Abstract
Powdery mildew (PM) is a very destructive disease of wheat (Triticum aestivum L.). Wheat-Thinopyrum ponticum introgression line CH7086 was shown to possess powdery mildew resistance possibly originating from Th. ponticum. Genomic in situ hybridization and molecular characterization of the alien introgression failed to identify alien chromatin. To study the genetics of resistance, CH7086 was crossed with susceptible genotypes. Segregation in F2 populations and F2:3 lines tested with Chinese Bgt race E09 under controlled conditions indicated that CH7086 carries a single dominant gene for powdery mildew resistance. Fourteen SSR and EST-PCR markers linked with the locus were identified. The genetic distances between the locus and the two flanking markers were 1.5 and 3.2 cM, respectively. Based on the locations of the markers by nullisomic-tetrasomic and deletion lines of ‘Chinese Spring’, the resistance gene was located in deletion bin 2BL-0.89-1.00. Conserved orthologous marker analysis indicated that the genomic region flanking the resistance gene has a high level of collinearity to that of rice chromosome 4 and Brachypodium chromosome 5. Both resistance specificities and tests of allelism suggested the resistance gene in CH7086 was different from previously reported powdery mildew resistance genes on 2BL, and the gene was provisionally designated PmCH86. Molecular analysis of PmCH86 compared with other genes for resistance to Bgt in the 2BL-0.89-1.00 region suggested that PmCH86 may be a new PM resistance gene, and it was therefore designated as Pm51. The closely linked flanking markers could be useful in exploiting this putative wheat-Thinopyrum translocation line for rapid transfer of Pm51 to wheat breeding programs.
Introduction
Powdery mildew (PM), caused by Blumeria graminis f. sp. tritici (Bgt), is a globally important disease of wheat (Triticum aestivum L.). Resistant varieties are the most feasible means of controlling the disease and reducing yield losses. To date, 54 formally designated Pm resistance genes have been identified. They have been mapped to 46 loci and assigned to specific chromosomes or chromosome arms [1]. Of these loci, 29 genes were transferred from relatives, including T. turgidum var. dicoccoides, T. timopheevii, T. monococcum, Aegilops tauschii, Ae. speltoides, Ae. longissima, Ae. ovata, and from more distantly related species, including Secale cereale, Dasypyrum villosum, and Thinopyrum intermedium [2]. However, many resistance genes become ineffective because of frequent changes in pathogen populations, especially when a single resistance gene is deployed over a wide area. Therefore, new sources of effective and durable resistance from both common wheat and wild relatives are required for resistance breeding.
Tall wheatgrass, Thinopyrum ponticum (Podp.) Z.-W. Liu & R.-C. Wang [syn. Agropyron elongatum (Host) Beauv., Lophopyrum ponticum (Podp.) A. Löve, and Elytrigia elongata (Host) Nevski], has been one of the most beneficial perennial species that conferred valuable genetic variability for wheat improvement. In addition to wheat rust resistances, Th. ponticum displays resistance to powdery mildew, eyespot, wheat streak mosaic virus (WSMV), wheat curl mite (WCM), Cephalosporium stripe, and Fusarium head blight - [3]. As for rust resistance transferred from Th. ponticum, three genes for resistance to leaf rust viz. Lr19, Lr24, and Lr29, and three genes for resistance to stem rust, viz. Sr24, Sr25, and Sr26, were reported in wheat-Th. ponticum translocation derivatives [4],[5]. However, there are few reports on the transfer of powdery mildew resistance from Th. ponticum to wheat [3].
So called cryptic translocations between wheat and alien chromatin have been reported on a number of occasions. These are alien transfers that cannot be visualized by cytological means, and are also usually not detectable with markers. Recent examples were the transfers of rust resistances from Ae. geniculata and Ae. triuncialis to wheat [6],[7]. Genomic rearrangements in wheat hybrids due to cryptic introgressions of small chromosome segments from Dasypyrum villosum and Th. ponticum to wheat were also reported recently [8],[9]. However, with materials having putative cryptic translocations that cannot be detected cytologically or with markers there is always the question of alien identity.
CH7086, a Th. ponticum-derived wheat introgression line, was resistant to powdery mildew under greenhouse conditions in Taiyuan, Shanxi province. The resistance gene was preliminarily assigned to chromosome arm 2BL [10]. The objectives of the present study were to characterize this potential new cryptic wheat-Th. ponticum translocation, and to determine its location using microsatellite and comparative genomic molecular marker analyses.
Materials and Methods
Plant materials and populations
The materials used in this study were Th. ponticum (accession R431) with the genomic formula JJJJsJs [11]; partial amphiploid, Xiaoyan 7430, derived from accession R431 and provided by the Crop Science Institute, Shanxi Academy of Agricultural Sciences, Taiyuan; wheat genotypes ‘CH7086’, ‘CH5241’, ‘Zhong 8701’, ‘Jimai 26’, ‘Xiangyang 4’, ‘Misuizao’, and ‘Chinese Spring (CS)’; and various ‘CS’ nullisomic-tetrasomic (NT) stocks and deletion lines, obtained from Dr. B. Friebe, Wheat Genetic and Genomic Resources Center, Kansas State University, USA. CH7086 and CH5241 are homogeneous BC2F5-derived wheat lines obtained from the cross Zhong 8701/Xiaoyan 7430//2*Jimai 26. CH7086 is resistant to powdery mildew whereas CH5241 is susceptible. Xiaoyan 7430, the resistance donor of CH7086, was derived from the cross Misuizao/R431//Xiangyang 4 [12].
To investigate the inheritance of powdery mildew resistance introgressed from Th. ponticum, CH7086 was crossed to susceptible cultivars CH5241, Taichung 29, SY95-71, and Jintai 170 to generate segregating populations. The F2 and F3 were tested for segregation of powdery mildew response. An F2 population of 154 plants and 148 derived F3 lines from CH7086/CH5241 were used for microsatellite screening and gene mapping. CH7086, Xiaoyan 7430, the original mildew resistant donor accession (R431) of Th. ponticum, and CS were also used for genomic in situ hybridization and C-banding analyses to determine the chromosomal composition in Xiaoyan 7430 and the size of any alien introgressions in CH7086.
Genomic in situ hybridization
Seedling root tips were collected, pretreated in ice water for 24 h and fixed in ethanol-acetic acid (3∶1) for one week. Root-tip squashes and the conventional Giemsa-C banding methods were performed according to Gill et al. [13]. For GISH analysis, total genomic DNA from Th. ponticum was labeled with fluorescein-12-dUTP by nick translation following the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN). Sheared genomic DNA of Chinese Spring wheat (AABBDD, 2n = 6x = 42) was used as blocking DNA, and the probe-to-blocker ratio was approximately 1∶120. The detection and visualization of GISH signals were performed as described by Han et al. [14]. Images of GISH and C-banded chromosomes were taken with an Olympus BX-51 microscope using a DP70 CCD camera (Olympus, Japan).
Testing for powdery mildew response
The seedling reactions of line CH7086 and the parental lines inoculated with four Bgt isolates, provided by the Plant Protection Institute, Chinese Academy of Agricultural Sciences, are shown in Table 1. Among the Bgt isolates tested, E09, a prevalent pathotype in the Beijing area, is virulent to Pm1, Pm3a, Pm3c, Pm3e, Pm5a, Pm6, Pm7, Pm8, Pm17, and Pm19 [15]; E20 and E21 are the most widely virulent pathotypes in China and are virulent to most of the Pm genes including Pm4a, Pm4b, PmPs5A, and Pm33. E26 is virulent to Pm4b and Pm33, but avirulent to Pm4a and PmPs5A [16],[17]; E15, avirulent toPm6, was used for the test of allelism between Pm6 and the resistance gene in CH7086 [16]. Procedures used in powdery mildew inoculation, incubation of inoculated plants, and reaction scoring were as described in He et al. [18]. All F2 plants and their parents were inoculated with isolate E09 at the one-leaf seedling stage to screen for powdery mildew reaction. For progeny testing, 15 to 20 F3 seedlings from each F2 plant were grown and tested with the same race. The infection types (ITs) were rated on a 0–4 scale, 7–10 days after inoculation when conidia were fully developed [18].
Table 1. Reactions of selected donor materials, parents and controls after with four Bgt isolates.
| Line | Chromosome | Genomic | Bgt isolate | |||
| number | formula | E09 | E20 | E21 | E26 | |
| Th. ponticum R431 | 70 | JJJJsJs | 0 | 0 | 0 | 0 |
| Xiaoyan (XY) 7430 | 56 | ABD +JJs | 0 | 0; | 0; | 0 |
| Xiangyang 4 | 42 | ABD | 4 | 3 | 3 | 4 |
| Misuizao | 42 | ABD | 4 | 3 | 4 | 3 |
| CH7086 | 42 | ABD | 0 | 0; | 0 | 0; |
| CH5241 | 42 | ABD | 4 | 4 | 4 | 3 |
| Zhong 8701 | 42 | ABD | 4 | 4 | 4 | 4 |
| Jimai 26 | 42 | ABD | 4 | 3 | 4 | 4 |
| SY95-71 | 42 | ABD | 4 | 4 | 4 | 4 |
| Jintai 170 | 42 | ABD | 4 | 4 | 4 | 3 |
| Taichung 29 | 42 | ABD | 4 | 4 | 4 | 4 |
Infection types were based on a 0–4 scale, where 0 = no visible symptoms, 0; = necrotic flecks, 1 = necrosis with low sporulation, 2 = necrosis with moderate sporulation, 3 = no necrosis with moderate to high sporulation, and 4 = no necrosis with full sporulation. Scores of 0–2 were classified as resistant and 3–4 as susceptible.
Molecular marker analysis
Wheat chromosome 2BL has good synteny with rice chromosome 4 [19],[20]. Thus, wheat ESTs that mapped to bin 2BL- 0.50–1.00 were aligned to the rice genome sequences using BLASTN. ESTs with orthologous genes in the syntenic region of rice chromosome 4 were used to develop STS (sequence tagged site) markers. PCR products from STS primers were separated in 1% agarose gels, whereas PCR products from EST and SSR primers were separated in 8% non-denaturing polyacrylamide gels.
In order to produce SCAR markers, amplified polymorphic bands between CH7086 and Taichung 29 were extracted from gels and re-amplified. The method of cloning and sequencing PCR products was as described by Liu et al. [21].
Chromosome assignment and linkage analysis
Chi-squared (χ2) tests for goodness-of-fit were used to test for deviations of observed data from theoretically expected segregations. Linkages between DNA markers and the resistance gene were established with JoinMap version 4.0 software (Wageningen, Netherlands) with a LOD threshold of 3.0. Map distances were determined using the Kosambi mapping function.
Results
Likely origin of the powdery mildew resistance
Seedling reactions of Th. ponticum, the partial amphiploid donor and nine wheat cultivars/lines to four Bgt isolates are summarized in Table 1. CH7086 and the Th. ponticum parent R431 were resistant to isolates E09, E20, E21, and E26 (IT 0-0;), whereas the wheat parents or lines, Xiangyang 4, Misuizao, Zhong 8701, and Jimai 26, were susceptible (IT 3-4). These results demonstrated that CH7086 was resistant to powdery mildew, with the ITs being similar to the donor Xiaoyan 7430 (IT 0-0;) as well as the donor Th. ponticum accession R431 (IT 0).
Attempted characterization of an alien introgression in CH7086
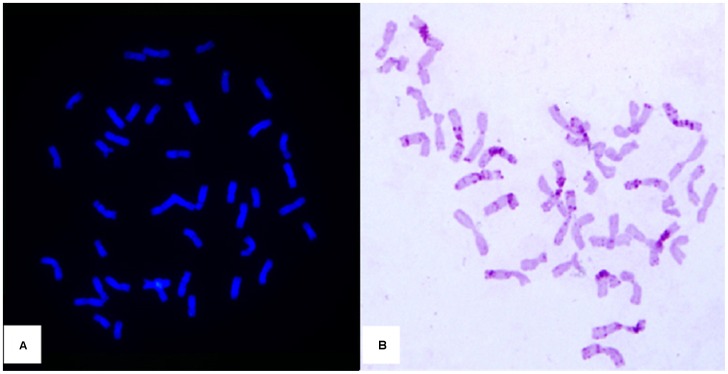
When GISH using Th. ponticum genomic DNA as probe was performed on the wheat-Th. ponticum partial amphiploid Xiaoyan 7430 (2n = 56) and line CH7086, 14 Th. ponticum chromosomes were clearly distinguishable in the mitotic metaphases of Xiaoyan 7034 (results not shown). However, no GISH signals were observed in CH7086 (Figure 1A). Giemsa-C banding (Figure 1B) indicated that CH7086 contained typical wheat chromosomes without visible bands indicative of Thinopyrum chromatin.
Figure 1. Cytogenetic analysis of CH7086.
A) GISH patterns of CH7086 using Th. ponticum genomic DNA (labeled with fluorescein-12-dUTP) as probe. Cell was counterstained with DAPI and fluoresces blue. No GISH signal was observed in CH7086. B) Giemsa-C banding of CH7086.
Genetic analysis of powdery mildew resistance in CH7086
Wheat-Th. ponticum introgression line CH7086, the F1 hybrid, and F2:3 families from CH7086 crossed respectively with wheat lines Taichung 29, CH5241, SY95-71, and Jintai 170 were inoculated with Bgt isolate E09. CH7086 was highly resistant (IT 0) and the wheat lines were all highly susceptible (IT 4) (Table 1). All F1 hybrid seedlings and adult plants were highly resistant, implying that the resistance in CH7086 was dominant. F2 populations from four crosses segregated 3 resistant: 1 susceptible and the pooled data of F2:3 lines from CH5241/CH7086 and CH7086/Taichung 29 segregated 63 homozygous resistant: 128 segregating: 61 homozygous susceptible, as expected for single gene segregation (χ2 1:2:1 = 0.10, P df2 = 0.95) (Table 2). The dominant gene for Pm resistance in CH7086 was temporarily designated as PmCH86.
Table 2. Mildew reactions and segregation ratios of F2 plants and derived F3 lines following inoculation with Bgt isolate E09.
| Parent or cross | No. of plants or lines | Expected | ?2 | P | |||
| Resistant | Segregating | Susceptible | ratio | ||||
| CH7086 | P1 | 19 | |||||
| SY95-71 | P2 | 15 | |||||
| CH5241 | P3 | 13 | |||||
| Taichung 29 | P4 | 13 | |||||
| Jintai 170 | P5 | 17 | |||||
| CH7086/Taichung 29 | F1 | 11 | |||||
| F2 | 88 | 26 | 3∶1 | 0.292 | 0.589 | ||
| F2:3 | 25 | 52 | 27 | 1∶2∶1 | 0.077 | 0.962 | |
| CH7086/SY95-71 | F1 | 7 | |||||
| F2 | 118 | 42 | 3∶1 | 0.133 | 0.715 | ||
| CH5241/CH7086 | F1 | 21 | |||||
| F2 | 111 | 43 | 3∶1 | 0.701 | 0.402 | ||
| F2:3 | 38 | 76 | 34 | 1∶2∶1 | 0.324 | 0.850 | |
| CH7086/Jintai 170 | F1 | 9 | |||||
| F2 | 149 | 40 | 3∶1 | 1.483 | 0.223 | ||
| Pooled F2 data | 466 | 151 | 3∶1 | 0.091 | 0.763 | ||
| Pooled F2:3 data | 63 | 128 | 61 | 1∶2∶1 | 0.095 | 0.953 | |
Values for significance of χ 2 at P = 0.05, and 3.83 for 1 df and 5.99 for 2 df, respectively.
Identification and physical bin mapping of polymorphic markers linked to PmCH86
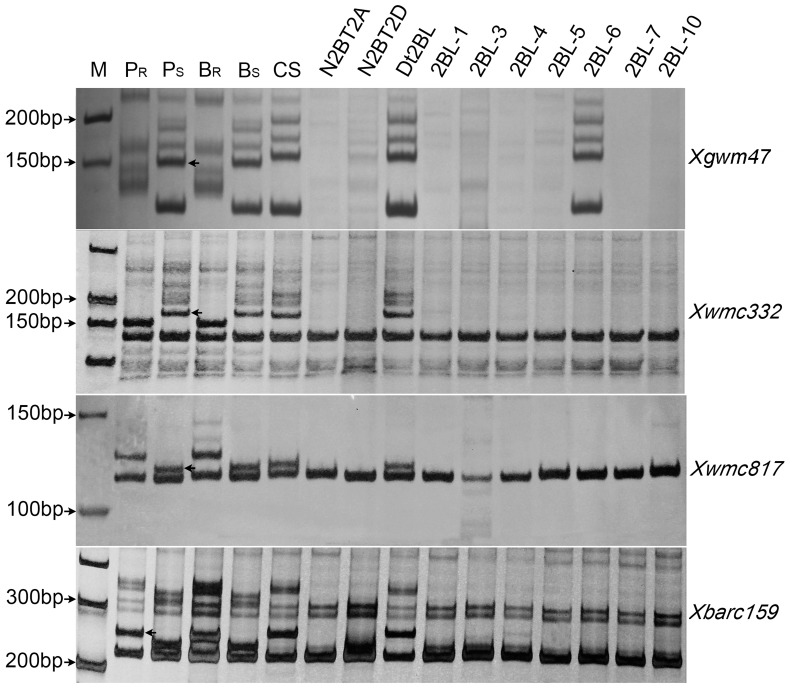
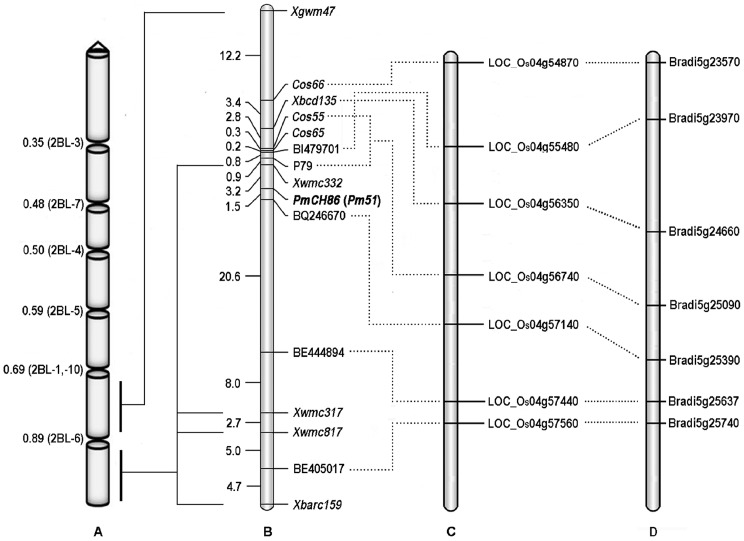
The 148 plants of the F2 population segregated 1∶2∶1 for all fourteen markers (Table 3, Table 4). Of the microsatellite markers tested, Xgwm47, Xwmc332, Xwmc317, Xwmc817,and Xbarc159 showed linkage with powdery mildew resistance in CH7086. As these markers all map to chromosome arm 2BL, PmCH86 must also be located in this arm. Based on the high-density microsatellite consensus map of common wheat [22], two microsatellite markers (Xwmc332 and Xbarc159) linked with the resistance in this study were mapped at a distance of 45.7 cM on chromosome arm 2BL. As shown in Figure 2, we bin mapped the five SSR markers by CS deletion lines. Xgwm47 was the only marker located in bin 0.59–0.89 and the others, Xwmc332, Xwmc317, Xwmc817, and Xbarc159, mapped to the 2BL-6 deletion bin FL 0.89–1.00. PmCH86 was placed in the interval between Xwmc332 and Xwmc317, thus the physical location of PmCH86 was in the 2BL-6 deletion bin, which is the most distal bin of the long arm accounting for approximately 11% of the physical length of this chromosome arm. Evaluation of the five linked microsatellite markers, including four codominant and one dominant (Table 3, Table 4, Figure 2), along the physical and genetic maps of chromosome 2B allowed us to estimate the genomic location of PmCH86.
Table 3. Segregations of powdery mildew resistance and markers linked to PmCH86 in F2 population or F2:3 lines from CH5241/CH7086.
| Marker | Resistance | Codominant marker | Dominant marker | Total | ?2 (1:2:1or 3:1) | P | |||||
| genotype | AA | Aa | aa | AA | Not AA | Not aa | aa | ||||
| Xgwm47 | PmPm | 24 | 6 | 8 | 38a | ||||||
| Pmpm | 9 | 59 | 8 | 76 | |||||||
| pmpm | 5 | 4 | 25 | 34 | |||||||
| Total | 38 | 69 | 41 | 148 | 0.797b | 0.671 | |||||
| Cos66 | PmPm | 31 | 7 | 0 | 38 | ||||||
| Pmpm | 8 | 62 | 6 | 76 | |||||||
| pmpm | 0 | 4 | 30 | 34 | |||||||
| Total | 39 | 73 | 36 | 148 | 0.149 | 0.928 | |||||
| Xbcd135 | PmPm | 38 | 0 | 38 | |||||||
| Pmpm | 73 | 3 | 76 | ||||||||
| pmpm | 4 | 30 | 34 | ||||||||
| Total | 115 | 33 | 148 | 0.577 | 0.448 | ||||||
| Cos55 | PmPm | 34 | 4 | 38 | |||||||
| Pmpm | 3 | 73 | 76 | ||||||||
| pmpm | 1 | 33 | 34 | ||||||||
| Total | 38 | 110 | 148 | 0.036 | 0.849 | ||||||
| Cos65 | PmPm | 34 | 3 | 1 | 38 | ||||||
| Pmpm | 3 | 72 | 1 | 76 | |||||||
| pmpm | 1 | 3 | 30 | 34 | |||||||
| Total | 38 | 78 | 32 | 148 | 0.919 | 0.632 | |||||
| BI479701 | PmPm | 37 | 1 | 38 | |||||||
| Pmpm | 75 | 1 | 76 | ||||||||
| pmpm | 4 | 30 | 34 | ||||||||
| Total | 116 | 32 | 148 | 0.901 | 0.343 | ||||||
| P79 | PmPm | 31 | 6 | 1 | 38 | ||||||
| Pmpm | 2 | 72 | 2 | 76 | |||||||
| pmpm | 1 | 2 | 31 | 34 | |||||||
| Total | 34 | 80 | 34 | 148 | 0.973 | 0.615 | |||||
AA homozygous for the CH7086 allele; aa homozygous for the CH5241 allele; Aa heterozygous.
aThe data of F2:3 lines from CH5241/CH7086;
bValues for significance of χ 2 at P = 0.05 is 3.84 for 1 df and 5.99 for 2 df, respectively.
Table 4. Segregations of powdery mildew resistance and markers linked to PmCH86 in F2 population or F2:3 lines from CH5241/CH7086.
| Marker | Resistance | Codominant marker | Dominant marker | Total | ?2 (1:2:1or 3:1) | P | |||||
| genotype | AA | Aa | aa | AA | Not AA | Not aa | aa | ||||
| Xwmc332 | PmPm | 34 | 3 | 1 | 38 | ||||||
| Pmpm | 1 | 73 | 2 | 76 | |||||||
| pmpm | 1 | 2 | 31 | 34 | |||||||
| Total | 36 | 78 | 34 | 148 | 0.486 | 0.784 | |||||
| BQ246670 | PmPm | 37 | 1 | 0 | 38 | ||||||
| Pmpm | 0 | 75 | 1 | 76 | |||||||
| pmpm | 1 | 0 | 33 | 34 | |||||||
| Total | 38 | 76 | 34 | 148 | 0.324 | 0.850 | |||||
| BE444894 | PmPm | 21 | 17 | 38 | |||||||
| Pmpm | 8 | 68 | 76 | ||||||||
| pmpm | 3 | 31 | 34 | ||||||||
| Total | 32 | 116 | 148 | 0.901 | 0.343 | ||||||
| Xwmc317 | PmPm | 30 | 8 | 38 | |||||||
| Pmpm | 65 | 11 | 76 | ||||||||
| pmpm | 12 | 22 | 34 | ||||||||
| PmPm | 107 | 41 | 148 | 0.577 | 0.448 | ||||||
| Xwmc817 | PmPm | 19 | 12 | 7 | 38 | ||||||
| Pmpm | 19 | 46 | 11 | 76 | |||||||
| pmpm | 6 | 5 | 23 | 34 | |||||||
| Total | 44 | 63 | 41 | 148 | 3.392 | 0.183 | |||||
| BE405017 | PmPm | 16 | 22 | 38 | |||||||
| Pmpm | 20 | 56 | 76 | ||||||||
| pmpm | 8 | 26 | 34 | ||||||||
| Total | 44 | 104 | 148 | 1.766 | 0.184 | ||||||
| Xbarc159 | PmPm | 10 | 17 | 11 | 38 | ||||||
| Pmpm | 19 | 42 | 15 | 76 | |||||||
| pmpm | 8 | 8 | 18 | 34 | |||||||
| Total | 37 | 67 | 44 | 148 | 1.986 | 0.370 | |||||
AA homozygous for the CH7086 allele; aa homozygous for the CH5241 allele; Aa heterozygous.
aThe data of F2:3 lines from CH5241/CH7086;
bValues for significance of χ 2 at P = 0.05 is 3.84 for 1 df and 5.99 for 2 df, respectively.
Figure 2. PCR amplification patterns of three linked microsatellite markers.
M: DNA ladder; PR: CH7086; PS: CH5241; BR: resistant bulk; BS: susceptible bulk; CS: Chinese Spring; N2BT2A and N2BT2D: nullisomic-tetrasomic lines; 2BL-1, 2BL-3, 2BL-4, 2BL-5, 2BL-6, 2BL-7 and 2BL-10: homozygous deletion lines of 2BL of CS; Arrows indicate the critical bands.
Comparative genomics analysis
Powdery mildew resistance genes Pm6, Pm33, and PmJM22 are also located in the distal region of chromosome 2BL [23],[24],[25]. To clarify the relationship of PmCH86 with these known genes, we designed the primers for molecular markers based on wheat EST and syntenic regions of the rice. An STS marker Xbcd135 which co-segregated with Pm6 was also developed (Table S1). Comparative mapping was reported between wheat chromosome arm 2BL, rice chromosome 4 and Brachypodium chromosome 5 [19],[25]. Eleven STS and EST-STS flanking markers, BF478581, DN949092, BM138525, CINAU140, BI479701, BQ169948, BQ169948, BQ246670, BE500840, BE444894, and BE405017, were used as queries to search for orthologous genes in rice and Brachypodium genomic sequences. Both Cos66 and BE405017 detected orthologs on the long arm terminal regions of rice chromosome 4 (LOC_Os04g54870 and LOC_Os04g57560) and Brachypodium chromosome 5 (Bradi5g23570 and Bradi5g25740). The closest EST markers flanking PmCH86 were BI479701 and BQ246670 at distances of 4.9 cM and 1.5 cM, respectively; BI479701 was ortholog of rice gene LOC_Os04g55480 and Brachypodium gene Bradi5g23970. The ortholog of BQ246670 was found in rice (LOC_Os04g57140) and Brachypodium (Bradi5g25390). The terminal region of wheat 2BL had a similar gene order to rice 4L and Brachypodium 5L. Thus, the collinear region of the powdery mildew resistance gene PmCH86 covered 214 kb genomic region (LOC_Os04g56740 to LOC_Os04g57140) of chromosome 4 L in rice and 198 kb genomic region (Bradi5g25090 to Bradi5g25390) of chromosome 5 L in Brachypodium (Table S1, Figure 3). The orthologous genomic regions of PmCH86 in the rice and Brachypodium genomes could be candidate regions for fine mapping of PmCH86.
Figure 3. Genetic and comparative mapping of the PmCH86 gene.
A) Chromosome physical map of 2BL (http://www.k-state.edu/wgrc/Germplasm/Deletions/grp2L.html). B) Genetic map of wheat chromosome 2BL, only the region relatively close to PmCH86 was shown. Genetic distances are shown to the left in cM. C) The homologous region of PmCH86 with rice chromosome 4 (http://rice.plantbiology.msu.edu/). D) The homologous region of PmCH86 with Brachypodium chromosome 5 (http://www.brachypodium.org/database).
Validation of the flanking markers in marker-assisted selection
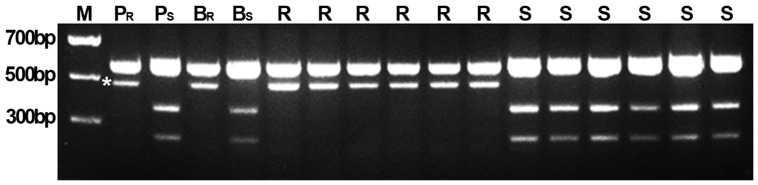
The closest markers Xwmc332 and BQ246670 were linked to PmCH86 with genetic distances of 3.2 and 1.5 cM, respectively (Figure 3). The genomic DNAs of resistant and susceptible F2 plants from crosses of CH7086 with susceptible lines CH5241 and Taichung 29, as well as the parents and resistant (BR) and susceptible (BS) bulks were tested for the presence of markers linked to PmCH86. The specific PmCH86-associated 500-bp band amplified by BQ246670 was inherited as a dominant marker (Table 4, Figure 4). Marker BQ246670 may be useful for marker assisted selection (MAS) and for pyramiding PmCH86 with other powdery mildew resistance genes in wheat depending on marker polymorphisms.
Figure 4. A profile of amplification with marker BQ246670 in F2 population from cross of CH7086/Taichung 29.
M: DNA ladder; PR: CH7086; PS: Taichung 29; BR: resistant bulk; BS: susceptible bulk; R: homozygous resistant F2 plants, S: homozygous susceptible F2 plants. Asterisk indicates the critical band linked with PmCH86.
Comparison of PmCH86 and other Pm genes on 2BL
Pm6 was resistant only to race E15, and susceptible to races E1-3, E5-7, E10, E13, E16-18, E20-21, E23, E26, E30-32, and E42 at the seedling stage [16]. Timgalen carrying Pm6 was highly susceptible (IT 3-4) to E21 and E26, whereas CH7086 was highly resistant (IT 0-0;) (Table 1). This indicated that PmCH86 differs in specificity from Pm6.
In order to further clarify the genetic relationship of PmCH86 and Pm6, 236 F2 plants from CH7086/Timgalen were inoculated with E15, an isolate avirulent to both parents. Two susceptible plants were found, confirming that PmCH86 and Pm6 were not allelic, but were also not genetically independent (χ2 15:1 = 11.76, P df1 = 0.001<0.01).
Discussion
Alien gene transfer has an important role in increasing the genetic diversity available for wheat improvement [4]. Th. ponticum is immune to wheat powdery mildew and certain wheat-Th. ponticum derivatives are highly resistant to Chinese Bgt isolates. A resistance gene was recently found in two Th. ponticum-derived partial amphiploids [26]. However, there is no published report of transfer of powdery mildew resistance from this species to a wheat chromosome. In this study, CH7086 was produced by crossing and backcrossing Xiaoyan 7430 with susceptible wheat cultivars and selecting for powdery mildew resistance. A novel powdery mildew resistance gene, presumably transferred from Th. ponticum into common wheat, was mapped on chromosome arm 2BL and closely linked SSR markers were identified. However, based on GISH and Giemsa-C banding analyses of CH7086, no cytological evidence was found for an alien translocation. The gene PmCH86 must be either present in a cryptic translocation involving a small chromosome segment from Th. ponticum, or a wheat gene derived from an unknown source. Cryptic alien transfers have been reported in other studies [6],[7]. Further studies are needed to determine the source of PmCH86.
Fivepowdery mildew resistance genes, Pm6, Pm26, MlZec1, Pm33, and MlLX9, were previously located on chromosome arm 2BL. Pm6 originated from the G genome of T. timopheevii and Pm33 was identified in T. carthlicum [27],[23]. Pm6 and its linked RFLP marker Xbcd135 were physically located at the region of deletion bin 2BL-6 (FL 0.89–1.00) [28], and this marker was subsequently converted into two STS markers, STSBCD135-1 and STSBCD135-2, which are closely linked to Pm6 with a genetic distance of 0.8 cM [27]. Recently, Qin et al. developed a high-density genetic linkage map of the Pm6 locus through a comparative genomics analysis using the genome sequences of rice and Brachypodium together with Triticeae ESTs, and localized Pm6 at 0.2 to 1.2 cM proximal to the Xbcd135 locus. However, in the present study, PmCH86 was 8.2 cM distal to Xbcd135 (Figure 3). The low infection type conferred by PmCH86 to all races tested (Table 1) was also different from that of Timgalen [16].
Based on a microsatellite map, T. carthlicum-derived powdery mildew resistance gene Pm33 was placed in the region between 18.1 cM distal to Xgwm526 and 1.1 cM proximal to Xwmc317 on chromosome 2BL, and the genetic distance between Pm33 and Pm6 was estimated to be 61.7 cM [23]. In our study, PmCH86 was placed between Xwmc332 and Xwmc317 with an estimated genetic distance being about 30 cM proximal to Xwmc317 (Figure 3). This indicates that PmCH86 should not be allelic to Pm33. Yin et al. [24] located PmJM22 on 2BL in wheat cultivar Jimai 22 using microsatellite markers. Since PmJM22 was close to the region of Pm33, they may be allelic. MlZec1, a dominant resistance gene derived from wild emmer, was mapped distally to SSR marker Xwmc356 on the terminal bin 2BL 0.89–1.00 [29]. Xwmc356 was distal to Xwmc317 [22], thus being at a different position fromPmCH86. The recently identified gene MlLX99 was derived from commercial winter wheat cultivars Liangxing 99. MlLX99 was located on chromosome 2BL in the deletion bin 2BLl2-0.36-0.50 and was linked to SSR marker Xgwm120 [30]. Based on these results and our own data, it appears that PmCH86 is different from other known powdery mildew resistance genes on chromosome 2BL and represents a new powdery mildew resistance locus, and was therefore designated as Pm51. The genetic map of 2BL presented here is in minor conflict with PmJM22 at map distance of BE405017 and BE444894 on the previously published map by Yin et al. [24]. This is likely due to our map being significantly longer. Similar phenomena were also hypothesised for small putative segmental introgressions carrying stem rust resistance from Ae. speltoides into wheat [31].
The wheat chromosome arm 2BL appears to be a hotspot region in disease resistance genes. Rust resistance genes, Yr5, Yr7, Yr43, Yr44 [32],[33], Lr48, and Sr28 [34],[35], have been mapped by molecular markers. Genes Sr9 (several alleles) and Sr16 were also placed in this arm [36]. The accumulation of functional markers in 2BL can be used to target resistance genes in the 2BL terminal regions. Comparative maps will be useful for isolating Pm51 from a gene-rich region in the terminal region of wheat chromosome 2BL by using the rice and Brachypodium genomes as references. Saturation mapping of Pm51 with more functional markers is underway to better understand the allelic relationships, gene structure and function in this gene-rich region.
We found that the new powdery mildew resistance gene Pm51 mapped distally on chromosome 2BL was close to the EST-PCR marker BQ246670 (1.5 cM distal). Tightly linked markers for the Pm51 locus characterized in this study could be used for MAS of Pm51 in wheat breeding programs or to pyramid multiple resistance genes in a single genotype in order to achieve more durable resistance.
Supporting Information
Molecular markers mapped to the PmCH86 region based on wheat, rice, and Brachypodium synteny.
(DOCX)
Acknowledgments
We are grateful to Prof. Robert McIntosh and Dr. Peng Zhang (Plant Breeding Institute, University of Sydney) for critical reviews of this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by The National Natural Science Foundation of China (No. 31171542, 31171839, 31101143); Shanxi Provincial Program of International S & T Cooperation (2013081007; 2012081006-2); Shanxi Provincial Program of Science and Technology (20130311001-5); The Opening Project of the State Key Laboratory of Plant Cell and Chromosome Engineering, Institute of Genetics and Developmental Biology, Chinese Academy of Science Foundation (2012-PCCE-KF-02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gao H, Zhu F, Jiang Y, Wu J, Yan W, et al. (2012) Genetic analysis and molecular mapping of a new powdery mildew resistant gene Pm46 in common wheat. Theor Appl Genet 125:967–973. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J, Morris C, et al. (2013) Catalogue of gene symbols for wheat. 12th International Wheat Genetics Symposium. Yokohama, Japan.
- 3. Li H, Wang X (2009) Thinopyrum ponticum and Th. intermedium: the promising source of resistance to fungal and viral diseases of wheat. J Genet Genomics 36:557–565. [DOI] [PubMed] [Google Scholar]
- 4. Jiang J, Friebe B, Gill BS (1994) Recent advances in alien gene transfer in wheat. Euphytica 73:199–212. [Google Scholar]
- 5. Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87. [Google Scholar]
- 6. Kuraparthy V, Chhuneja P, Dhaliwal HS, Kaur S, Bowden RL, et al. (2007) Characterization and mapping of cryptic alien introgression from Aegilops geniculata with leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor Appl Genet 114:1379–1389. [DOI] [PubMed] [Google Scholar]
- 7. Kuraparthy V, Sood S, Chhuneja P, Dhaliwal HS, Kaur S, et al. (2007) A cryptic wheat-Aegilops triuncialis translocation with leaf rust resistance gene Lr58 . Crop Sci 47:1995–2003. [Google Scholar]
- 8. Caceres ME, Pupilli F, Ceccarelli M, Vaccino P, Sarri V, et al. (2012) Cryptic introgression of Dasypyrum villosum parental DNA in wheat lines derived from intergeneric hybridization. Cytogenet Genome Res 136:75–81. [DOI] [PubMed] [Google Scholar]
- 9. Chen G, Zheng Q, Bao Y, Liu S, Wang H, et al. (2012) Molecular cytogenetic identification of a novel dwarf wheat line with introgressed Thinopyrum ponticum chromatin. J Bio Sci 37:149–155. [DOI] [PubMed] [Google Scholar]
- 10. Zhan HX, Chang ZJ, Liu HM, Zhang XJ, Dong CL (2008) Genetic analysis and SSR mapping of powdery mildew resistant genes in CH7086, a Thinopyrum ponticum-derived wheat line. Acta Bot Boreal-Occident Sin 28:1960–1966. [Google Scholar]
- 11. Chen Q, Conner RL, Laroche A, Thomas JB (1998) Genome analysis of Thinopyrum intermedium and Th. ponticum using genomic in situ hybridization. Genome 41:580–586. [PubMed] [Google Scholar]
- 12.Li ZS, Rong S, Chen SY, Zhong GC, Mu SM (1985) Wheat wide hybridization. Chinese Scientific Press, China, pp 59–67.
- 13. Gill BS, Friebe B, Endo TR (1991) Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 34:830–839. [Google Scholar]
- 14. Han F, Gao Z, Birchler JA (2009) Centromere inactivation and reactivation reveal both genetic and epigenetic components for centromere specification. Plant Cell 21:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hua W, Liu Z, Zhu J, Xie C, Yang T, et al. (2009) Identification and genetic mapping of pm42, a new recessive wheat powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides). Theor Appl Genet 119:223–230. [DOI] [PubMed] [Google Scholar]
- 16. Wang ZL, Li LH, He ZH, Duan XY, Zhou YL, et al. (2005) Seedling and adult plant resistance to powdery mildew in Chinese bread wheat cultivars and lines. Plant Dis 89:457–463. [DOI] [PubMed] [Google Scholar]
- 17. Zhou R, Zhu Z, Kong X, Huo N, Tian Q, et al. (2005) Development of wheat near-isogenic lines for powdery mildew resistance. Theor Appl Genet 110:640–648. [DOI] [PubMed] [Google Scholar]
- 18. He R, Chang Z, Yang Z, Liu Z, Zhan H, et al. (2009) Inheritance and mapping of a powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet 118:1173–1180. [DOI] [PubMed] [Google Scholar]
- 19. Conley EJ, Nduati V, Gonzalez-Hernandez JL, Mesfin A, Trudeau-Spanjers M, et al. (2004) A 2600-locus chromosome bin map of wheat homoeologous group 2 reveals interstitial gene-rich islands and colinearity with rice. Genetics 168:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee TG, Lee YJ, Kim DY, Seo YW (2010) Comparative physical mapping between wheat chromosome arm 2BL and rice chromosome 4. Genetica 138:1277–1296. [DOI] [PubMed] [Google Scholar]
- 21. Liu C, Yang ZJ, Li GR, Zeng ZX, Zhang Y, et al. (2008) Isolation of a new repetitive DNA sequence from Secale africanum enables targeting of Secale chromatin in wheat background. Euphytica 159:249–258. [Google Scholar]
- 22. Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114. [DOI] [PubMed] [Google Scholar]
- 23. Zhu Z, Zhou R, Kong X, Dong Y, Jia J (2005) Microsatellite markers linked to 2 powdery mildew resistance genes introgressed from Triticum carthlicum accession PS5 into common wheat. Genome 48:585–590. [DOI] [PubMed] [Google Scholar]
- 24. Yin GH, Li GY, He ZH, Liu JJ, Wang H, et al. (2009) Molecular mapping of powdery mildew resistance gene in wheat cultivar Jimai 22. Acta Agronomica Sinica 35:1425–1431. [Google Scholar]
- 25. Qin B, Cao A, Wang H, Chen T, You FM, et al. (2011) Collinearity-based marker mining for the fine mapping of Pm6, a powdery mildew resistance gene in wheat. Theor Appl Genet 123:207–218. [DOI] [PubMed] [Google Scholar]
- 26. He F, Xu JQ, Qi XL, Bao YG, Li XF, et al. (2013) Molecular cytogenetic characterization of two partial wheat Elytrigia elongatea amphiploids resistant to powdery mildew. Plant Breeding 132:553–557. [Google Scholar]
- 27. Ji J, Qin B, Wang H, Cao A, Wang S, et al. (2008) STS markers for powdery mildew resistance gene Pm6 in wheat. Euphytica 163:159–165. [Google Scholar]
- 28. Ji J, Cao A, Wang H, Qin B, Wang S, et al. (2007) Discrimination of the Triticum aestivum-T. timopheevii introgression lines using PCR-based molecular markers. Hereditas 29:1256–1262. [DOI] [PubMed] [Google Scholar]
- 29. Mohler V, Zeller FJ, Wenzel G, Hsam SLK (2005) Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 9. Gene MlZec1 from the Triticum dicoccoides-derived wheat line Zecoi-1. Euphytica 142:161–167. [Google Scholar]
- 30. Zhao ZH, Sun HG, Song W, Lu M, Huang J, et al. (2013) Genetic analysis and detection of the gene MlLX99 on chromosome 2BL conferring resistance to powdery mildew in the wheat cultivar Liangxing 99. Theor Appl Gene 126:3081–3089. [DOI] [PubMed] [Google Scholar]
- 31. Faris JD, Xu SS, Cai X, Friesen TL, Jin Y (2008) Molecular and cytogenetic characterization of a durum wheat-Aegilops speltoides chromosome translocation conferring resistance to stem rust. Chromosome Res 16:1097–1105. [DOI] [PubMed] [Google Scholar]
- 32. Zhang P, McIntosh RA, Hoxha S, Dong C (2009) Wheat stripe rust resistance genes Yr5 and Yr7 are allelic. Theor Appl Genet 120:25–29. [DOI] [PubMed] [Google Scholar]
- 33. Cheng P, Chen XM (2010) Molecular mapping of a gene for stripe rust resistance in spring wheat cultivar IDO377s. Theor Appl Genet 121:195–204. [DOI] [PubMed] [Google Scholar]
- 34. Singh A, Pallavi JK, Gupta P, Prabhu KV (2011) Identification of microsatellite markers linked to leaf rust adult plant resistance (APR) gene Lr48 in wheat. Plant Breeding 130:31–34. [Google Scholar]
- 35. Rouse MN, Nava IC, Chao S, Anderson JA, Jin Y (2012) Identification of markers linked to the race Ug99 effective stem rust resistance gene Sr28 in wheat (Triticum aestivum L.). Theor Appl Genet 125:877–885. [DOI] [PubMed] [Google Scholar]
- 36. Sears ER, Loegering WQ (1968) Mapping of stem-rust genes Sr9 and Sr16 of wheat. Crop Sci 8:371–373. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Molecular markers mapped to the PmCH86 region based on wheat, rice, and Brachypodium synteny.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.