Abstract
Ground squirrel, a hibernating mammalian species, is more resistant to ischemic brain stress than rat. Gaining insight into the adaptive mechanisms of ground squirrels may help us design treatment strategies to reduce brain damage in patients suffering ischemic stroke. To understand the anti-stress mechanisms in ground squirrel neurons, we studied glutamate toxicity in primary cultured neurons of the Daurian ground squirrel (Spermophilus dauricus). At the neuronal level, for the first time, we found that ground squirrel was more resistant to glutamate excitotoxicity than rat. Mechanistically, ground squirrel neurons displayed a similar calcium influx to the rat neurons in response to glutamate or N-methyl-D-aspartate (NMDA) perfusion. However, the rate of calcium removal in ground squirrel neurons was markedly faster than in rat neurons. This allows ground squirrel neurons to maintain lower level of intracellular calcium concentration ([Ca2+]i) upon glutamate insult. Moreover, we found that Na+/Ca2+ exchanger (NCX) activity was higher in ground squirrel neurons than in rat neurons. We also proved that overexpression of ground squirrel NCX2, rather than NCX1 or NCX3, in rat neurons promoted neuron survival against glutamate toxicity. Taken together, our results indicate that ground squirrel neurons are better at maintaining calcium homeostasis than rat neurons and this is likely achieved through the activity of ground squirrel NCX2. Our findings not only reveal an adaptive mechanism of mammalian hibernators at the cellular level, but also suggest that NCX2 of ground squirrel may have therapeutic value for suppressing brain ischemic damage.
Introduction
Hibernation is a physiological process characterized by inactivity with a low metabolism and body temperature (the torpor state). It is regularly interrupted by brief periods of arousal when the metabolism and body temperature return to normal. During hibernation, blood flow to the brain is dramatically reduced and can be as low as one tenth of the normal level [1]. Hibernators can endure tremendous decrease in oxygen-glucose supply without neurological injury, as observed in vitro and in vivo [1]–[5]. Therefore, hibernators can be served as an ideal model for studying tolerance to brain ischemia and ischemia-related injuries. Interruption of the blood flow to the brain during ischemic stroke severely compromises the energy supply to the brain, which further disrupts ionic gradients across plasma membrane. This leads to neuronal depolarization, enhanced excitatory neurotransmitter release and reduced transmitter reuptake. Excessive extracellular accumulation of glutamate overactivates glutamate receptors, elicits calcium overload, and can ultimately lead to neuronal death [6]–[9].
In neurons, calcium functions as a signal in a variety of processes, including transmitter release, synaptic plasticity and gene transcription. At rest, the global intracellular calcium concentration ([Ca2+]i) of most neurons is approximately 50∼100 nM [10], which, after excitation, rises transiently to a level that is dozens of times higher [11]. Prolonged exposure to a high concentration of calcium overactivates endonucleases, proteases, lipases and phosphatases, which leads to lethal downstream reactions driven by oxidative stress or mitochondrial dysfunction [12]. The balance between calcium influx to the cytosol and calcium removal from the cytosol maintains calcium homeostasis. Calcium influx through the channels on the plasma membrane and calcium release from intracellular calcium stores are the two sources of [Ca2+]i elevation. Once neurons are activated, calcium from the extracellular fluid enters the cell through multiple ways, including voltage-gated calcium channels, ionotropic glutamate receptors (such as N-methyl-D-aspartate (NMDA) receptors and calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors), nicotinic acetylcholine receptors [13], transient receptor potential channels [14] and store-operated channels [15], [16]. In addition, when ryanodine receptors or inositol trisphosphate receptors are activated, calcium is released from the endoplasmic reticulum (ER). After activation, the calcium concentration returns to resting level by pumping calcium out of the cell and/or back into internal stores via the Na+/Ca2+ exchanger (NCX), the plasma membrane calcium ATPase (PMCA) and the sarco/endoplasmic reticulum calcium ATPase (SERCA), respectively. Meanwhile, mitochondria can buffer calcium by transiently taking up calcium through the uniporter and slowly releasing it back into the cytosol through the mitochondrial NCX [10].
To date, our understanding of the adaptive mechanisms of mammalian hibernators to brain ischemic injury is limited. Studies in injury models have been performed mostly on brain slices, and the tolerance at the cellular level was undetermined. Moreover, most studies have focused on injury tolerance during the hibernating state with few concerning the euthermic state [2], [17]. In addition, the role of excitotoxicity-induced calcium overload (a central event in ischemic injury) has not been well investigated in hibernators. In the present study, we determined the tolerance against glutamate toxicity of Daurian ground squirrels (which are hibernators) in primary cultured cortical neurons and investigated the adaptive mechanisms of ground squirrels at the neuronal level.
Materials and Methods
Animals and ethics statement
Daurian Ground squirrels [(Spermophilus dauricus (Brandt 1843)] and Sprague-Dawley rats [Rattus norvegicus (Berkenhout 1769)] were manipulated in accordance with the strict guidelines of the 7th edition of the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health. Animal trapping and experiments were approved by the Institutional Animal Care and Use Committee of Peking University (Permit Numbers: lsc-wangsq-1 and lac-tianyl-2). The ground squirrel used in the present study, Spermophilus dauricus, is a species widely distributed in China, Mongolia and Russia. In approximately mid-May, pregnant ground squirrels were caught in Zhangbei County (41.2°N, 114.8°E; Hebei province, China) and fed cabbages and rodent chow, following a 7-day quarantine period.
Primary culture of cortical neurons and glutamate exposure
Primary cortical neurons were prepared from 18-day-old Sprague-Dawley rat embryos (Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) and postnatal day 1 ground squirrels as previously described [18]. To minimize suffering, the fetal rats and neonatal ground squirrels were rapidly decapitated before cortices were removed. The dissociated cerebral cortices were then digested with 0.2% trypsin (Gibco, Gaithersburg, MD, USA) at 37°C for 2 minutes, and the digestion was terminated with 0.1% trypsin inhibitor (Sigma, St. Louis, MO, USA). The dispersed cells were collected and plated on poly-D-lysine pre-coated coverslips or 24-well plates at a density of 0.5–0.8×105 cells/cm2 in neurobasal medium (Gibco) supplemented with 2% B27 (Gibco), 2 mM glutamine (Sigma), 50 units/ml penicillin and 50 µg/ml streptomycin (Gibco). Forty-eight hour later, 10 µM cytosine β-D-arabinofuranoside (Sigma) was added, followed by changing the culture medium after 24 hours. Neurons were kept in a 5% CO2, humidified incubator at 37°C for 8–10 days before experiments, and half of the culture medium was replaced every 3 days. Immunofluorescence analysis showed that astrocyte contamination was less than 3% in rat neurons [18], the purity of ground squirrel neurons was more than 98%. After culturing for 9 days in vitro, the primary neurons were exposed to 200 µM L-glutamate (Sigma), and the neuronal viability was determined 3 hours or 24 hours later.
Cell viability by MTT, ATP luminescence and LDH release assays
For the MTT assay, neuronal metabolic activity was determined by measuring the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into a purple formazan product. Briefly, MTT (sigma) was dissolved in neuronal culture medium at a concentration of 0.5 mg/ml and 300 µl was added to each well. After 3 hours of incubation at 37°C, the culture medium was removed, and 300 µl/well of DMSO (Merck, Whitehouse Station, NJ, USA) was added to dissolve the formazan crystal. The absorbance was read at a wavelength of 570 nm on a microplate reader (BIO-RAD Model 680, Hercules, CA, USA).
For the ATP assay, ATP levels in neurons were determined using the CellTiter-Glo luminescent cell viability assay (Promega, Fitchburg, WI., USA) according to the manufacturer's instructions. The primary neurons were lysed for 2 min with a mixture of CellTiter-Glo reagent and neurobasal medium after exposure to glutamate for 24 hours, followed by 10 min incubation in the same mixture at room temperature to stabilize the luminescent signal. Wells containing CellTiter-Glo reagent and medium without cells were used as control for measuring the background luminescence. The overall luminescence was recorded in opaque-walled multiwell plates (Corning Inc., Corning, NY, USA) with a Thermo, Varioskan Flash microplate reader (Thermo Scientific, USA). The ATP level in glutamate treated neurons was expressed as a percentage of that in control neurons.
For the lactate dehydrogenase (LDH) release assay, media from cultured neurons were collected, and the LDH activity was measured using a commercial kit (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega) in accordance with the manufacturer's instructions.
Measurement of the reactive oxygen species
Dihydroethidium (DHE; Sigma) was used to measure the reactive oxygen species (ROS) levels in primary neurons of rat and ground squirrel. Neurons loaded with 10 µM DHE for 10 min at 37°C were imaged with an inverted microscope (20/NA 0.45, Olympus IX71,Tokyo, Japan) in artificial cerebrospinal fluid (ACSF; 141 mM NaCl, 2.5 mM KCl, 1.3 mM MgCl2, 1.25 mM NaH2PO4, 10 mM glucose, 2 mM CaCl2 and 10 mM HEPES; pH 7.4). Neurons treated with glutamate were loaded with DHE solution with 200 µM glutamate and recorded in ACSF supplemented with 200 µM glutamate.
Measurement of mitochondrial membrane potential (Ψm)
A ratiometric probe 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolyl-carbocyanine iodide (JC-1, Biotium Inc., Hayward, CA, USA) was used to detect the mitochondrial membrane potential (Ψm) in primary neurons. The monomeric green form and the aggregate red form of JC-1 have maximum emissions at wavelengths of 530 and 590 nm. Primary neurons loaded with JC-1 were kept in the incubator for 15 min before imaging with a confocal microscope (40/NA 1.0 water-immersion lens, Zeiss 710, Oberkochen, Germany). JC-1 was excited at 488 nm, and the red/green components of the emission fluorescence were spectrally separated. Neurons treated with glutamate were loaded with JC-1 solution supplemented with 200 µM glutamate, and then recorded in ACSF with 200 µM glutamate.
In transfection experiments, neurons were loaded with 100 nM tetramethylrhodamine ethyl ester (TMRE, Invitrogen, Carlsbad, CA, USA) for 10 min at 37°C to measure Ψm.
[Ca2+]i measurement
The resting Ca2+ concentration was measured with the ratiometric calcium indicator fura-2 AM. The cultured neurons were rinsed with ACSF after the culture medium removal. Then, 5 µM fura-2 AM (Invitrogen, Carlsbad, CA, USA) was added for 10 min at 37°C avoiding light. Next, the cultures were placed on an inverted microscope (20/NA 0.45, Olympus IX71, Tokyo, Japan) after being rinsed twice with ACSF. The same process was performed for glutamate treated neurons, except for same concentration of glutamate (200 µM) added in all of the solutions contained. 3-amino-6-chloro-5-((4-chlorobenzyl)amino)-N-(((2,4-dimethylbenzyl)amino)iminomethyl)-pyrazinecarboxamide (CB-DMB, Sigma) was used to block NCXs; and thapsigargin (TG, Sigma) was used to block SERCA. All images were taken with the Cell∧R system (Olympus). The excitation light from a xenon lamp was filtered with a rotating wheel containing 340 nm and 380 nm filters (Semrock, Rochester, NY, USA). The emitted fluorescence was measured at a wavelength approximately 510 nm. All the images were analyzed with Xcellence software (Olympus). The [Ca2+]i was calculated using the Eqn 1:
| (1) |
where R = F340/F380; K d is the dissociation constant of fura-2; β = F380min/F380max, and R min and R max are the minimal and maximal F340/F380. Following the method described by Sipido [19], the parameters in Eqn 1 were determined and shown in Eqn 2:
| (2) |
To calculate the calcium removal rate, fluo-4 AM (Invitrogen), a calcium indicator with a faster rate of Ca2+ dissociation than fura-2, was used. After being rinsed twice with ACSF, the neurons on the culture coverslips were loaded with 10 µM fluo-4 AM for 10 min at 37°C and then placed on an inverted microscope. Upon glutamate perfusion, calcium transients were recorded with a laser scanning confocal microscope (Zeiss 710, 40 water-immersion lens/NA 1.0). All of the experiments were performed at 25°C. The acquired images were processed with IDL 7.0 software (Research Systems, Inc., Boulder, CO, USA). The calcium concentration was calculated according to the Eqn 3:
| (3) |
in which R = F/F0; K d, the dissociation constant of fluo-4, is almost temperature-independent at physiological pH, and an approximate value of 1.1 µM was used in the formula [20]. The resting calcium concentration in primary neurons, [Ca2+]rest, was determined as 94 nM by using the calcium ratiometric indicator fura-2 AM. Rhod-2 AM (Invitrogen) was used as a calcium indicator to calculate the calcium removal rate in neurons 2 days after transfection with plasmids carrying EGFP. The K d value for rhod-2 was 1.3 µM [21]. The decay phase of the [Ca2+]i transient was fitted with a 1-phase exponential decay curve and the time constant (τ) was then obtained using the GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA).
Electrophysiology
Whole-cell NMDA current recordings were conducted on the primary neurons cultured for 9–10 days in vitro in ACSF. Patch electrodes (4–6 MΩ) were fabricated on a horizontal puller (Sutter, Instruments). The pipette solution contained the following components: 115 mM K-gluconate, 20 mM KCl, 1.5 mM MgCl2, 10 mM HEPES, 0.025 mM EGTA, 0.2 mM Li4-GTP, 2 mM Mg-ATP and 10 mM Na2-phosphocreatine. The pH was adjusted to 7.2–7.3 with KOH. NMDA currents were evoked with magnesium-free ACSF with 100 µM NMDA and 10 µM glycine at the holding potential of −70 mV. Whole-cell patch-clamp recordings were made with an EPC-10 amplifier (HEKA Electronics, Lambrecht, Germany) and digitized at 10 kHz with Patchmaster software (HEKA). Charge transfer density was calculated as the area under the curve (pA*s) normalized to cell capacitance (pF).
NCX cloning, plasmid preparation, cell transfection and determination of cell viability
Total RNA was extracted from the cerebral cortical tissue of rats and ground squirrels (male, approximately 200 g) using TRIzol (Invitrogen) according to the manufacturer's instructions. The purified RNA was reverse transcribed into cDNA with oligo (dT) 15 primers. Rat and ground squirrel NCXs were cloned using the primers listed below (5′-3′; ground squirrel: GS): Rat-NCX1-F ATGCTTCGACTAAGTCTC; Rat-NCX1-R TTAGAAGCCTTTTATGTG; Rat-NCX2-F ATGGCTCCCTTGGCTTTG; Rat-NCX2-R CTAGAAGCCCCGAATGTG; Rat-NCX3-F TGTATGGCGTGGTTACGG; Rat-NCX3-R GCCCTGTGGAGGTCTTGT; GS-NCX1-F ATGCCTCGGTTAAGCCTC; GS-NCX1-R TTAGAAGCCTTTTATGTG; GS-NCX2-F ATGGCTCCCCTGGCTTTGGTG; GS-NCX2-R CCTAGAAACCCCGGATGTGGC; GS-NCX3-F ATGGCGTGGTTAAGGTTG; GS-NCX3-R GTGGATTTGTTGCTGTTG. The sequence data of rat NCXs are available on GenBank (NCX1-NCX3: accession numbers: NM_001270774, NM_078619, NM_078620; version numbers: NM_001270774.1, NM_078619.1, NM_078620.1). The primers for ground squirrel NCXs were designed according to the sequence data of squirrel on Ensembl (Ensembl version: ENSSTOG00000025770.1, ENSSTOG00000020165.1, ENSSTOG00000003907.2). PrimeSTAR Max DNA Polymerase (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China) was used to amplify the cDNA template according to the following PCR protocol: 35 cycles of 98°C for 10 s, 55–60°C for 15 s and 72°C for 20 s. Nucleotide sequence encoding for 2A peptide from porcine teschovirus-1 (P2A; GCTACTAACTTCAGCCTGCTGAAGCAGGCTGGAGACGTGGAGGAGAACCCTGGACCT) were synthesized by Ruibio Tech (Beijing, China), and annealed before they were inserted into a modified version of pFUGW ([22]; courtesy of Prof. Zhang from Peking University) at the BsrGI/BamHI (restriction endonucleases from NEB, Beverly, MA, USA) sites; this construct was termed pFUGW-P2A when mentioned. NCX cDNAs were cloned into pFUGW-P2A at the NheI/BamHI sites. After 6 days in vitro, cortical neurons were transfected with lipofectamine 2000 (Invitrogen) for 12 hours.
Neuronal viability was determined by assessing cell and nucleus morphology after staining with 0.4 µg/ml Hoechst 33342 (Sigma) for 10 min at 37°C. All of the images were taken under a fluorescence microscope (Olympus IX71, 10/NA 0.30).
Single-cell PCR analysis of ground squirrel NCX2 expression
Primary neurons were suspended in RNase-free PBS following digestion with trypsin-EDTA at 4–6 hours after removing the transfection medium (Gibco, Gaithersburg, MD, USA). Individual target neuron was sucked into a patch electrode (tip diameter ranging 15–20 µm) under a fluorescence microscope (Olympus IX71, 20/NA 0.45), and then transferred immediately to liquid nitrogen in a PCR tube (Axygen, Hangzhou, China). After incubating at 70°C for 90 s, oligo (dT) 15 primers were added for reverse transcription using SuperScript III (Invitrogen). PrimeSTAR Max DNA Polymerase (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China) were added for target amplification. The expression level of ground squirrel NCX2 was determined by PCR amplification with primers GS-NCX2 (F: 5′TCATCGCTGACCGCTTCA3′; R: 5′GCAGGCGTCCTCATAGTGC3′) according to the following protocol: 30 cycles of 98°C for 10 s, 59°C for 15 s and 72°C for 20 s. As an internal control, beta-actin expression was determined with primers (set 1, F1: 5′GAAATCGTGCGTGACATTA3′, R1: 5′ACTCATCGTACTCCTGCTTG3′; set 2, F2: 5′ GTAAAGACCTCTATGCCAACA3′, R2: 5′GGACTCATCGTACTCCTGCT3′) according to the following nested PCR protocol: first run: 30 cycles of 98°C for 10 s, 59°C for 15 s and 72°C for 5 s; second run: 35 cycles of 98°C for 10 s, 57°C for 15 s and 72°C for 5 s.
Real-time PCR was performed using Mx3000p QPCR system (Stratagene Corporation, La Jolla, CA, USA) with Brilliant II QPCR Master Mix (Agilent Technologies, Palo Alto, CA, USA). The target sequence was amplified in a total reaction volume of 10 µl containing 5 µl SYBR Green PCR Master Mix (2×), 0.5 µl cDNA (or cell contents not subjected to reverse transcribed) template and 0.5 µl forward/reverse primers (F: 5′CATCGCCAACTACTACGCTCTA 3′; R: 5′CGCCGTCATCCTCATCCT 3′) using the following PCR protocol: 40 cycles of 95°C for 30 s, 61°C for 30 s and 72°C for 20 s.
Statistical analysis
Data were analyzed with the two-tailed unpaired Student's t-test using Sigmastat 3.5 software (Systat Software, Inc., San Jose, CA, USA), unless otherwise stated. Two-way ANOVA with post-hoc Holm-Sidak test was used to compare [Ca2+]i between rat and ground squirrel neurons. All data are presented as mean ± s.e.m. The sample size used for statistical analysis was n. P<0.05 was considered to be statistically significant.
Results
Ground squirrel neurons were more tolerant to glutamate toxicity than rat neurons
Hibernators have been reported to be resistant to ischemic damage in vivo [2] and in brain slices [5], however, whether hibernators are resistant to ischemic insults or excitotoxicity (the key event in ischemia) at the cellular level is still undetermined. To test whether ground squirrel have an adaptive advantage against excitotoxicity over rat at the neuronal level, primary neurons were treated with glutamate to induce cell damage. Under basal conditions, no significant differences in cell viability were detected between rat and ground squirrel neurons, as determined by the MTT assay (Rat: 0.72±0.04; Ground squirrel: 0.77±0.32. P = 0.39, n = 3). However, following glutamate treatment for 24 hours, considerably less morphological defects were observed in ground squirrel neurons than in rat neurons (Fig. 1A). We further quantified the difference in cell viability using the MTT assay (Fig. 1B, Rat: 27.97±1.52%; Ground squirrel: 63.87±3.71%) and the LDH release assay (Fig. 1C, Rat: 231.34±20.37%; Ground squirrel: 131.29±11.50%). These results show the neuronal viability is much higher in ground squirrel, indicating that at the neuronal level ground squirrel is also more resistant to glutamate toxicity than rat.
Figure 1. Ground squirrel primary cortical neurons were more tolerant to glutamate toxicity than rat neurons.
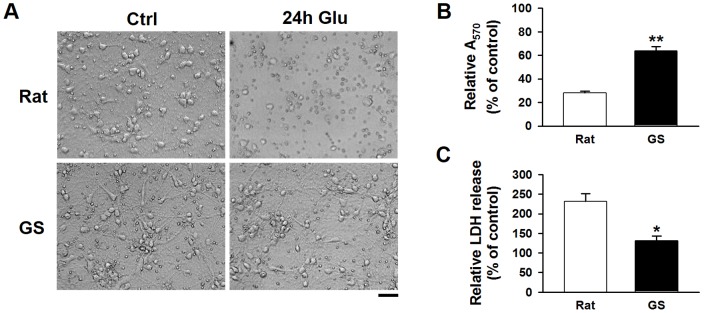
After the primary cortical neurons were cultured for 9 days in vitro, glutamate (200 µM) was added to the culture for 24 hours. (A) Representative phase contrast images show ground squirrel and rat cultured cortical neurons. The images show that the morphology of the ground squirrel neurons is better maintained than that of the rat neurons. Scale bar, 50 µm. (B) MTT assay, A570 refers to the absorbance at 570 nm. n = 3 separate experiments, P<0.01. (C) LDH release assay. Rat: n = 3 separate experiments, GS: n = 2 separate experiments, P<0.05. The results demonstrate that ground squirrel neurons survived glutamate toxicity better than rat neurons. GS: ground squirrel.
Extensive activation of glutamate receptors can lead to overproduction of ROS and cause damage to lipids, proteins and DNA; therefore, ROS can be used as a marker of severe stress [23], [24]. To assess ROS changes in the cytoplasm, neurons were stained with DHE [25], [26]. Indeed, the DHE fluorescence intensity of rat neurons increased by more than 1.5 fold over 3 hours of glutamate treatment (Fig. S1A, 164±10.16%). However, no significant increase in the DHE fluorescence was detected in the ground squirrel neurons (Fig. S1A, 102±4.17%). This result suggests that ground squirrel neurons generate less ROS under glutamate toxicity. Furthermore, the ATP assay indicates that ATP level is higher in ground squirrel neurons (Fig. S1B, Rat: 32.93±3.47%; Ground squirrel: 62.51±3.82%). Mitochondrial dysfunction promotes cell death in various diseases [27]–[29]. To assess the mitochondrial membrane potential (Ψm), we performed JC-1 staining [30]. The results showed that ground squirrel neurons maintained a stable Ψm after 3- or 24-hour glutamate treatment, whereas rat neurons displayed 37.62% or 41.31% reduction in the Ψm after the 3- or 24-hour glutamate treatment (Fig. S1C). Together, our results suggest that ground squirrel neurons have several adaptive advantages for resisting glutamate-induced cell death, including better maintenance of mitochondrial membrane potential and lower ROS production.
Ground squirrel neurons maintained calcium homeostasis better than rat neurons
Glutamate-induced mitochondrial damage and cell death are primarily caused by calcium overload [31]–[33], the higher viability of ground squirrel neurons under glutamate treatment described above thus may result from the better regulation of calcium homeostasis in these cells. To test whether the [Ca2+]i in ground squirrel neurons is different from that in rat neurons during the glutamate treatment, we measured [Ca2+]i with fura-2, a ratiometric calcium indicator. At the resting state, the [Ca2+]i of rat and ground squirrel neurons displayed a similar basal level (Fig. 2). However, the increase in [Ca2+]i was notably less in ground squirrel neurons than in rat neurons after 3-hour or 24-hour glutamate treatment (Fig. 2, 0 hours: Rat: 75.91±8.29 nM; Ground squirrel: 79.96±6.22 nM; 3 hours: Rat: 2.35±0.38 µM; Ground squirrel: 0.72±0.13 µM; 24 hours: Rat: 3.03±0.62 µM; Ground squirrel: 0.54±0.05 µM). This result indicates that ground squirrel neurons can better maintain calcium homeostasis than rat neurons during glutamate exposure.
Figure 2. The [Ca2+]i was much lower in ground squirrel neurons 3 and 24 hours after glutamate exposure.
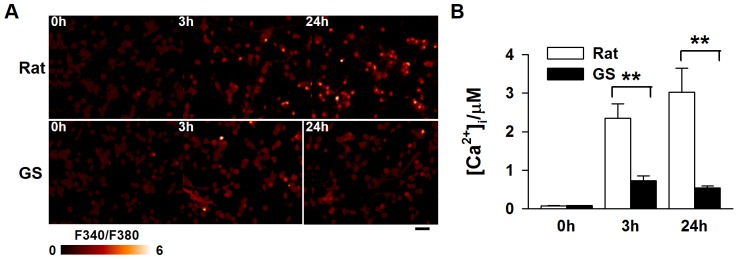
(A) Representative fluorescence ratio images of fura-2. Scale bar: 50 µm. (B) Statistical results show that the relative intracellular calcium concentration ([Ca2+]i) in neurons after glutamate exposure is increased in both ground squirrel and rat neurons. However, the level of the [Ca2+]i increase was much lower in the ground squirrel neurons than in the rat neurons. n = 174–180 neurons, from 3 separate experiments. Two-way ANOVA test with Post hoc Holm-Sidak comparison, P<0.01. Fura-2 AM was used as a calcium indicator.
Glutamate and NMDA induced [Ca2+]i elevations were similar in ground squirrel and rat neurons
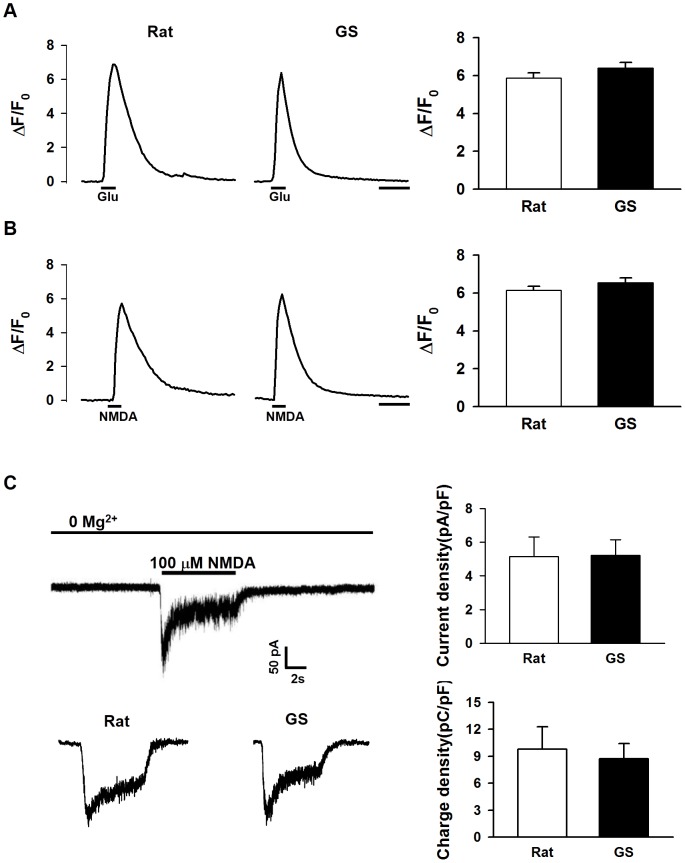
[Ca2+]i is determined by the combined effects of calcium influx/release into and the removal from the cytosol. Technically speaking, calcium overload can be achieved by over-activation of calcium influx/release and/or decrease of calcium removal. Another calcium indicator with lower affinity (fluo-4) than fura-2 was used to measure the [Ca2+]i elevation. We found no significant difference in the amplitude of the [Ca2+]i elevation between ground squirrel and rat neurons upon glutamate perfusion (Fig. 3A, Rat: 5.87±0.27; Ground squirrel: 6.40±0.28). NMDA receptor is the main glutamate receptor mediating Ca2+ influx and [Ca2+]i elevation. The amplitude of the [Ca2+]i elevation induced by NMDA perfusion was thus measured and showed no difference between the two types of neurons either (Fig. 3B, Rat: 6.14±0.22; Ground squirrel: 6.54±0.27). We also measured NMDA receptor-mediated currents by whole-cell patch clamp recording. In coincidence with previous results, there were no differences in current density (Fig. 3C, Rat: 5.15±1.16 pA/pF; Ground squirrel: 5.21±0.92 pA/pF) or charge transfer density (Fig. 3C, Rat: 9.80±2.48 pC/pF; Ground squirrel: 8.74±1.66 pC/pF) between ground squirrel and rat neurons. Together, these results suggest that the influx of calcium and [Ca2+]i elevation in response to glutamate stimulation is similar in ground squirrel and rat neurons.
Figure 3. A similar [Ca2+]i elevation in rat and ground squirrel neurons was induced by glutamate and NMDA.
(A) Glutamate (Glu, 200 µM) induced [Ca2+]i changes in ground squirrel and rat neurons. n = 58–65 neurons, from 3 separate experiments. Scale bar, 10 s. (B) NMDA (100 µM) induced [Ca2+]i elevation in both types of neurons. Scale bar, 10 s. The results show that the elevation in [Ca2+]i induced by glutamate or NMDA is similar in the two types of neurons. n = 55–62 neurons, from 3 separate experiments. (C) The neuronal response to NMDA perfusion was measured by whole-cell patch clamp. The results show that no significant differences exist in current density or charge density between ground squirrel and rat neurons. Eighteen rat neurons (from 5 separate experiments) and 26 ground squirrel neurons (from 7 separate experiments) were examined and used for statistical analysis. Fluo-4 AM was used in A and B as a calcium indicator.
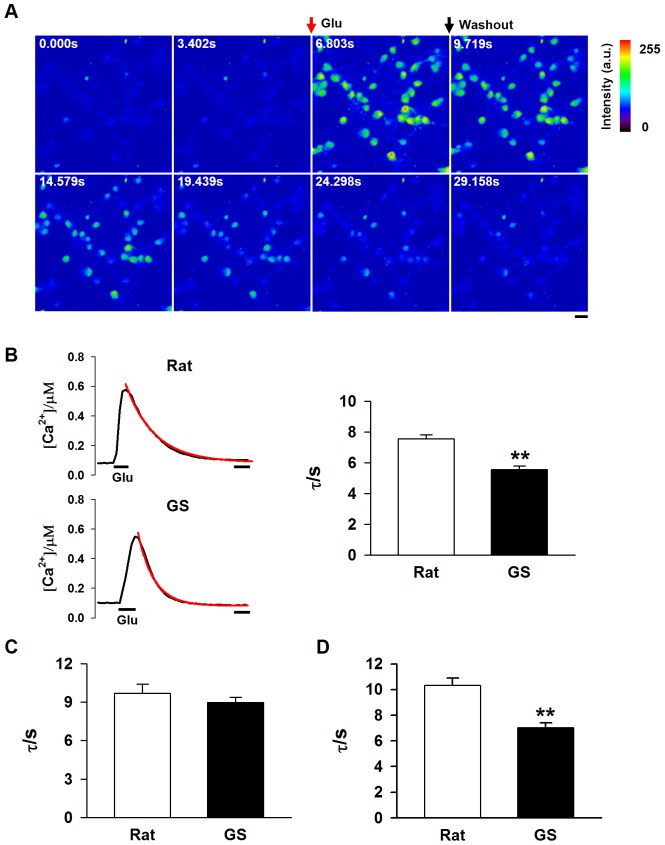
Calcium removal mediated by NCX was faster in ground squirrel neurons than in rat neurons
The rate of calcium removal in the cytoplasm can be assessed by the time constant (τ), which is estimated by fitting the [Ca2+]i decay phase with a single exponential function [34]. Glutamate perfusion induced a calcium transient in ground squirrel and rat neurons. As illustrated in Fig. 4A and 4B, the decay τ of ground squirrel neurons was lower, indicating a faster calcium removal rate than in rat neurons after glutamate washout (Fig. 4B, τrat = 7.56±0.27 s; τground squirrel = 5.56±0.22 s).
Figure 4. The velocity of calcium removal was faster in ground squirrel neurons than in rat neurons.
(A) A series of image frames of fluo-4 AM loaded neurons acquired by confocal microscopy. Glutamate (Glu) was applied between the second and the third frame (indicated by the red arrow). Then, glutamate was washed out approximately 2 s later (indicated by the black arrow). Scale bar, 20 µm. (B) Left: a typical example of [Ca2+]i dynamics in response to glutamate administration (the black line). The decay phase is fit with a single exponential function (red line) to estimate the time constant (τ). Right: statistical results suggest that the calcium removal rate is markedly faster in ground squirrel neurons than rat neurons. n = 76 to 131 neurons, from 3 separate experiments, P<0.01. Scale bar, 5 s. (C) Effect of NCX blockade on the [Ca2+]i decay rate. The result shows that the reduced τ value in ground squirrel neurons is abolished by administration of CB-DMB, a specific NCX blocker. n = 73 to 96 neurons, from 5 separate experiments. (D) Effect on the [Ca2+]i decay rate of the inhibition of SERCA. The results show that the reduced calcium removal rate is similar in rat and ground squirrel neurons in response to TG, a SERCA blocker. n = 56 to 70 neurons, from 4 (rat) and 3 (GS) separate experiments.
The major calcium removal pathways are through NCX on the plasma membrane and SERCA on the ER. First, we examined the effect of blocking NCX by CB-DMB, a pan- specific inhibitor of the NCXs in excitable cells [35], [36]. We found that CB-DMB could significantly prolong the decay time in both ground squirrel and rat neurons, resulting in comparable values of τ in the two species (Fig. 4C, τrat = 9.69±0.74 s; τground squirrel = 8.98±0.39 s). The blockade of NCX was sufficient to eliminate the difference of τ between ground squirrel and rat neurons, indicating that the activity of NCXs is likely higher in ground squirrel neurons than in rat neurons. However, TG, a specific SERCA inhibitor, equally increased τ in both ground squirrel and rat neurons (Fig. 4D, Rat-TG: τ = 10.33±0.55 s; Ground squirrel-TG: τ = 7.38±0.37 s). The decrease of calcium removal rate was similar in ground squirrel and rat neurons (Rat: 0.028±0.011 s−1; Ground squirrel: 0.015±0.0098 s−1. P = 0.36, n = 3), suggesting that ground squirrel and rat neurons have similar SERCA activity.
Expression of ground squirrel NCX2 in rat neurons increased neuron survival against glutamate toxicity
The rat genome contains three NCX genes, so does the ground squirrel genome. To test which NCX gene plays a protective role against glutamate toxicity, we transfected the cDNA of these NCX genes individually into rat neurons using EGFP as an indicator of the NCX expression (the transfection rate is approximately 10%). The neuronal survival after glutamate treatment was quantified by counting live cells [37]. We observed that the survival rate was similar among untransfected neurons in all groups (Fig. 5A, B). However, neurons overexpressing ground squirrel NCX2 displayed resistance to glutamate toxicity, and the viability of these cells was 224.59% of the control group (Fig. 5B). The mRNA expression of ground squirrel NCX2 was confirmed using single-cell PCR method (Fig. S2A and B). We further evaluated the calcium removal rate with the red-shift calcium indicator rhod-2 in rat neurons transfected with the empty vector or the vector containing ground squirrel NCX2. The calcium removal rate was similar in untransfected and empty vector-transfected neurons (untransfected neurons: 10.44±0.54 s; empty vector-transfected neurons: 10.26±0.89 s. P = 0.86, n = 5); however, calcium removal was markedly faster in the neurons expressing ground squirrel NCX2 (Fig. S3, Mock: 10.97±1.24 s; Ground squirrel NCX2: 6.22±0.50 s). Together, these results suggest that the expression of ground squirrel NCX2 increases rat neuron survival against glutamate toxicity.
Figure 5. Ground squirrel NCX2 expression in rat primary neurons increased the viability of neurons exposed to glutamate.
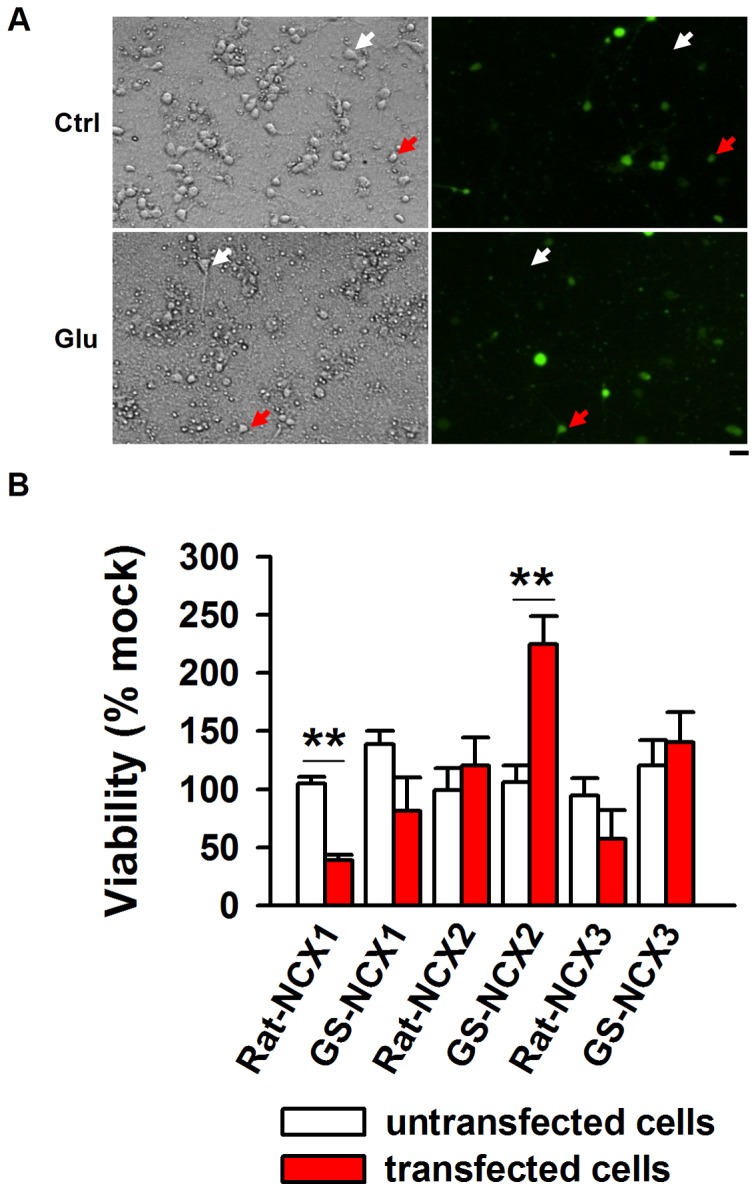
(A) Expression of ground squirrel NCX2 in rat primary neurons. Neurons transfected with ground squirrel NCX2 were labeled by EGFP, and neuronal survival was determined 6 hours after glutamate treatment. Scale bar, 25 µm. The results show that neurons transfected with ground squirrel NCX2 (EGFP positive cells identified by red arrows) are less susceptible to glutamate injury than were untransfected neurons (identified by white arrows). In addition, the effect of overexpressing NCX1, NCX2 and NCX3 from both rat and ground squirrel neurons were tested. The results are shown in the bar graph (B). The data suggest that the expression of ground squirrel NCX2 promotes survival under glutamate toxicity conditions, whereas overexpression of rat NCX1 enhances cell death. In contrast, overexpression of other rat and ground squirrel NCXs has no effect. n = 5 separate experiments, P<0.01.
Discussion
The present study unveils ground squirrel is more tolerant to glutamate toxicity than rat also at the neuronal level, based on a lower [Ca2+]i level during glutamate exposure. This maintenance of calcium homeostasis could be explained by a faster calcium removal rate resulted from higher activity of NCX in ground squirrel neurons. Expression of ground squirrel NCX2 in rat neurons reduces rat neuron death induced by glutamate excitotoxicity. Our findings might reveal an adaptive mechanism protecting against ischemic injury during hibernation, at the neuronal level.
It is well accepted that hibernators survive ischemia much better than nonhibernators, as determined in vitro [4], [17] and in vivo [2]. However, much remains to be learned about the adaptive mechanisms involved. In the present study, we focused on intracellular calcium homeostasis during glutamate exposure in primary cultured cortical neurons of rats and ground squirrels. We demonstrated that ground squirrel neurons were more resistant to glutamate excitotoxicity than rat neurons (Fig. 1). Mechanistically, the protective effect was a result of maintaining low [Ca2+]i in ground squirrel neurons during glutamate exposure (Fig. 2). A previous study has demonstrated that glutamate could induced a lower [Ca2+]i elevation in the hippocampal slices of hibernating and interbout euthermic AGS than in rats [38]. In our study, we showed that the amplitude of the [Ca2+]i elevation elicited by glutamate administration was similar in rat and ground squirrel primary neurons (Fig. 3A). In addition to the amplitude of calcium elevation [39], the source of calcium [40] is another determinant of glutamate toxicity. The NMDA receptor-mediated calcium influx has a particular propensity to cause neuronal injury [41]. However, the role of the NMDA receptor in mediating neuronal death in hibernators and non-hibernators remains controversial. Some studies proposed that the NMDA receptor played a key role, because the expression of the NR1 subunit of the NMDA receptor [38] and NMDA receptor-mediated neuronal death [4] were lower in hibernating and interbout euthermic AGS than in rats. However, no difference in the NR1 expression between rats and euthermic hamsters, while euthermic hamsters were more tolerant to OGD (oxygen and glucose deprivation) than rats [42] was also reported. Our data suggest that the NMDA receptor-mediated currents between rat and ground squirrel neurons are identical (Fig. 3B, C), indicating a similar role of the NMDA receptor in mediating glutamate neurotoxicity in the primary neurons of these two species.
During sustained glutamate exposure, high-capacity calcium removal apparatuses are required to remove the overloaded calcium elicited by glutamate. In contrast to PMCA clustering at the active zone, NCX localizes at the neuronal soma. Distinct from PMCA with a high affinity for Ca2+ (K d≈100 nM) and a low turnover rate (30–250 s−1), NCX has a lower affinity for Ca2+ (K d≈1000 nM) and a much higher turnover rate (2,000–5,000 s−1) [43]. These properties allow NCX to quickly remove calcium upon calcium overload. We found that a higher NCX activity in ground squirrel neurons (Fig. 4) facilitated calcium extrusion. NCX is a bi-directional membrane ion transporter and can shift to a reverse mode (injury promoting) under certain conditions [44], [45]. Furthermore, NCX isoforms could be cleaved by diverse proteases [46], [47]. For instance, NCX1 and NCX3 were degraded in focal brain ischemia, and NCX3 was cleaved in glutamate excitotoxicity in cerebellar granule neurons; in contrast, NCX2 was not cleaved during focal brain ischemia or glutamate exposure [48]. The role of NCX1 in ischemia injury is highly debated. Overexpression of cardiac NCX1 was reported to exacerbate ischemia/reperfusion injury in male transgenic mice [49], while knockdown of NCX1 in cortical neurons decreased cell death by ∼30% in response to OGD/reperfusion injury [50], both of which were consistent with our observation that overexpression NCX1 in rat primary neurons increased cell death (Fig. 5A and B). It is still unknown what causes the difference between NCX1 and the other two isoforms, one possibility is that the equilibrium potential for the three isoforms might be different.
Furthermore, our data suggest that the expression of ground squirrel NCX2, rather than other NCXs, promotes neuronal survival from glutamate toxicity (Fig. 5A and B). We did sequence analysis to explore the possible causes. After multiple sequence alignment analysis of NCX2, we next performed protein phosphorylation sites prediction with DISPHOS and Netphos 2.0. As a result, a predicted phosphorylation site (DISPHOS: 0.725; Netphos 2.0: 0.989) was uncovered in ground squirrel and another hibernator (thirteen-lined ground squirrel, Ictidomys tridecemlineatus) without shown in rat or human. Aligned with dog (Canis lupus familiaris) NCX1 3D structure (NCX2 3D structure are unknown), this phosphorylation site locates near the beginning of calcium binding domain 1 (CBD1), which may be involved in its activity regulation. We are working on this possibility in collaboration with another group. Bano et al. [48] reported that overexpression of rat NCX2 protected cerebellar granule neurons from glutamate toxicity, which we did not observe. A more severe damage in our model (200 µM glutamate for 6 hours in our system vs. 150 µM for 2.5 hours in their system) and a different cell type (the cortical neurons in our system vs. cerebellar granule neurons in their experiment) we used might account for this inconsistency.
Actually, in addition to the failure of intracellular calcium homeostasis, the increase of ROS production, the decrease of mitochondrial membrane potential and ATP level, as well as the crosstalk among them also contributed to glutamate-induced neuronal injury [51], [52]. We observed less ROS production (Fig. S1A), higher ATP level (Fig. S1B) and better maintained mitochondrial membrane potential (Fig. S1C) in ground squirrel neurons than in rat neurons under glutamate treatment. We also found a lower ROS level and comparable mitochondrial membrane potential in rat primary neurons transfected with ground squirrel NCX2 (Fig. S4A and B), indicating increased ROS level, rather than loss of mitochondrial membrane potential, was a consequence of limited calcium clearance. Furthermore, the decreased ROS level in rat primary neurons expressed ground squirrel NCX2 was also consistent with our previous observation that ROS formation in ground squirrel neurons was less than in rat neurons (Fig. S4A).
Glutamate excitotoxicity widely occurs in neurodegenerative disorders, such as seizures, ischemia and traumatic brain injury. To develop strategies preventing neuronal cells from death, most studies have focused on reducing calcium entry, such as inhibition of the NMDA receptor. Here, our research indicates that increased calcium extrusion can also protect neurons from glutamate toxicity. Our research may promote further investigation into the function of ground squirrel NCX2 and provide novel targets for treating excitotoxicity-mediated neurological disorders.
Supporting Information
Compared with rat neurons, ground squirrel neurons maintained lower reactive oxygen species (ROS) production (A. DHE was used as a ROS indicator. n = 146 to 153 neurons, from 3 separate experiments, P <0.05), higher ATP level (B. Rat: n = 3 separate experiments; Ground squirrel: n = 5 separate experiments, P <0.01) and more stable mitochondrial membrane potential (Ψm) (C. JC-1 was used as Ψm indicator. Rat: n = 3 separate experiments; Ground squirrel: n = 5 separate experiments, P<0.05) under glutamate treatment. GS: ground squirrel.
(TIF)
The expression of ground squirrel NCX2 mRNA was detected in transfected rat neurons. (A) Ground squirrel (GS) NCX2 specific band was detected only in reverse-transcribed preparations from transfected neurons. 0: control (no cells); B: nontransfected neurons; E: mock transfected neurons; G: GS NCX2 transfected neurons. n = 3 separate experiments. (B) Both the GS NCX2 plasmid and the mRNA transcribed from GS NCX2 could serve as templates for the PCR amplification. Their roles were determined with Q-PCR. cDNA: mRNA of GS NCX2 as template; P: plasmid GS-NCX2 as template. n = 7–9 neurons, from 3 separate experiments, P<0.01. GS: ground squirrel.
(TIF)
Expression of ground squirrel NCX2 in rat primary neurons reduced the τ value of calcium removal. Neurons were loaded with 10 µM rhod-2 AM for 10 min at 37°C. n = 24–28 neuron, from 5 separate experiments, P<0.01.
(TIF)
Ground squirrel NCX2 expression in rat primary neurons decreased ROS, and had no effect on mitochondrial membrane potential (Ψm). (A) Left: ROS level of rat primary neurons was significantly higher than that in ground squirrel neurons. Rat: n = 153 neurons, from 4 separate experiments; GS: n = 147 neurons, from 3 separate experiments, P<0.01. Right: expression of GS NCX2 in rat primary neurons lowered ROS level. n = 20 to 55 neurons, from 3 separate experiments, P<0.01. (B) Expression of GS NCX2 in rat primary neurons did not change Ψm. n = 109, 32, 120, 44 neurons respectively, from 3 separate experiments. Two-way ANOVA test. GS: ground squirrel.
(TIF)
Acknowledgments
We thank Dr. Lei Liu, Prof. Shiqiang Wang and Prof. Jingchu Luo for critical suggestions, and Dr. Fuchou Tang for technical supports. We thank Yu Guo and Jun Wei for assistance with neuronal culture.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by National Basic Research Program (973 Program) of China [Grant number: 2012CB518200, 2006CB504100], URL:http://www.973.gov.cn/English/Index.aspx; National Natural Science Foundation of China [Grant number: 30730013] URL: http://www.nsfc.gov.cn/. ZC received the fundings. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM (1994) Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab 14:193–205. [DOI] [PubMed] [Google Scholar]
- 2. Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA (2006) The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke 37:1261–1265. [DOI] [PubMed] [Google Scholar]
- 3. Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, et al. (2005) Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol 289:R1297–1306. [DOI] [PubMed] [Google Scholar]
- 4. Ross AP, Christian SL, Zhao HW, Drew KL (2006) Persistent tolerance to oxygen and nutrient deprivation and N-methyl-D-aspartate in cultured hippocampal slices from hibernating Arctic ground squirrel. J Cereb Blood Flow Metab 26:1148–1156. [DOI] [PubMed] [Google Scholar]
- 5. Frerichs KU, Hallenbeck JM (1998) Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab 18:168–175. [DOI] [PubMed] [Google Scholar]
- 6. Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4:399–415. [DOI] [PubMed] [Google Scholar]
- 7. Rossi DJ, Oshima T, Attwell D (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403:316–321. [DOI] [PubMed] [Google Scholar]
- 8. Simon RP, Swan JH, Griffiths T, Meldrum BS (1984) Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 226:850–852. [DOI] [PubMed] [Google Scholar]
- 9. Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164:719–721. [DOI] [PubMed] [Google Scholar]
- 10. Grienberger C, Konnerth A (2012) Imaging calcium in neurons. Neuron 73:862–885. [DOI] [PubMed] [Google Scholar]
- 11. Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21. [DOI] [PubMed] [Google Scholar]
- 12. Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4:552–565. [DOI] [PubMed] [Google Scholar]
- 13. Fucile S (2004) Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium 35:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647. [DOI] [PubMed] [Google Scholar]
- 15. Gemes G, Bangaru ML, Wu HE, Tang Q, Weihrauch D, et al. (2011) Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J Neurosci 31:3536–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Emptage NJ, Reid CA, Fine A (2001) Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron 29:197–208. [DOI] [PubMed] [Google Scholar]
- 17. Christian SL, Ross AP, Zhao HW, Kristenson HJ, Zhan X, et al. (2008) Arctic ground squirrel (Spermophilus parryii) hippocampal neurons tolerate prolonged oxygen-glucose deprivation and maintain baseline ERK1/2 and JNK activation despite drastic ATP loss. J Cereb Blood Flow Metab 28:1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou C, Ye HH, Wang SQ, Chai Z (2006) Interleukin-1beta regulation of N-type Ca2+ channels in cortical neurons. Neurosci Lett 403:181–185. [DOI] [PubMed] [Google Scholar]
- 19. Sipido KR, Callewaert G (1995) How to measure intracellular [Ca2+] in single cardiac cells with fura-2 or indo-1. Cardiovasc Res 29:717–726. [PubMed] [Google Scholar]
- 20. Wang SQ, Song LS, Lakatta EG, Cheng H (2001) Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410:592–596. [DOI] [PubMed] [Google Scholar]
- 21. Escobar AL, Monck JR, Fernandez JM, Vergara JL (1994) Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal muscle fibres. Nature 367:739–741. [DOI] [PubMed] [Google Scholar]
- 22. Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, et al. (2009) Presenilins are essential for regulating neurotransmitter release. Nature 460:632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, et al. (2009) NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 12:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds IJ, Hastings TG (1995) Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15:3318–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, et al. (2008) Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem 106:45–55. [DOI] [PubMed] [Google Scholar]
- 26. Furuichi T, Liu W, Shi H, Miyake M, Liu KJ (2005) Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res 79:816–824. [DOI] [PubMed] [Google Scholar]
- 27. Niizuma K, Endo H, Chan PH (2009) Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem 109 Suppl 1133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheng ZH, Cai Q (2012) Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci 13:77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicholls DG, Ward MW (2000) Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci 23:166–174. [DOI] [PubMed] [Google Scholar]
- 30. Iijima T (2006) Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res 55:234–243. [DOI] [PubMed] [Google Scholar]
- 31. Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA (2006) Calcium in cell injury and death. Annu Rev Pathol 1:405–434. [DOI] [PubMed] [Google Scholar]
- 32. Vergun O, Keelan J, Khodorov BI, Duchen MR (1999) Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol 519 Pt 2:451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manev H, Favaron M, Guidotti A, Costa E (1989) Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol 36:106–112. [PubMed] [Google Scholar]
- 34. Majewska A, Brown E, Ross J, Yuste R (2000) Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci 20:1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Secondo A, Pannaccione A, Molinaro P, Ambrosino P, Lippiello P, et al. (2009) Molecular pharmacology of the amiloride analog 3-amino-6-chloro-5-[(4-chloro-benzyl)amino]-n-[[(2,4-dimethylbenzyl)-amino]iminom ethyl]-pyrazinecarboxamide (CB-DMB) as a pan inhibitor of the Na+-Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in stably transfected cells. J Pharmacol Exp Ther 331:212–221. [DOI] [PubMed] [Google Scholar]
- 36. Annunziato L, Pignataro G, Di Renzo GF (2004) Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev 56:633–654. [DOI] [PubMed] [Google Scholar]
- 37. Li LL, Ginet V, Liu X, Vergun O, Tuittila M, et al. (2013) The nNOS-p38MAPK pathway is mediated by NOS1AP during neuronal death. J Neurosci 33:8185–8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao HW, Ross AP, Christian SL, Buchholz JN, Drew KL (2006) Decreased NR1 phosphorylation and decreased NMDAR function in hibernating Arctic ground squirrels. J Neurosci Res 84:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeon D, Yang YM, Jeong MJ, Philipson KD, Rhim H, et al. (2003) Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38:965–976. [DOI] [PubMed] [Google Scholar]
- 40. Tymianski M, Charlton MP, Carlen PL, Tator CH (1993) Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci 13:2085–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sattler R, Charlton MP, Hafner M, Tymianski M (1998) Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J Neurochem 71:2349–2364. [DOI] [PubMed] [Google Scholar]
- 42. Mielke JG (2013) Susceptibility to oxygen-glucose deprivation is reduced in acute hippocampal slices from euthermic Syrian golden hamsters relative to slices from Sprague-Dawley rats. Neurosci Lett 553:13–17. [DOI] [PubMed] [Google Scholar]
- 43. Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF (2002) Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci 976:356–366. [DOI] [PubMed] [Google Scholar]
- 44. Yu SP, Choi DW (1997) Na(+)-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. Eur J Neurosci 9:1273–1281. [DOI] [PubMed] [Google Scholar]
- 45. Czyz A, Kiedrowski L (2002) In depolarized and glucose-deprived neurons, Na+ influx reverses plasmalemmal K+-dependent and K+-independent Na+/Ca2+ exchangers and contributes to NMDA excitotoxicity. J Neurochem 83:1321–1328. [DOI] [PubMed] [Google Scholar]
- 46. Brustovetsky T, Bolshakov A, Brustovetsky N (2010) Calpain activation and Na+/Ca2+ exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res 88:1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bano D, Munarriz E, Chen HL, Ziviani E, Lippi G, et al. (2007) The plasma membrane Na+/Ca2+ exchanger is cleaved by distinct protease families in neuronal cell death. Ann N Y Acad Sci 1099:451–455. [DOI] [PubMed] [Google Scholar]
- 48. Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, et al. (2005) Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120:275–285. [DOI] [PubMed] [Google Scholar]
- 49. Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E (1998) Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res 83:1215–1223. [DOI] [PubMed] [Google Scholar]
- 50. Luo J, Wang Y, Chen X, Chen H, Kintner DB, et al. (2007) Increased tolerance to ischemic neuronal damage by knockdown of Na+-Ca2+ exchanger isoform 1. Ann N Y Acad Sci 1099:292–305. [DOI] [PubMed] [Google Scholar]
- 51. Mattson MP, Lovell MA, Furukawa K, Markesbery WR (1995) Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem 65:1740–1751. [DOI] [PubMed] [Google Scholar]
- 52. Ward MW, Rego AC, Frenguelli BG, Nicholls DG (2000) Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 20:7208–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Compared with rat neurons, ground squirrel neurons maintained lower reactive oxygen species (ROS) production (A. DHE was used as a ROS indicator. n = 146 to 153 neurons, from 3 separate experiments, P <0.05), higher ATP level (B. Rat: n = 3 separate experiments; Ground squirrel: n = 5 separate experiments, P <0.01) and more stable mitochondrial membrane potential (Ψm) (C. JC-1 was used as Ψm indicator. Rat: n = 3 separate experiments; Ground squirrel: n = 5 separate experiments, P<0.05) under glutamate treatment. GS: ground squirrel.
(TIF)
The expression of ground squirrel NCX2 mRNA was detected in transfected rat neurons. (A) Ground squirrel (GS) NCX2 specific band was detected only in reverse-transcribed preparations from transfected neurons. 0: control (no cells); B: nontransfected neurons; E: mock transfected neurons; G: GS NCX2 transfected neurons. n = 3 separate experiments. (B) Both the GS NCX2 plasmid and the mRNA transcribed from GS NCX2 could serve as templates for the PCR amplification. Their roles were determined with Q-PCR. cDNA: mRNA of GS NCX2 as template; P: plasmid GS-NCX2 as template. n = 7–9 neurons, from 3 separate experiments, P<0.01. GS: ground squirrel.
(TIF)
Expression of ground squirrel NCX2 in rat primary neurons reduced the τ value of calcium removal. Neurons were loaded with 10 µM rhod-2 AM for 10 min at 37°C. n = 24–28 neuron, from 5 separate experiments, P<0.01.
(TIF)
Ground squirrel NCX2 expression in rat primary neurons decreased ROS, and had no effect on mitochondrial membrane potential (Ψm). (A) Left: ROS level of rat primary neurons was significantly higher than that in ground squirrel neurons. Rat: n = 153 neurons, from 4 separate experiments; GS: n = 147 neurons, from 3 separate experiments, P<0.01. Right: expression of GS NCX2 in rat primary neurons lowered ROS level. n = 20 to 55 neurons, from 3 separate experiments, P<0.01. (B) Expression of GS NCX2 in rat primary neurons did not change Ψm. n = 109, 32, 120, 44 neurons respectively, from 3 separate experiments. Two-way ANOVA test. GS: ground squirrel.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.