analyses were performed using Stata 12.1 (StataCorp. 2011. College Station, TX).
Results
Forty-five patients with a mean age 60.2 ± 13.5 years underwent LVAD placement for New York Heart Association (NYHA) class IV heart failure. Of these, 86.6% were male and 53% had heart failure due to ischemic cardiomyopathy. Baseline patient characteristics including hemodynamic parameters and concomitant medications are listed in Table 1. The high osmotic fragility patients had significantly more hemolysis in 0.45% NaCl than the low osmotic fragility patients (p<0.001), indicating we had good separation between groups based on our definition. The high osmotic fragility patients were older (64.4 vs 55.9) by an average of 8.5 years (p=0.03). The high osmotic fragility patients were also more likely to be on a beta-blocker at the time of admission prior to VAD placement (35% vs 5%, p=0.01). Otherwise these two groups were similar in their baseline characteristics.
Table 1.
Baseline Patient Characteristics
| Variable | Overall (n = 45) | Low Fragility (n = 22) | High Fragility (n = 23) | p-value |
|---|---|---|---|---|
| % Hemolysis (0.45% NaCl) | 15.68 ± 12.96 | 5.86 ± 3.75 | 25.09 ± 11.56 | <0.001** |
| Age, mean | 60.2 ± 13.5 | 55.9 ± 16.1 | 64.5 ± 9.1 | 0.03* |
| Sex % Male | 86.6% | 91% | 83% | 0.67 |
| Etiology | ||||
| Ischemic cardiomyopathy | 24 (53%) | 12 | 12 | 0.87 |
| Non-ischemic | 21 (47%) | 10 | 11 | 0.87 |
| BMI | 26.7 ± 4.9 | 26.5 ± 5.25 | 26.8 ± 4.6 | 0.76 |
| NYHA Class IV | 45 (100%) | 22 (100%) | 23 (100%) | >0.99 |
| HR | 81.8 ± 19.2 | 86.0 ± 17.5 | 77.8 ± 20.3 | 0.15 |
| SBP | 99.1 ± 12.2 | 98.6 ± 10.5 | 99.5 ± 13.8 | 0.81 |
| DBP | 60.0 ± 9.4 | 59.3 ± 8.6 | 60.5 ± 10.4 | 0.67 |
| EF | 17.5 ± 6.6 | 17.7 ± 6.7 | 17.3 ± 6.4 | 0.83 |
| CI | 1.73 ± 0.61 | 1.80 ± 0.74 | 1.64 ± 0.44 | 0.41 |
| PCWP | 21.2 ± 8.4 | 21.3 ± 9.0 | 21.1 ± 8.0 | 0.94 |
| PVR | 4.8 ± 3.4 | 4.6 ± 3.1 | 4.9 ± 3.9 | 0.85 |
| Cr | 1.34 ± 0.46 | 1.35 ± 0.48 | 1.33 ± 0.45 | 0.86 |
| Concomitant Medications (%) | ||||
| Loop diuretics | 84% | 82% | 87% | 0.70 |
| Aldosterone antagonists | 58% | 50% | 65% | 0.30 |
| ACE Inhibitors | 36% | 36% | 35% | 0.91 |
| ARB | 20% | 18% | 22% | 0.77 |
| Amiodarone | 38% | 41% | 35% | 0.67 |
| Beta-blocker | 20% | 5% | 35% | 0.01* |
| IV Inotrope | 76% | 86% | 65% | 0.10 |
p<0.001,
p<0.05
Three continuous flow VAD device types were utilized: Heartmate II, HeartWare & Jarvik 2000. The majority of patients (75.6%) received the Heartmate II device, while 13.3% received the HeartWare device and 11.1% received a Jarvik 2000. The different devices were utilized at similar rates among the high and low osmotic fragility groups, Table 2.
Table 2.
VAD Device Type
| Device Type | Overall (n= 45) | Low Fragility (n = 22) | High Fragility (n =23) | p-value |
|---|---|---|---|---|
| Heartmate II | 34 (75.6%) | 16 (72.7%) | 18 (78.3%) | 0.66 |
| HeartWare | 6 (13.3%) | 3 (13.6%) | 3 (13.0%) | >0.99 |
| Jarvik | 5 (11.1%) | 3 (13.6%) | 2 (8.7%) | 0.67 |
There was no difference in post-operative LDH levels between the high osmotic fragility and low osmotic fragility patients: median562 (IQR 506–936) versus 704 (427–1014), p= 0.81. Individual percent hemolysis in 0.45% NaCl vs Post-op LDH was graphed using a scatter plot (Figure 1). The scatter plot revealed no clear relationship, nor need for data transformation. Linear regression confirmed no predictive relationship between pre-op osmotic fragility and post-op LDH (p=0.71).
Figure 1.
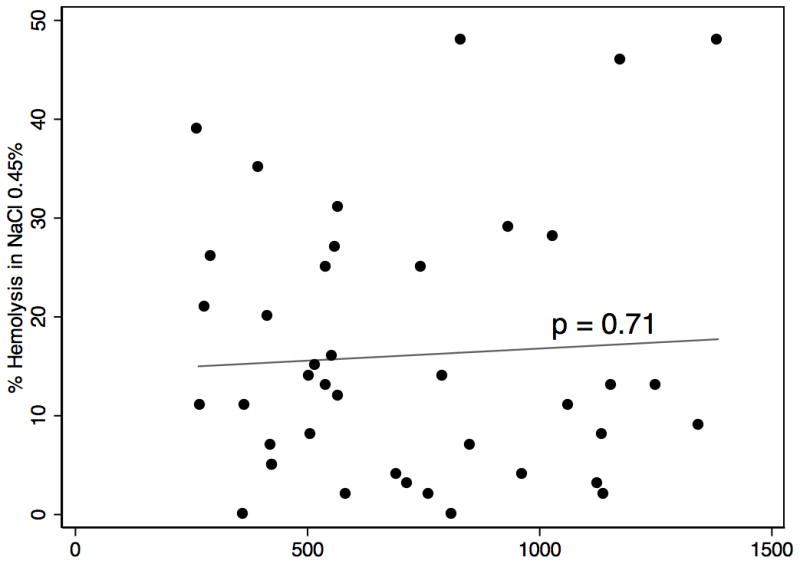
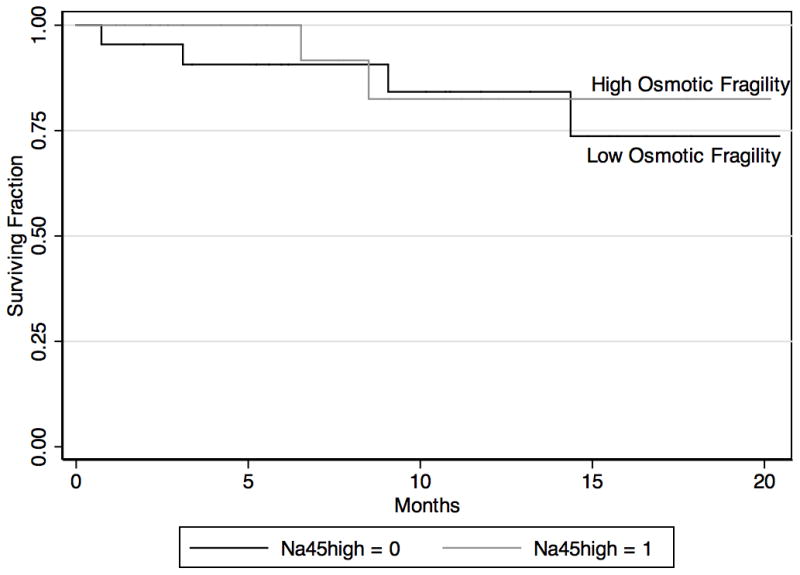
Median follow-up was 9.1 months (IQR 3.4–14.4 months) overall and was similar in both groups. Length of stay and post-operative complications were similar between groups, (Table 3). As depicted in figure 2, there was no difference in survival between the high and low osmotic fragility groups (p=0.64).
Table 3.
Outcomes
| Variable | Overall (n= 45) | Low Fragility (n = 22) | High Fragility (n =23) | p-value |
|---|---|---|---|---|
| LDH, median, (IQR) | 583 (427–965) | 704 (427–1014) | 562 (506–936) | 0.81 |
| Follow-up, months, median, (IQR) | 9.1 (3.4–14.4) | 10.8 (5.6–15.5) | 6.5 (2.4–11.8) | 0.13 |
| Length of stay, median, (IQR) | 14 (10–22) | 17(12–24) | 12 (8–21) | 0.24 |
| RVAD needed | 2 (4.4%) | 2 (9.1%) | 0 (0%) | 0.23 |
| Stroke | 3 (6.7%) | 2 (9.2%) | 1 (4.4%) | 0.61 |
| VAD Thrombosis | 2 (4.4%) | 2 (9.1%) | 0 (0%) | 0.23 |
| Renal Failure | 1 (2.2%) | 1 (4.6%) | 0 (0%) | 0.49 |
| Survival, Median (IQR) | Not Reached | Not Reached | Not Reached |
p<0.05
Figure 2.
Discussion
We hypothesized that baseline red cell osmotic fragility would impact post-implant hemolysis rates in patients receiving continuous flow LVADs. Alternatively, we observed, in this relatively small group of patients, that preoperative osmotic fragility has no predictable influence on post-LVAD hemolysis.
The majority of patients with durable LVADs have supra-physiologic, and quite possibly clinically irrelevant, levels of hemolysis as witnessed by elevations in clinical markers of hemolysis. Even more marked elevations in LDH and/or plasma free hemoglobin are often identified at the time of thrombotic complications, including pump thrombosis. This increase in hemolysis is typically thought to be a consequence and a marker of the thrombotic event.
While increased hemolysis in patients undergoing VAD therapy often prompts an investigation for complications such as device thrombosis, hemolysis itself can actually drive thrombosis secondary to adenosine diphosphate release, which is a potent platelet activator. Additionally, plasma free hemoglobin is liberated, which lowers the bioavailability of nitric oxide, in turn enhancing platelet activation.16 In this way, the supra-physiologic levels of hemolysis generated by current generation ventricular assist devices may actually contribute to thrombosis of the device itself through platelet activation. Currently, it is not know if these or other mechanisms are responsible for the reported recent increase in left ventricular assist device thrombosis, but this increase has been associated with a significant increase in morbidity and mortality.5,17
While much interest is focused on a particular pump’s proclivity to pump-induced hemolysis, we observed no differences between second and third generation pumps (admittedly the relative numbers are too small to draw firm conclusions). Theoretically, the axial flow pumps might provide a more “harsh” environment, that if there was a proclivity to RBC fragility, might lead to more hemolysis.18,19
To date, few pre-operative risk factors have been identified which predict increased levels of postoperative hemolysis. Recently, glucose 6-phosphatase deficiency (G6PD), while present in only a small segment of patients, has been associated with increase hemolytic events following VAD placement.20 The underlying ethnic mix of this cohort is primarily Caucasian, which may be a limitation as some conditions that predispose individuals to hemolysis, such as G6PD, are more prevalent in non-Caucasian populations. In our cohort, patients with high osmotic fragility were older, but this ultimately did result in differences in post-VAD hemolysis.
This study has several limitations. LDH is not a perfect marker for RBC hemolysis. While LDH is commonly used to screen for RBC hemolysis, INTERMACS and other organizations use elevations in plasma free hemoglobin to define hemolysis. At the time of this study plasma free hemoglobin was utilized in our center as a confirmatory test when clinical concern of marked hemolysis was high, while LDH was obtained routinely.21,22 Additionally, factors such as recent transfusion and serum osmolality may affect the osmotic fragility assay result and our patients’ transfusion status and osmolality at the time of their osmotic fragility assay is unknown.23
Finally, osmotic fragility measures one specific aspect of RBC fragility, that being the hemolytic response of the RBC when placed under various degrees of osmotic stress. While the effects of osmotic fragility on hemolysis in LVAD patients has not been previously published, in other model systems osmotic fragility does not necessarily predict red blood cell deformability not the hemolytic response to mechanical stress.24,25 While we saw no predictive relationship between pre-implant osmotic fragility and post-VAD hemolysis, other measurements of RBC fragility to mechanical/sheer stresses may be more likely to predict post-VAD hemolysis.
Conclusions
The ability to pre-operatively identify which patients are at higher risk for post-operative hemolysis and thromboembolic complications would significantly aid in patient selection and counseling as well as provide valuable clinical information to guide individualized post-implantation anti-platelet and anticoagulation strategies. The current study demonstrates that preoperative variations in osmotic fragility do not appear to account for differences in hemolysis following LVAD placement. Mechanical forces generated by existing LVADs result in similar levels of biochemical hemolysis, as assessed by LDH, despite baseline differences in a patient’s red cell osmotic fragility.
Footnotes
Disclosure: This work is funded in part by the National Institute of Health, R01HL089592 (CHS), U54 HL112311 and 2R01 HL066277 (ASW), NIH K23HL092161 and R03AG040631 (MTR); the Veterans Affairs Administration Merit 1I01CX000710-01A1 (JS); and Doris Duke Foundation Clinical Scientist Development Grant 7/2013 and Deseret Foundation #00571 (SGD),
References
- 1.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. Journal of Heart and Lung Transplantation. 2013;32(2):141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Eckman PM, John R. Bleeding and Thrombosis in Patients With Continuous-Flow Ventricular Assist Devices. Circulation. 2012;125(24):3038–3047. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 5.Starling RC, Moazami N, Silvestry SC, et al. Unexpected Abrupt Increase in Left Ventricular Assist Device Thrombosis. N Engl J Med. 2013:131127140053002. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 6.Holman WL, Naftel DC, Eckert CE, Kormos RL, Goldstein DJ, Kirklin JK. Durability of left ventricular assist devices: Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 2006 to 2011. J Thorac Cardiovasc Surg. 2013;146(2):437–41. e1. doi: 10.1016/j.jtcvs.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Heilmann C, Geisen U, Benk C, et al. Haemolysis in patients with ventricular assist devices: major differences between systems⋆. European Journal of Cardio-Thoracic Surgery. 2009;36(3):580–584. doi: 10.1016/j.ejcts.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Stepanenko A, Krabatsch T, Hennig E, et al. Retrospective Hemolysis Comparison Between Patients With Centrifugal Biventricular Assist and Left Ventricular Assist Devices. Asaio J. 2011;57(5):382–387. doi: 10.1097/MAT.0b013e31822c4994. [DOI] [PubMed] [Google Scholar]
- 9.Chan CHH, Hilton A, Foster G, Hawkins K. Reevaluation of the Harboe Assay as a Standardized Method of Assessment for the Hemolytic Performance of Ventricular Assist Devices. Artif Organs. 2012;36(8):724–730. doi: 10.1111/j.1525-1594.2012.01515.x. [DOI] [PubMed] [Google Scholar]
- 10.The PediaFlow Consortium. Maul TM, Kocyildirim E, et al. In Vitro and In Vivo Performance Evaluation of the Second Developmental Version of the PediaFlow Pediatric Ventricular Assist Device. Cardiovasc Eng Tech. 2011;2(4):253–262. doi: 10.1007/s13239-011-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klotz S, Klotz S, Vahlhaus C, et al. Pre-operative prediction of post-VAD implant mortality using easily accessible clinical parameters. J Heart Lung Transplant. 2010;29(1):45–52. doi: 10.1016/j.healun.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Feldman D, Pamboukian SV, Pamboukian SV, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J Heart Lung Transplant. 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Mountis MM, Starling RC. Management of left ventricular assist devices after surgery: bridge, destination, and recovery. Curr Opin Cardiol. 2009;24(3):252–256. doi: 10.1097/HCO.0b013e32832c7c09. [DOI] [PubMed] [Google Scholar]
- 14.Shim YJ, Won DI. Flow cytometric osmotic fragility testing does reflect the clinical severity of hereditary spherocytosis. Cytometry. 2013 doi: 10.1002/cyto.b.21143. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 15.King MJ, Zanella A. Hereditary red cell membrane disorders and laboratory diagnostic testing. Int Jnl Lab Hem. 2013;35(3):237–243. doi: 10.1111/ijlh.12070. [DOI] [PubMed] [Google Scholar]
- 16.Helms CC, Marvel M, Zhao W, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013 doi: 10.1111/jth.12422. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechter MA, Daneshmand MA, Patel CB, Blue LJ, Rogers JG, Milano CA. Outcomes After Implantable Left Ventricular Assist Device Replacement Procedures. Asaio J. 2013:1. doi: 10.1097/MAT.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanfield JR, Selzman CH, Pardyjak ER, Bamberg S. Flow Characteristics of Continuous-Flow Left Ventricular Assist Devices in a Novel Open-Loop System. Asaio J. 2012;58(6):590–596. doi: 10.1097/MAT.0b013e31826dcbd9. [DOI] [PubMed] [Google Scholar]
- 19.Stanfield JR, Selzman CH. In Vitro Pulsatility Analysis of Axial-Flow and Centrifugal-Flow Left Ventricular Assist Devices. J Biomech Eng. 2013;135(3):034505. doi: 10.1115/1.4023525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhosaini H, Katz JN, Jensen BC, Stansfield W, Sheridan BC, Chang PP. A Novel Link between G6PD Deficiency and Hemolysis Events in Patients Supported with Continuous-Flow Left Ventricular Assist Devices. The Journal of Heart and Lung Transplantation. 2013;32(4):S38–1. doi: 10.1016/j.healun.2013.01.897. [DOI] [PubMed] [Google Scholar]
- 21.INTERMACS. Appendix A - Adverse Event Definitions. 2010. [Google Scholar]
- 22.Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. Journal of Heart and Lung Transplantation. 2013:1–3. doi: 10.1016/j.healun.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Almizraq R, Tchir JDR, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013 doi: 10.1111/trf.12080. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 24.Gu L, Smith WA, Chatzimavroudis GP. Mechanical Fragility Calibration of Red Blood Cells. Asaio J. 2005;51(3):194–201. doi: 10.1097/01.MAT.0000161940.30190.6D. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Beutler E. Erythrocyte cellular and membrane deformability in hereditary spherocytosis. Blood. 1979;53(3):481–485. [PubMed] [Google Scholar]


