Abstract
Background:
Although controversial, many studies have shown effectiveness of colloid loading as a substitute for crystalloids on reducing the incidence of hypotension in spinal anesthesia. This study was conducted to compare the effects of three intravenous fluid regimens on hemodynamic changes following spinal anesthesia in cesarean section. The regimens included 6% Hydroxyethylstarch 130/0.4 (HES) as a colloid and two crystalloids (lactated ringer’s solution and sodium chloride 0.9%).
Material & Method:
In a double-blind clinical trial, 90 otherwise healthy parturients candidate of elective caesarean section were randomly allocated to receive lactated ringer’s solution (1000 ml), sodium chloride 0.9% (1000 ml) or HES (7.5 mL/Kg) as preloading before spinal anesthesia. Hemodynamic parameters including blood pressure and heart rate, umbilical cord blood pH and the neonatal Apgar score were compared among the three groups.
Results:
There was no difference in the basic hemodynamic measurements among the three groups. The incidence of hypotension and required dose of ephedrine was lower in HES group (p=0.008). There was no significant difference in umbilical cord blood PH or Apgar scores among intervention groups.
Conclusion:
Preloading with HES is more effective than crystalloids in prevention hypotension after spinal anesthesia without significant difference in Apgar score and umblical cord blood pH.
Keywords: Caesarian section, Hydroxyethylstarch, Crystalloid, spinal anesthesia, blood pressure
1. INTRODUCTION
Spinal anesthesia offers many advantages for cesarean delivery. It has a very rapid onset and provides a dense neural block. Because of the small doses used, there is little risk of local anesthetic toxicity and minimal transfer of drug to the fetus. Disadvantages of this technique include the finite duration of anesthesia and a higher incidence of hypotension (1, 2, 3).
Hypotension if remain untreated could produce fetal distress and reduce Apgar Score (4). Expansion of intravascular volume, physical methods or prophylactic administration of vasoactive drugs such as ephedrine or phenylephrine are used to prevent hypotension (5, 6). Intravascular volume expansion could take place by different types of fluids. Crystalloids are effective for prevention of hypotension in parturient (7), although less expensive and more available they need to be infused in larger amount and so are more time consuming. Crystalloid replacement can induce fluid retention edema and may also produce electrolyte derangement (8). Some studies indicate that crystalloids by entering extravascular space may not completely replace intravascular deficit, so there is still some doubt about the value of relying just on crystalloids for volume expansion (9, 10).
Colloids remain for a longer period of time in circulation and the required volume is equal to the volume of deficit or blood loss (8). One of the disadvantages of colloids is their detrimental effect on haemostatic system. However, this concern may not be relevant for Hydroxyethyl starch (6% HES 130/0.4) because of its low molecular weight (11). Although controversial, some studies have reported the beneficial effects of colloids on reducing the incidence of hypotension (12, 13). The issue needs to be validated in further studies.
In spite of prophylactic measures, there is still a high incidence of hypotension up to 80% in cesarean section following spinal anesthesia (14, 15). This study aimed to compare the effects of three intravenous fluid regimens including HES, lactated ringer and sodium chloride 0.9% on hemodynamic changes following spinal anesthesia in cesarean section. Apgar scores and umbilical cord blood pH were also evaluated as secondary outcomes.
2. METHODS
2.1. Patients
In a double-blind clinical trial, 90 otherwise healthy parturients candidate of elective caesarean section were enrolled. Inclusion criteria consisted of normal single pregnancy, gestational age of more than 37 weeks, and no history of hypertension. Patients with any contraindication for spinal anesthesia, third trimester bleeding, body mass index (BMI) more than 30, previous allergy to HES preparations, known cardiomyopathy, height less than 155cm and sympathetic block higher than T4 level were excluded from the study. The local ethics committee approved the study protocol. Informed consent was obtained from all participants before inclusion.
2.2. Intervention and measurements
After admitting to operating room, for each patient two intravenous lines 20G and standard monitoring (noninvasive blood pressure, pulse oximetry, ECG) were established and baseline blood pressure (BP) and heart rate (HR) were recorded. Using block randomization table, 90 patients were allocated to three groups of HES, NS and LR. In the preoperative holding area patients were hydrated with 1000 ml lactated Ringer’s in LR group, 1000 ml Sodium chloride 0.9% in NS group and 7.5 ml/kg HES 6%, 130/0.4 (Voluven, Fresenius Kabi, Germany) in HES group in 15 minutes. After volume loading, patients were transferred to the operating room. Spinal anesthesia was performed in lateral position with Quincke needle No. 25 in either L3-L4 or L4-L5 interspace. A total of 12mg hyperbaric Bupivacaine 0.5% was injected in subarachnoid space. The patients immediately turned to supine position.
Blood pressure and HR monitoring were performed in predetermined intervals (every 1 minute for the first 10 minutes, every 2 minute for the second 10 minutes and every 5 minute for the rest of surgery). The level of sympathetic block was checked by cotton swab. More than 20% reduction in SBP or BP less than 100 mmHg was considered as hypotension. In such cases 5mg intravenous ephedrine was administered to manage hypotension. Total dose of administered ephedrine and level of block were recorded. Maintenance Fluid was calculated according to 4-2-1 method. Umbilical cord blood pH and the neonatal Apgar score were recorded.
2.3. Statistical analysis
Data were presented as mean (standard deviation) or frequency (percentage), as appropriate. Age, blood pressure, heart rate, Apgar scores and umbilical cord pH were compared among the three groups with ANOVA or Kruskal-Wallis tests, according to the result of Kolmogrov-smirnov test. For comparing the incidence of hypotension Chi-square test was used. Apgar score and umbilical cord blood pH was compared in patients with or without hypotension with Man-Whitney U test. P value less than 0.05 was considered statistically significant. All the comparisons were two-tailed. Analyses were performed using SPSS version 11 (SPSS Inc., Chicago, Il).
3. RESULTS
Baseline measurements including age, BP and HR were not statistically different among the three groups (Table 1). The level of sympathetic block in all patients was between T4 and T6. Hypotension was detected in 31 patients after spinal anesthesia. The incidence of systolic hypotension and ephedrine administration was significantly lower in HES group than either LR or NS groups (13.3% vs. 46.6% and 40%, respectively; P<0.05). Changes in diastolic and mean BP as well as HR were also lower in HES than either crystalloid groups (Table 2).
Table 1.
Baseline variables in three groups
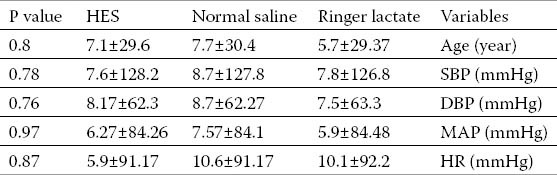
Table 2.
Maternal hemodynamic parameters and neonatal outcome measures following spinal anesthesia
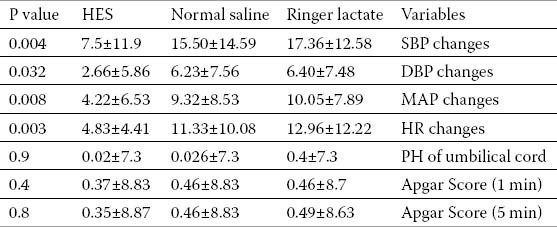
There was no significant difference in umbilical cord blood PH. Mean Apgar scores in all 3 groups were above eight with no statistically significant difference. (Table 2) Further analyses in patients with and without hypotension showed that mean Apgar score in the 5th min (8.7±0.4 vs 8.8±0.4) and umbilical blood pH (7.3±0.033 vs 7.3±0.034) may not be affected by BP changes (p<0.05)
4. DISCUSSION
The cardiovascular effects of neuraxial blocks are similar in some ways to the combined use of intravenous α1- and β-adrenergic blockers including decreased heart rate and arterial blood pressure. The sympathectomy that accompanies the techniques depends on the height of the block. This sympathectomy causes venous and arterial vasodilation, the venodilation effect predominates as a result of the limited amount of smooth muscle in venules. That the decrease in arterial blood pressure after neuraxial block can be minimized by the administration of crystalloids intravenously is probably not a valid concept. Specifically, 250- to 2000-mL preblock hydration regimens appear to temporarily increase preload and cardiac output without consistently increasing arterial blood pressure or preventing hypotension. The extent to which arterial blood pressure decreases with nuroaxial technique depends on multiple factors, including patient age and intravascular volume status (15).
Hypotension is present when systolic blood pressure decreases to less than 100 mm Hg or to more than 20% less than baseline readings. Hypotension occurs in many patients after neuraxial anesthesia. The incidence and severity of hypotension depends on the height of the block, the position of the parturient, and whether prophylactic measures were taken to avoid such hypotension. Measures that decrease the risk of hypotension to varying degrees include intravenous administration of fluids, avoidance of aortocaval compression (left uterine displacement), and vigilant monitoring of blood pressure at frequent intervals after placement of a regional anesthetic. Intravenous ephedrine in 5- to 10-mg increments remains the first-line treatment; recent evidence supports the use of phenylephrine (14).
Fluid preloading with 15-20 ml of crystalloids is an accepted method of reducing the incidence of hypotension. However, there is no consensus about the most effective solution against hemodynamic changes during spinal anesthesia. Although controversial (16, 17), several earlier studies have reported the superiority of different colloid solutions over crystalloids on reducing the incidence of spinal induced hypotension (18-24). Voluven (6% HES 130/0.4 in 0.9% sodium chloride) is used for intravenous volume expansion with almost least complications seen with other colloids. In the present study, in spite of less administered infused volume in the HES group versus crystalloid groups, the hemodynamic parameters including BP and HR were more stable.
Noteworthy, our findings suggest that hemodynamic changes are not associated with lower neonatal Apgar scores and umbilical cord PH. It seems that transient maternal hypotension, if recognized and treated promptly, may not be associated with neonatal morbidity. Another study on 60 parturients candidate of caesarian section reported that HES and crystalloid solutions comparably maintain cardiac output following spinal anesthesia (25).
Our findings indicated that preloading with HES in parturients candidate for caesarean section under spinal anesthesia can significantly reduce the incidence of spinal induced hypotension compared to crystalloid solutions. This will reduce the need for vasoactive medications such as ephedrine. However, apgar scores and umbilical cord PH may not be affected by the type of IV fluid therapy. It seems reasonable to conclude that in the case of prompt treatment of maternal hypotension, HES, LR and NS all effectively maintain uteroplacental perfusion in otherwise healthy parturients. Possibly economic concerns and parturient’ co-morbidities such as preeclampsia play a role in selecting the most appropriate choice of solution before spinal anesthesia. The issue needs to be addressed in further clinical trials and cost-effective analyses.
5. CONCLUSION
Preloading with HES is more effective than crystalloids in prevention hypotension after spinal anesthesia without significant difference in Apgar score and umblical cord blood pH.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Afolabi BB, Lesi FE. Regional versus general anaesthesia for caesarean section. Cochrane Database Syst Rev. 2012 Oct 17;10:CD004350. doi: 10.1002/14651858.CD004350.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Langesæter E, Dyer RA. Maternal haemodynamic changesduring spinal anaesthesia for caesarean section. Curr Opin Anaesthesiol. 2011 Jun;24(3):242–248. doi: 10.1097/ACO.0b013e32834588c5. [DOI] [PubMed] [Google Scholar]
- 3.Gogarten W. Spinal anaesthesia for obstetrics. Best Pract Res Clin Anaesthesiol. 2003 Sep;17(3):377–392. doi: 10.1016/s1521-6896(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 4.Preston R, Crosby ET, Kotarba D, Dudas H, Elliott RD. Maternal positioning affects fetal heart rate changes after epidural analgesia for labour. Can J Anaesth. 1993 Dec;40(12):1136–1141. doi: 10.1007/BF03009602. [DOI] [PubMed] [Google Scholar]
- 5.Burns SM, Cowan CM, Wilkes RG. Prevention and management of hypotension during spinal anaesthesia forelective Caesarean section: a survey of practice. Anaesthesia. 2001 Aug;56(8):794–798. doi: 10.1046/j.1365-2044.2001.02058-5.x. [DOI] [PubMed] [Google Scholar]
- 6.Morgan PJ, Halpern SH, Tarshis J. The effects of an increase of central blood volume before spinal anesthesiafor cesarean delivery: a qualitative systematic review. Anesth Analg. 2001 Apr;92(4):997–1005. doi: 10.1097/00000539-200104000-00036. [DOI] [PubMed] [Google Scholar]
- 7.Rout CC, Rocke DA, Levin J, Gouws E, Reddy D. A reevaluation of the role of crystalloid preload in the prevention of hypotension associated withspinal anesthesia for elective cesarean section. Anesthesiology. 1993 Aug;79(2):262–269. doi: 10.1097/00000542-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013 Feb 28;2:CD000567. doi: 10.1002/14651858.CD000567.pub6. doi:10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 9.Rout CC, Akoojee SS, Rocke DA, Gouws E. Rapid administration of crystalloid preload does not decrease the incidence of hypotension after spinal anaesthesia for elective caesarean section. Br J Anaesth. 1992 Apr;68(4):394–397. doi: 10.1093/bja/68.4.394. [DOI] [PubMed] [Google Scholar]
- 10.Jackson R, Reid JA, Thorburn J. Volume preloading is not essential to prevent spinal-induced hypotension at caesarean section. Br J Anaesth. 1995 Sep;75(3):262–265. doi: 10.1093/bja/75.3.262. [DOI] [PubMed] [Google Scholar]
- 11.Boldt J, Haisch G, Suttner S, Kumle B, Schellhaass A. Effects of a new modified, balanced hydroxyethyl starch preparation (Hextend) on measures of coagulation. Br J Anaesth. 2002 Nov;89(5):722–728. doi: 10.1093/bja/aef242. [DOI] [PubMed] [Google Scholar]
- 12.Dahlgren G, Granath F, Wessel H, Irestedt L. Prediction of hypotension during spinal anesthesia for Cesarean section and its relation to the effect of crystalloid or colloid preload. Int J Obstet Anesth. 2007 Apr;16(2):128–134. doi: 10.1016/j.ijoa.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Karinen J, Räsänen J, Alahuhta S, Jouppila R, Jouppila P. Effect of crystalloid and colloid preloading on uteroplacental and maternal haemodynamic stateduring spinal anaesthesia for caesarean section. Br J Anaesth. 1995 Nov;75(5):531–535. doi: 10.1093/bja/75.5.531. [DOI] [PubMed] [Google Scholar]
- 14.Ngan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. AnesthAnalg. 2000 Jun;90(6):1390–1395. doi: 10.1097/00000539-200006000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Brown DL. Spinal, epidural and caudal anesthesia. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010. pp. 1611–1638. [Google Scholar]
- 16.Tamilselvan P, Fernando R, Bray J, Sodhi M, Columb M. The effectsof crystalloid and colloid preload on cardiac output in the parturient undergoing planned cesarean delivery under spinal anesthesia: a randomized trial. Anesth Analg. 2009 Dec;109(6):1916–1921. doi: 10.1213/ANE.0b013e3181bbfdf6. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama N, Nishikawa K, Saito Y, Saito S, Goto F. [Comparison of the effects of colloid and crystalloid solution for volume preloading on maternalhemodynamics and neonatal outcome in spinal anesthesia for cesarean section] Masui. 2004 Sep;53(9):1019–1024. [PubMed] [Google Scholar]
- 18.French GW, White JB, Howell SJ, Popat M. Comparison of pentastarch and Hartmann's solution for volume preloading inspinal anaesthesia for elective caesarean section. Br J Anaesth. 1999 Sep;83(3):475–477. doi: 10.1093/bja/83.3.475. [DOI] [PubMed] [Google Scholar]
- 19.Dahlgren G, Granath F, Pregner K, Rösblad PG, Wessel H, Irestedt L. Colloid vs. crystalloid preloading to prevent maternal hypotension during spinal anesthesia forelective cesarean section. Acta Anaesthesiol Scand. 2005 Sep;49(8):1200–1206. doi: 10.1111/j.1399-6576.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa K, Yokoyama N, Saito S, Goto F. Comparison of effects of rapid colloid loading before and after spinal anesthesia on maternalhemodynamics and neonatal outcomes in cesarean section. J Clin Monit Comput. 2007 Apr;21(2):125–129. doi: 10.1007/s10877-006-9066-4. [DOI] [PubMed] [Google Scholar]
- 21.Siddik SM, Aouad MT, Kai GE, Sfeir MM, Baraka AS. Hydroxyethylstarch 10% is superior to Ringer's solution for preloading before spinal anesthesiafor Cesarean section. Can J Anaesth. 2000 Jul;47(7):616–621. doi: 10.1007/BF03018992. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SK, Gajraj NM, Sidawi JE. Prevention of hypotension during spinal anesthesia: a comparison of intravascular administration of hetastarch versus lactated Ringer's solution. Anesth Analg. 1997 Jan;84(1):111–114. doi: 10.1097/00000539-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Madi-Jebara S, Ghosn A, Sleilaty G, Richa F, Cherfane A, Haddad F, et al. Prevention of hypotension after spinal anesthesia for cesarean section: 6% hydroxyethyl starch130/0.4 (Voluven) versus lactated Ringer's solution. J Med Liban. 2008 Oct-Dec;56(4):203–207. [PubMed] [Google Scholar]
- 24.Bouchnak M, Magouri M, Abassi S, Khemiri K, Tlili F, Troudi H, et al. [Preloading with HES 130/0.4 versus normal saline solution to prevent hypotension duringspinal anaesthesia for elective caesarean section] Ann Fr Anesth Reanim. 2012 Jun;31(6):523–527. doi: 10.1016/j.annfar.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 25.McDonald S, Fernando R, Ashpole K, Columb M. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective cesarean delivery: a randomized controlled trial. Anesth Analg. 2011 Oct;113(4):803–810. doi: 10.1213/ANE.0b013e31822c0f08. [DOI] [PubMed] [Google Scholar]


