Abstract
Background:
Childhood urinary infections are among the most common febrile illnesses occurring during this period with varying susceptibility to antibiotic.
Aim:
The aim of this study was to identify uropathogens responsible to for urinarytract infection (UTIs) in children less than 5 years of age, and determine the antibiograms of the isolates to commonly used antibiotics.
Patients and methods:
Hundred and four children (2 months - 5 years old) seen at the Gadarif Teaching Hospital from January 2012 and December 2013 were evaluated. A urine specimen was obtained by a plastic bag with an adhesive backing around an opening or by direct voiding into sterile container. Urine was examined microscopically and those with significant pyuria and bacteruria were further cultured and microorganisms were identified and tested for antimicrobial susceptibility.
Results:
Out of 304 children suffering from UTIs; 145(47.7%) had significant pyuria of them; 54(17.8 %) had positive bacterial growth. The frequency of sex and residency were almost the same. E. coli (42.6%) was the most common uropathogen, sensitive to ciprofloxacin (91.3%), followed by Pseudomonas aeruginosa (29.6%) sensitive to Ciprofloxacin (75%)and Norofloxacin (68.8%), Klebsiellapneumoniae (18.5%) sensitive to Ciprofloxacin and Norofloxacin and Nalidixic acid (90%) and Proteus mirabilis sensitive to Ciprofloxacin and Norofloxacin (90%), Amoxicillin / clavulanic acid (Augmentin(80%).
Conclusion:
The most common uropathogens were E. coli, Pseudomonas aeruginosa,Klebsiellapneumoniae, and Proteus mirabilis. Ciprofloxacin is the recommended initial empirical therapy while awaiting the culture and sensitivity results.
Keywords: pediatrics, urinary tract infection, antimicrobial, sensitivity, resistance
1. INTRODUCTION
Urinary tract infection is the most common febrile illness in pediatric second to otitis media and pharyngitis. It accounted for 10% of all febrile illnesses in children (1, 2, 3). Long-term complications of urinary tract infection in children include unilateral renal parenchymal defect and unilateral kidney retarded growth. The glomerular filtration rate and blood pressure do not change indicating a very low for serious renal damage (4).
It is more common in preterm babies (4-25%) than term ones (1%) (5). In early life, it is more common in males than female then it decline rapidly in the prepubertal life girls experienced more episodes of UTIs than males, 8% compared to 2% respectively (6, 7).
Escherichia coli for many years remain the most common isolates causing UTI in children (60–92%). Other common organisms include Klebsiella, Proteus, Enterobacter spp. and Enterococcus (8-10). Diagnosis of UTI may in children is simple usually urine analysis and cultures are enough to establish the diagnosis. The most difficult task is to established appropriate therapy. The development of multidrug resistance strains makes treatment issue among the most controversial issues in children.
In Africa, UTIs in children is a common and complex problem due to co-infection with other febrile illnesses (11). High frequency rate of infection was reported by many authors due to co-existence of multiple risks factors. In Sudan, no prior studies on UTIs in children were conducted. The aim of this study was to identify uropathogens responsible for urinary tract infection in children fewer than 5 years of age, and determine the antibiograms of the uropathogens to commonly used antibiotics. Moreover, to provide the foundation for prevention programs, and making policy decisions.
2. METHODS AND SUBJECTS
This prospective cross-sectional study was conducted at Gadarif Teaching Hospital, in Gadarif State, Eastern Sudan, during the period from June 2011 to June 2012. It is the biggest hospital of the state and there are 800 beds to maintain health services for the whole Gadarif State. The study was approved by theHospital Ethical committee.
A total of 304 children under 5 years of age who fulfilled criteria for case definition of UTI were included in the study. Inclusion criteria were children under 5 years of age who suffered from (dysuria), (fever and dysuria), (dysuria and vomiting), (Dysuria, fever and vomiting), (Polyuria and suprabubic pain), (Fever), (Fever and vomiting), (Fever and diarrhea), (Fever, vomiting and diarrhea), (Vomiting), (Vomiting and diarrhea) or (Diarrhea) were eligible to be enrolled in the study (12). Children excluded from the study, were those already on antibiotic or known to suffer from other underlying conditions.
2.1. Specimens’ collection
The urine sample was collected by two different techniques. For children less than 3 years of age the specimens were collected by a disposable apparatus consisting of a plastic bag with an adhesive backing around an opening that can be fastened to the perineal area or around the penis to permit direct voiding into the bag. The uretheral area was cleaned thoroughly before applying the collection bag. The specimen bag was carefully removed, and the urine transferred to a sterile urine container. Then the collected urine was analyzed immediately (Fischbach and Dunning , 2004) (13). For children above the age of 3 years 3-5 ml of urine were collected into sterile container.
2.2. Urine examination
Urine samples were centrifuged at 2000 revolution per minute for 5 minutes. Wet film of urine deposits were microscopically examined for pus cells, RBCs, crystals, cast, bacterial cells. Finding of more than 5 white blood cells per high-power field in centrifuged fresh urine was considered a satisfactory positive screening test for UTI (15).
Semi-quantitative method was done byusing a calibrated loop (0.001ml) of un centrifuged urine , a loopfull was spread on Cystine lactose electrolyte deficient (CLED) agar. This CLED was incubated aerobically over night at 37° C if no growth further incubation for 48 hours, the growth of 100 colony forming units by this method indicates the presence of 105 bacteria per ml of urine (Michael L et. al, 2004) [16]. The bacterial growth estimation of (105)bacteria per ml or more was considered UTI; A count of 10.000–49.000bacteria/ ml was considered significant with a signs of acute infection (presence of pyuria). The count of 10 colonies and above with presence of pyuria indicate significant bacteriuria.
Bacteriological Identification for significant specimen on presumptive colony was identified using: colonial morphology (16), gram stain (17), and biochemical test s such as: indole, citrate, oxidase, H2S production, MRVP, lactose fermentation, urea hydrolysis, gas production, catalase, coagulase previously discuss by Bartet al. (18).
2.3 The Analytical Profile Index (API) systems
Analytical Profile Index (API-20E) system was performed for confirmation of identification as previously mentioned by (Ahmed MI, 2012) to identify members of the family Enterobacteriaceae and associated organisms (BioMerieux, Inc. Hazelwood, MO., France).
2.4 Antimicrobial susceptibility test
The “disc diffusion method” were employed by standardize filter paper discs impregnated with fixed amounts of antimicrobial drugs.
3. RESULTS
A total of 304 (100%) children suffering from UTIs with the mean age 1.97±1.061 years were included in this study. The majority of these children 203 (66.7 %) were less than two years of age, and more than half of them were male 174 (57.2%). Significant pyuria and bacteriuria was detected in 47.7 %(n=145) of the total studied subjects; in 250 (82.2%) no potential pathogens were isolated. The frequency of UTI was also equal in urban and rural settings (51% vs. 49%). Of them; only 17.5% (n=54) had positive bacterial growth. The majority 46.3 % (n=25) of subjects were less than 1 year of age, followed by age group 1 to 2 years 20.4%,and 16.7% for both 2 to 3 and more than 3 years of age.
The most common bacterial isolates were Escherichia coli - 23 (42.6%) followed by Pseudomonas aeruginosa -16 (29.6%), Klebsiellapneumoniae - 10(18.5%), and Proteus mirabilis - 5 (9.3%) as identified by conventional biochemical tests Figure 1. Furthermore, the rapid and accurate test of identification organisms (API identification system) for Enterobacteria used to confirm the conventional identification methods results showed typical results.
Figure 1.
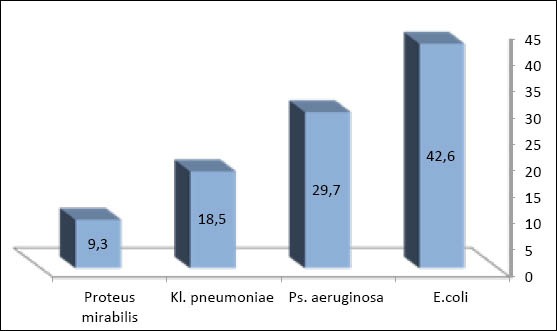
Types of bacterial isolates
In males, the predominant uropathogens during the first year of life was Klebsiella pneumonia 46.7 %, while E.coli and Proteus mirabilis constituted 13.3% of isolates for each, after the first year of life the Escherichia coli isolates predominated 100% Figure 2.
Figure 2.
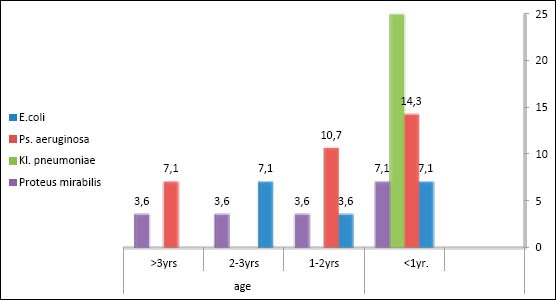
Distribution of uropathogens in males according to age group
In females, both Escherichia coli and Pseudomonas aeruginosa were the predominating isolates up to 5 years with few Proteus mirabilis infection Figure 3.
Figure 3.
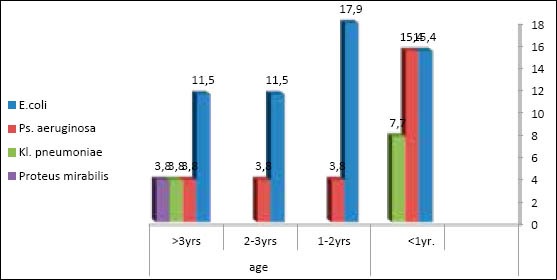
Distribution of uropathogens in females according to age group
3.1. Antimicrobial Susceptibility
The antimicrobial susceptibility of all isolates is shown in Tables 1a and 1b. E. coli isolates was resistance against; Augmentin 91.3%, cotrimoxazole 100%, Cephalexin 95.7%, and Nalidixic acid 26.1%. However E. coli isolates were highly susceptible to Norofloxacin91.3%, ciroflxacin 87% and Nitrofurantoin 73.9% intermediate sensitivity is shown on the tables.
Table 1a.
Uropthogens and their susceptibility to antimicrobials agents in children with urinary tract infection
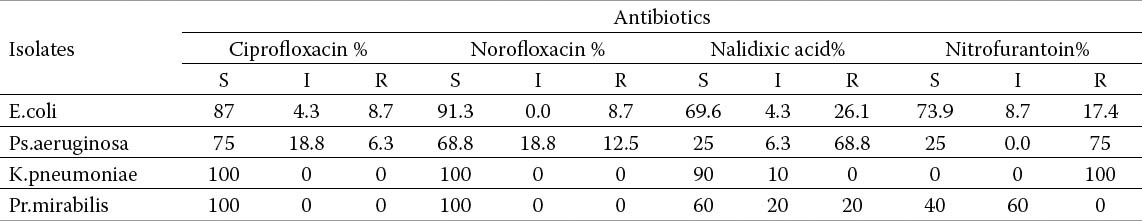
Table 1b.
Uropthogens and their susceptibility to antimicrobials agents in children with urinary tract infection
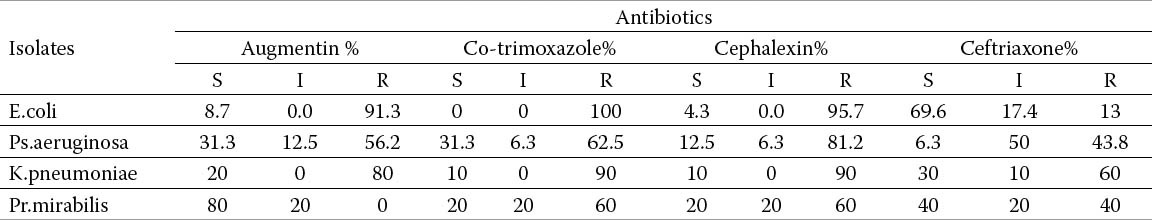
Ps.aeruginosa was shown resistant against; Cephalexin 81.2%, Co-trimoxazole 62, 5%, Nitrofurantoin 75%, Augmentin56.2% and Ceftriaxone43.8%. On other hand Ps.aeruginosa isolates were susceptible; to Ciprofloxacin 75%, and norfloxacin 68.8%.
Both K.pneumoniae and Pr.mirabilis were high susceptible; to ciprofloxacin (100%),and (Norofloxacin) 100%. K.pneumoniae isolates were 90% sensitive to Nalidixic acid compared to 60% for Pr.mirabilis. K.pneumoniae isolates showed 100% resistance to Nitrofurantoin.
4. DISCUSSION
In this study, of all subjects with significant pyuria (145, 47.7%), 54(17.8%) had positive bacterial growth. The risk of developing UTIs in these children under was almost equal among males (51.9%) and females (48.1%) and rural and urban residency was similarly affected. The majority of subjects 36 (66.7 %) were less than two years of age. E. coli (42.6%) was the most predominant uropathogen, sensitive to ciprofloxacin (91.3%), followed by Pseudomonas aeruginosa (29.6%) sensitive to Ciprofloxacin (75%) and Norfloxacin (68.8%), Klebsiellapneumoniae (18.5%) sensitive to Ciprofloxacin and Norfloxacin and Nalidixic acid (90%) and Proteus mirabilis sensitive to Ciprofloxacin and Norfloxacin (90%), Amoxicillin / clavulanic acid (Augmentin(80%].
Furthermore, the study showed that the incidence of first episode of UTIs was similar in both sexes in during the first year of life and thereafter, females had the higher incidence of UTIs than males. Studies from Sweden have shown similar findings (19, 20).
This study documented a high frequent occurrence of urinary tract infections (17.8%), and bacteremia among febrile under-fives attending Maternity and Children hospital Gadarif, Eastern Sudan. This finding does not represent the true prevalence of UTIs in Gadarif district because the nature of the study was a hospital-based, only patients with severe disease and those who live nearby reported to the hospital while those living far or have financial constraints, or lack of transportation. Empirical treatment of all fever with antibiotics and antimalarial is a common practice in the developing nations. A higher prevalence rate (20.3%) of UTIS was reported in Afric (21). Frequency of UTIs in the developing countries is very high than 3.3% and 9% rates quoted from developed countries (22, 23).
It has been found that the earlier the development of UTIs in life, the higher chance of developing recurrent UTIs, furthermore, the risk of recurrent UTIs increased significantly when the first infection is caused by a non-E. Coli strain (25). In this study, we found that the dominant uropathogen was E. coli followed by Pseudomonas aeruginosa, Klebsiellapneumoniae and Proteus mirabilis. In Sudan, a similar study conducted on adult populations showed that E. coli of least occurrences (6%), while the dominant organism was P. aeurginosa (37%) (26).
Our findings are in agreement with some previous studies, which have shown that Escherichia coli were the commonest organism isolated from urine samples (24, 27). Furthermore, and in a study it was found that it accounted for 75% of all UTIs in all pediatrics of all age group followed by Klebsiellapneumoniae, Proteus mirabilis and Pseudomonas aeruginosa (28, 22). A recent study examined the pathogens and their susceptibility in the first episode of UTIS showed that 96.1% of the isolate were Escherichia coli with high susceptibility to aminoglycosides, ciprofloxacin and nitrofurantoin; third generation cephalosporins, and low susceptibility to cephaloxin (29).
E. coli isolates in this study were highly sensitive (near 100%) to ciprofloxacin, Norfloxacin, and nalidixic acid. Unfortunately, it showed high resistant to commonly used antibiotics (Augmentin, Cephalexin and -trimoxazole) (near 100%). Similar findings from Tanzania reported that E. coli is the dominant organism as a cause of UTIs in children under-fives, and it is sensitive to fluoroquinolones and resistant to third- and fourth-generation cephalosporin’s (1). Similar studies were concluded in Marrakech [30] and in Kashmir (31) concluded that Escherichia coli were the most predominant isolate, followed by Klebsiellapneumoniae and Pseudomonas aeruginosa. However, they differ in the antibiotic sensitivity pattern, in Marrakech third generation cephalosporin’s and aminosides kept their effectiveness on the majority of isolates were the most effective treatment while, in the latter study, E. coli isolates were fully sensitive to ofloxacin, and cefuroxime. The high sensitivity to ciprofloxacin In this study may be explained by the fact that it is not one the commonly used drug for children due to their questionable safety in children.
The development of multi-drugs resistance (MDR) to commonly used antibiotics in the developing world could be due to drug misuse, this misuse over time, will lead to greater levels of mutation in bacteria, leading to high levels of bacterial resistance. Moreover, antibiotics are used as growth promoters to control infectious disease may lead to the development of MDR due to change in the genetic composition of bacteria.
In this study, Pseudomonas aeruginosa was found to be resistant to many antibiotics, and only appreciable susceptibility was found with Ciprofloxacin and Norfloxacin (near70%). Many studies have demonstrated that Pseudomonas aeruginosa is becoming multi-drug resistant organism (31, 32). Furthermore, results from United States and European hospitals indicated that Pseudomonas aeruginosa exhibited lower susceptibility rates to many antimicrobial drugs in Europe compared to) compared to USA(33-35). There is no report on current literature showed there are satisfactory antimicrobials that provided a satisfactory coverage for this organism. This means that treatment of UTI in the absence of susceptibility will results in long-term complications of UTIs and the emergence of multi-resistant organisms.
Antibiotic resistance rates in uropathogens are rapidly growing, especially with regard to E. coli infections. For all uropathogens culture and sensitivity of the isolates remains the only solution to decrease multi-drug resistance strains, and it should be done as a routine before advocating the therapy. Antibiotic abuse is of serious concern in the developing world, and a restriction of their uses in the community to a retard development of further drug resistance is important. Drug-resistant E. coli are readily acquired through the food and water; therefore, public and personal hygiene are the essential component of this circuit. Prescribing medications while awaiting culture may lead to increase in MDR strains. Future researchers may be able to change our practice in solving this problem.
5. CONCLUSION
The most common uropathogens were E. coli, Pseudomonas aeruginosa, Klebsiellapneumoniae, and Proteus mirabilis. Ciprofloxacin is the recommended initial empirical therapy while awaiting the culture and sensitivity results.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Wolff O, Maclennan C. Evidence behind the WHO guidelines: hospital care for children: what is the appropriate empiric antibiotic therapy in uncomplicated urinary tract infections in children in developing countries? J Trop Pediatr. 2007;53(3):150–152. doi: 10.1093/tropej/fmm030. [DOI] [PubMed] [Google Scholar]
- 2.Harmsen M, Wensing M, Braspenning JC, Wolters RJ, van der Wouden JC, Grol GPTM. Management of children's urinary tract infections in Dutch family practice: a cohort study. BMCF Am Pract. 2007;8:9. doi: 10.1186/1471-2296-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper BS, Curry SH. Urinary tract infection in children. Am Fam Physician. 2005;72(12):2483–2488. [PubMed] [Google Scholar]
- 4.Hannula A, Perhomaa M, Venhola M, Pokka T, Renko M, Uhari M. Long-term follow-up of patients after childhood urinary tract infection. Arch PediatrAdolesc Med. 2012;166:1117–1122. doi: 10.1001/archpediatrics.2012.1383. [DOI] [PubMed] [Google Scholar]
- 5.Sastre JB, Aparicio AR, Cotallo GD, Colomer BF, Hernandez MC. Urinary tract infection in the newborn: clinical and radio imaging studies. PediatrNephrol. 2007;22(10):1735–1741. doi: 10.1007/s00467-007-0556-5. [DOI] [PubMed] [Google Scholar]
- 6.Bauer R, Kogan BA. New developments in the diagnosis and management of pediatric UTIs. Urol Clin North Am. 2008;35(1):47–58. doi: 10.1016/j.ucl.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Masson P, Matheson S, Webster AC, Craig JC. Meta-analyses in prevention and treatment of urinary tract infections. Infect Dis Clin North Am. 2009;23(2):355–385. doi: 10.1016/j.idc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J NMC Hospital. Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6:4. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakupurakal R, Ahmed M, Sobithadevi DN, Chinnappan S, Reynolds T. Urinary tract pathogens and resistance pattern. J ClinPathol. 2010;63:652–654. doi: 10.1136/jcp.2009.074617. [DOI] [PubMed] [Google Scholar]
- 10.Lutter SA, Currie ML, Mitz LB, Greenbaum LA. Antibiotic resistance patterns in children hospitalized for urinary tract infections. Arch Pediatr Adolesc Med. 2005;159:924–928. doi: 10.1001/archpedi.159.10.924. [DOI] [PubMed] [Google Scholar]
- 11.Okunola PO, Ibadin MO, Ofovwe GE, Ukoh G. Co-existence of urinary tract infection and malaria among children under five years old: a report from Benin City, Nigeria. Saudi J Kidney Dis Transpl. 2012 May;23(3):629–634. [PubMed] [Google Scholar]
- 12.Megged O. Staphylococcus aureus urinary tract infections in children are associated with urinary tract abnormalities and vesico-ureteral reflux. Pediatr Nephrol. 2014 Feb;29(2):269–272. doi: 10.1007/s00467-013-2655-9. [DOI] [PubMed] [Google Scholar]
- 13.Fischbach FT, Dunning MB. 7th edition. Vol. 201. Lippincott Williams and Wilikins; 2004. Urine studies and Microbiologic studies. A manual of laboratory and diagnostic tests; pp. 503–505. [Google Scholar]
- 14.El Mishad AM. Manual of practical Microbiology, 7th editon, Alahram Commercial Press. Egypt. 2002:112–151. [Google Scholar]
- 15.Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infections although hospitalized for suspected sepsis. J Pediatr. 1985;107:855–860. doi: 10.1016/s0022-3476(85)80175-x. [DOI] [PubMed] [Google Scholar]
- 16.Michael LW, Loretta G. Laboratory Diagnosis of Urinary Tract Infections in Adult Patients. Clinical Infectious Diseases. 2004;38:1150–1158. doi: 10.1086/383029. [DOI] [PubMed] [Google Scholar]
- 17.Ananthanaryan R, Jayaram P. Textbook of Microbiology. 5th edition. India: Orient Longman; 1997. pp. 258–261. [Google Scholar]
- 18.Ochei J, Kolhatkar A. India: Tata McGraw-Hill Publishing Company; 2000. Medical Laboratory Science Theory and Practice; p. 635. [Google Scholar]
- 19.Bart J Knottnerus, Patrick JE Bindels, Suzanne E Geerlings, Eric P, Moll van Charante, Gerbenter Riet. Optimizing the diagnostic work-up of acute uncomplicated urinary tract infections. BMCF Am Pract. 2008;9:64. doi: 10.1186/1471-2296-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed MI. Prevalence of nosocomial wound infection among postoperative patients and antibiotics patterns at teaching hospital in Sudan. North Am J Med Sci. 2012;4:29–34. doi: 10.4103/1947-2714.92900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakobsson B, Esbjorner E, Hansson S, Ferreira C, Matos P, Monteiro T. Minimum incidence and diagnostic rate of first urinary tract infection. Nascer e Crescer. 2000;9:51–52. [Google Scholar]
- 22.Marild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. ActaPaediatr. 1998;87:549–552. doi: 10.1080/08035259850158272. [DOI] [PubMed] [Google Scholar]
- 23.Msaki BP, Mshana SE, Hokororo A, Mazigo HD, Morona H. Prevalence and predictors of urinary tract infection and severe malaria among febrile children attending Makongoro health centre in Mwanza city, North-Western Tanzania. Arch Public Health. 2012;70(1):4. doi: 10.1186/0778-7367-70-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panahi Y, MoharamzadBeiraghdar, Matinzadeh ZK, Einollahi B. The incidence of urinary tract infections in febrile children during a two-year period in Tehran, Iran. Tropica Doctor. 2008;38(4):247–249. doi: 10.1258/td.2008.070356. [DOI] [PubMed] [Google Scholar]
- 25.Poulsen LL, Bisgaard M, Son NT, Trung NV, An HM, Dalsmgaard A. Enterococcus and Streptococcus spp. associated with c hronic and self-medicated urinary tract infections in Vietnam. BMC Infect Dis. 2012 Nov;23(12):320. doi: 10.1186/1471-2334-12-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okwara FN, Obimbo EM, Murila FV. Bacteremia, UTI and malaria in hospitalized febrile children in Nairobi. Is there an association? East AfrMed J. 2004;81:47–51. doi: 10.4314/eamj.v81i1.8795. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Song SH, Lee C, Kim JW, Kim KS. Bacterial pathogens in first febrile urinary tract infection affect breakthrough infections in infants with vesicoureteral reflux treated with prophylactic antibiotics. Urology. 2013 Jun;81(6):1342–1345. doi: 10.1016/j.urology.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed MI, Mohsin S. Pattern of Nosocomial Urinary Tract Infections among Sudanese Patients. British Microbiology Research Journal. 2012;2(2):53–61. [Google Scholar]
- 29.Festo E, Kidenya BR, Hokororo A, Mshana SE. Predictors of urinary tract infection among febrile children attending at Bugando Medical Centre, Northwestern Tanzania. Archives Clin Microbiol. 2011;2(5) doi:10.3823/239. [Google Scholar]
- 30.Gallegos J, Márquez S, Morales K, Peña A. Etiologic and antibiotic susceptibility profile of the first episode of febrile urinary tract infection. Rev Chilena Infectol. 2013 Oct;30(5):474–479. doi: 10.4067/S0716-10182013000500002. [DOI] [PubMed] [Google Scholar]
- 31.Bouskraoui M, Ait Sab I, Draiss G, Bourrouss M, Sbihi M. Epidemiology of urinary tract infection in children in Marrakech. Arch Pediatr. 2010 Sep;17(Suppl 4):S177–178. doi: 10.1016/S0929-693X(10)70921-0. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad S. Pattern of urinary tract infection in Kashmir andantimicrobialsusceptibility. Bangladesh Med Res Counc Bull. 2012 Dec;38(3):79–83. doi: 10.3329/bmrcb.v38i3.14330. [DOI] [PubMed] [Google Scholar]
- 33.Ada-Adegbola HO, Muili KA. Antibiotic susceptibility pattern of urinary tract pathogens in Ibadan, Nigeria. Afr J Med Med Sci. 2010 Sep;39(3):173–179. [PubMed] [Google Scholar]
- 34.Jombo GT, Jonah P, Ayeni JA. Multiple resistant Pseudomonas aeruginosain contemporary medical practice: findings from urinary isolates at a Nigerian University Teaching Hospital. Niger J Physiol Sci. 2008 Jun-Dec;23(1-2):105–109. doi: 10.4314/njps.v23i1-2.54944. [DOI] [PubMed] [Google Scholar]
- 35.Sader HS, Flamm RK, Jones RN. Frequency of occurrence and antimicrobial susceptibility of Gram-negative bacteremia isolates in patients with urinary tract infection: results from United States and European hospitals. J Chemother. 2013 Aug 5; doi: 10.1179/1973947813Y.0000000121. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


