Abstract
Background
The impact of the various elements of metabolic syndrome (MetS) on chronic kidney disease (CKD) has been conflicting. Therefore, in the present study we aimed to examine the association of MetS and its components with decreased glomerular filtration rate (GFR).
Methods
A total of 75,468 urban workers who underwent annual health examinations under the auspices of the local governments between March 2010 and September 2012 at the health examination center of Xuzhou center hospital were enrolled in the cross-sectional survey. Decreased GFR was defined as an estimated GFR <60 mL/min per 1.73 m2. The definition of MetS was based on the most-recent interim joint consensus definition, requiring any three of the five components, consisting of elevated blood pressure (BP), fasting plasma glucose (FPG), or triglycerides (TG), reduced high density lipoprotein-cholesterol (HDL-c), and obesity.
Results
MetS was related to the reduced GFR with an odds ratio [95% confidence interval (CI)] of 1.43 (1.13–1.83). In multivariable analyses, individual components of MetS that were independently associated with decreased GFR were elevated BP (OR 1.34, 95% CI 1.00–1.78), low HDL-c (OR 1.88, 95% CI 1.44–2.43), and elevated FPG (OR 1.42, 95% CI 1.09–1.85). The age-adjusted population-attributable risk percent (PARP) for reduced GFR was 27.55%, 19.67% and14.31% for elevated BP, low HDL-c and elevated FPG respectively. The multivariate-adjusted odds ratios (95% CI) of decreased GFR were 1.70(1.11–2.60), 2.38(1.53–3.71), or 4.11(2.42–6.98) for those with 1, 2, or 3 critical elements (versus zero), respectively. The corresponding multivariate-adjusted odds ratios (95% CI) of decreased GFR were 1.11(0.84–1.48) and 0.89(0.63–1.27) for those with 1 or 2 noncritical components (versus zero), respectively.
Conclusions
We concluded that various elements of MetS and the cumulative number of MetS should not be considered indiscriminately as risk factors for reduced GFR.
Introduction
Chronic kidney disease (CKD) has received increased attention as a major public health issue worldwide. Until recently, the population prevalence of CKD has exceeded 10%, more than 50% of whom were in high-risk sub-populations [1]. Moreover, global deaths from kidney disease have risen by 83% since 1990 [2]. A better understanding of the nature of CKD, its risk factors, and implementation of prevention strategies are, therefore, keys to saving many lives [3].
Subsequent articles suggested that hypertension and diabetes were deemed to be the leading causes of CKD in all developed and many developing countries [4]–[6]. Increasingly, the important contribution of metabolic syndrome (MetS) in CKD was recognized, which helped to obtain a further understanding of the aetiology of CKD. Epidemiologic observation suggested an independent association for MetS and CKD [7], [8]. However, studies on the association between various elements of MetS and CKD, to some extent, were conflicting. For example, in a cross-sectional study of 15,160 Chinese adults aged 35–74 years, Chen et al [9] reported a significant association of CKD with low high density lipoprotein-cholesterol (HDL-c), elevated fasting plasma glucose (FPG) and abdominal obesity. Nevertheless, Kitiyakara et al [10] found that only high blood pressure (BP) was associated with the prevalence of CKD among all five MetS components in a Southeast Asian cohort. Meanwhile, it was well established that the odds ratio of CKD was proportional to the cumulative number of MetS components [11]. However, the association between CKD and the number of critical or noncritical components of MetS was not well known. Thus, further supporting evidence is needed.
In the present study, our objective was to evaluate the association of MetS with decreased GFR in a large cross-sectional study among 75,468 Chinese adults. The ad hoc objectives were to examine the relationship between individual elements of MetS and decreased GFR, and to determine if there was a biologic gradient in the association between the odds of decreased GFR and the number of critical or noncritical components of MetS.
Material and Methods
Study population
The data for this retrospective study was an electronic record of urban workers who underwent annual health examinations under the auspices of the local governments between March 2010 and September 2012 at the health examination center of Xuzhou Center Hospital, the affiliated hospital of Xuzhou Medical College in Xuzhou, Jiangsu, China. The electronic record form consisted of four main parts, namely, participants registration files (register code, name, age, sex, office address, and register date), anthropometric measurements files (body height, body weight, systolic BP, and diastolic BP), laboratory files (blood and urine tests results), and other files (personal history of chronic disease, cardiovascular disease, renal transplant or dialysis, and medication history). After excluding participants with a renal transplant or on dialysis, a history of established cardiovascular disease, or those without related information (serum creatinine and all parameters related to MetS), data from 75,468 study subjects aged 18 years or older were used in our analysis. The ethics committee of Xuzhou Medical College of China approved this study, and the participants provided written informed consent.
Study measurements
Demographic, anthropometric, and laboratory data were collected from all participants. Information regarding age, gender, and medical history was obtained. Height, body weight, and blood pressures were included in the physical examinations, in which BP was measured 3 times by professional nurses using a random-zero sphygmomanometer after a 5-minute sitting, and their averages were recorded. Blood specimens were drawn after a 12 h overnight fast for examining lipids, glucose, and serum creatinine. Triglycerides (TG) levels were analyzed enzymatically using commercially available reagents. HDL-c was determined by immunoturbidimetry. FPG was measured using a modified hexokinase enzymatic method. Serum creatinine(Cr) was measured with Jaffe's kinetic method. Body mass index (BMI) was computed as weight [Kg]/(height[m])2.
Diagnosis of metabolic syndrome
The definition of MetS in our study was modeled after the Adult Treatment Panel III (ATP III) as the presence of three or more of the following risk factors [12]: 1) Obesity: BMI ≥25 kg/m2, 2) Elevated TG: Serum TG level ≥1.70 mmol/L (150 mg/dl), 3)Reduced HDL-c: HDL-c level <1.04 mmol/L (40 mg/dl) in men or <1.30 mmol/L (50 mg/dl) in women, 4) Elevated BP: BP ≥130/85 mmHg and/or use of antihypertensive medications, and 5) Elevated FPG: Serum glucose level ≥6.11 mmol/L (110 mg/dl) and/or use of insulin or hypoglycemic medication. BMI was used in our definition as the data set did not have enough measurements of waist circumference. BMI ≥25 Kg/m2 was proposed as a cutoff for the diagnosis of obesity in Asians [13].
Definition of decreased GFR
The estimated GFR (eGFR), as an indicator of kidney function, was calculated using a formula developed by adaptation of the Modification of the Diet Renal Disease (MDRD) equation on the basis of data from Chinese chronic kidney disease patients [14]: eGFR (mL/min/1.73 m2) = 175× (serum Cr)−1.234× (age)−0.179×0.79 (if female). Serum Cr: serum creatinine (mg/dl), 1 mg/dl = 88.4 umol/L. Decreased GFR was defined as an eGFR <60 mL/min/1.73 m2.
Statistical analysis
Statistical analyses were conducted by SPSS 18.0 for Windows. Baseline characteristics were compared between participants with and without MetS. Continuous variables were expressed as mean ± SD and compared using a Student's t test. Categorical variables were described as proportions and compared by the chi-square test. Curve diagrams for the prevalence of decreased GFR were drawn according to age and gender. The prevalence of decreased GFR for participants was calculated by the status (absence and presence) of each of the five MetS components, as well as the number of MetS components present.
To explore the association of individual MetS components with decreased GFR, odds ratio (OR) and 95% confidence interval (CI) were first estimated in univariate logistic regression models. Age and sex adjusted odds ratios of decreased GFR were calculated in multivariable logistic regression to circumvent the potential confounders (age and sex). We then constructed multivariable logistic regression models by including all five MetS elements along with age and gender to assess the independent effect of each element. Age-adjusted population-attributable risk percent (PARP) of critical components of MetS was calculated using the following formula [15].
Pj and 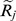 represents the proportion of all cases and relative risk in stratum j respectively.
represents the proportion of all cases and relative risk in stratum j respectively.
The association between the cumulative number of MetS components and decreased GFR was first examined with univariate logistic regression models and then examined with multivariable logistic regression models adjusting for age and gender. Later, all five MetS components were divided into critical components arm and noncritical components arm. OR and 95% CI of reduced GFR were calculated across MetS elements' categories after adjusting for age and gender. All tests were two sided with P<0.05 considered statistically significant.
Results
Baseline characteristics
Eligible data from 75,468 subjects were used. Mean age was 48.79±13.76 yr with 41.1% youth (18–44 yr), 36.8% middle-aged (45–59 yr), and 22.1% elderly (60- yr). There was 56.3% (n = 42,488) male participants. The percentages of elevated BP, elevated TG, reduced HDL-c, elevated FPG, and obesity were 51.3%, 30.2%, 29.9%, 14.8%, and 44.2%, respectively. Of the subjects, 21,497 (28.5%) subjects met criteria for the MetS, and 273 (0.4%) had decreased GFR.
General characteristics of study participants by the presence of MetS
Table 1 presents the general characteristics of all participants by MetS status. Participants with MetS had a higher mean age, as well as a higher proportion of males (P<0.001). The mean levels of SBP, DBP, TG, HDL-c, FPG, and BMI were significantly different between the two groups (P<0.001). Furthermore, eGFR was slightly lower for those with MetS (P<0.001).
Table 1. Baseline characteristics of study participants by presence of the metabolic syndrome.
| Characteristics | Metabolic Syndrome Present (n = 21497) | Metabolic Syndrome Absent (n = 53971) | P- value |
| Age (years) | 52.32±13.17 | 47.39±13.73 | <0.001 |
| Men | 14264 (66.35) | 28249 (52.34) | <0.001 |
| Body height (cm) | 166.54±8.97 | 165.61±8.39 | <0.001 |
| Body weight (kg) | 76.17±12.04 | 65.00±11.16 | <0.001 |
| Body mass index (kg/m2) | 27.36±2.73 | 23.61±2.94 | <0.001 |
| Systolic blood pressure(mm Hg) | 138.73±15.69 | 123.07±16.30 | <0.001 |
| Diastolic blood pressure(mm Hg) | 87.22±10.27 | 78.06±10.36 | <0.001 |
| Triglyceride (mmol/L) | 2.60±2.06 | 1.18±0.81 | <0.001 |
| Fasting plasma glucose (mmol/L) | 6.24±1.84 | 5.31±0.91 | <0.001 |
| HDL-cholesterol (mmol/L) | 1.11±0.25 | 1.42±0.32 | <0.001 |
| eGFR (ml/min/1.73 m2) | 134.23±34.69 | 137.97±31.95 | <0.001 |
Data are n (%) or mean ± SD; HDL, high-density lipoprotein. eGFR, estimated glomerular filtration rate.
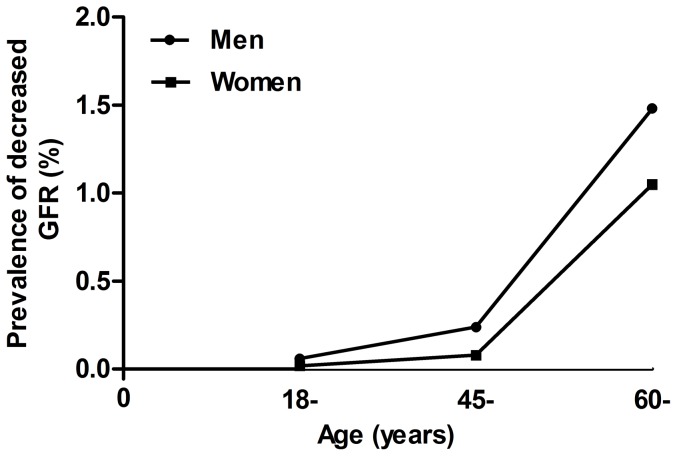
Prevalence of decreased GFR stratified by age and gender
Figure 1 depicts the prevalence of decreased GFR stratified by age and gender. The prevalence of decreased GFR increased with age in both men and women. Men had a higher prevalence of reduced GFR than women (0.42% vs 0.29%, P = 0.005).
Figure 1. of decreased GFR by age and gender.
GFR, glomerular filtration rate. Decreased GFR was defined as an estimated glomerular filtration rate <60 ml/min per 1.73 m2 according to a formula developed by adaptation of the Modification of the Diet Renal Disease (MDRD) equation on the basis of data from Chinese chronic kidney disease patients.
Association between individual components of MetS and decreased GFR
In univariate analyses, each component of MetS was associated with decreased GFR. After adjusted by age and gender, most results appeared consistent except obesity (Table 2). In multivariable analyses for individual MetS components (Table 3), there was a significant association between elevated BP, reduced HDL-c, elevated FPG and decreased GFR, with estimated ORs of 1.34(1.00–1.78), 1.88(1.44–2.43) and 1.42(1.09–1.85), respectively; the other 2 components seemed to be noncritical factors. The age-adjusted population-attributable risk percent (PARP) of elevated BP, reduced HDL-c, and elevated FPG for reduced GFR was 27.55%, 19.67%, and 14.31%, respectively.
Table 2. Prevalence and odds ratio of decreased GFR among participants with and without components of the metabolic syndrome.
| Variables | N | Prevalence (%) | OR (95% CI) | |
| Unadjusted | Age and Sex Adjusted | |||
| Metabolic syndrome | ||||
| No | 53971 | 0.28 | 1.00 | 1.00 |
| Yes | 21497 | 0.57 | 2.03 (1.60–2.58) | 1.43 (1.13–1.83) |
| Elevated blood pressure | ||||
| No | 36739 | 0.19 | 1.00 | 1.00 |
| Yes | 38729 | 0.53 | 2.87 (2.18–3.78) | 1.37 (1.03–1.82) |
| Elevated triglyceride | ||||
| No | 52683 | 0.32 | 1.00 | 1.00 |
| Yes | 22785 | 0.46 | 1.45 (1.13–1.85) | 1.43 (1.12–1.82) |
| Reduced HDL-cholesterol | ||||
| No | 52936 | 0.28 | 1.00 | 1.00 |
| Yes | 22532 | 0.55 | 1.93 (1.52–2.45) | 1.93 (1.51–2.47) |
| Elevated fasting glucose | ||||
| No | 64263 | 0.29 | 1.00 | 1.00 |
| Yes | 11205 | 0.78 | 2.70 (2.09–3.48) | 1.51 (1.16–1.95) |
| Obesity | ||||
| No | 42095 | 0.31 | 1.00 | 1.00 |
| Yes | 33373 | 0.42 | 1.34 (1.05–1.69) | 0.97 (0.76–1.23) |
| No. of Components | ||||
| 0 | 17348 | 0.10 | 1.00 | 1.00 |
| 1 | 19008 | 0.25 | 2.38 (1.38–4.10) | 1.34 (0.77–2.31) |
| 2 | 17615 | 0.49 | 4.71 (2.83–4.10) | 2.10 (1.25–3.51) |
| 3 | 13100 | 0.48 | 4.63 (2.74–7.83) | 2.03 (1.20–3.45) |
| 4 | 6899 | 0.61 | 5.86 (3.37–10.19) | 2.40 (1.37–4.20) |
| 5 | 1498 | 1.13 | 11.00 (5.66–21.39) | 3.91 (2.00–7.67) |
GFR, glomerular filtration rate; CI, confidence interval; OR, odds ratio; HDL, high-density lipoprotein.
Table 3. Univariate and multivariable association between individual components of metabolic syndrome and decreased GFR.
| Variables | Univariate Models* | Multivariable Models+ | ||
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Elevated blood pressure | 1.37 (1.03–1.82) | 0.028 | 1.34 (1.00–1.78) | 0.047 |
| Elevated triglyceride | 1.43 (1.12–1.82) | 0.005 | 1.14 (0.88–1.49) | 0.319 |
| Reduced HDL-cholesterol | 1.93 (1.51–2.47) | <0. 001 | 1.88 (1.44–2.43) | <0.001 |
| Elevated fasting glucose | 1.51 (1.16–1.95) | 0.002 | 1.42 (1.09–1.85) | 0.009 |
| Obesity | 0.97 (0.76–1.23) | 0.795 | 0.78 (0.61–1.00) | 0.054 |
GFR, glomerular filtration rate; CI, confidence interval; OR, odds ratio.
*Univariate models were adjusted for age and gender.
Multivatiate models were adjusted for age, gender and all the five metabolic syndrome components.
Association between the number of MetS components and decreased GFR
There seemed to be a biologic gradient between the number of MetS traits and the prevalence of decreased GFR, irrespective of age and gender (Table 2). However, in analyses stratified by the type of components of MetS (Table 4), the multivariate-adjusted ORs (95% CI) of decreased GFR were 1.70(1.11–2.60), 2.38(1.53–3.71), or 4.11(2.42–6.98) for those with 1, 2, or 3 essential components (versus zero), including high BP, low HDL-c, high FPG, respectively. Conversely, the corresponding multivariate-adjusted ORs (95% CI) of decreased GFR were 1.11(0.84–1.48) and 0.89(0.63–1.27) for those with 1 or 2 non-essential components (versus zero), including high TG and obesity. After randomly sampling 50% of cases from the established database, the models were tautologically reconstructed 20 times, and consistent results indicated that this result was robust and stable.
Table 4. Multivariable association between the number of critical or noncritical metabolic syndrome components and decreased GFR.
| Variables | Multivariable* | |
| OR (95%CI) | P-value | |
| Critical group | ||
| 0 component (reference) | 1.00 | |
| 1 component | 1.70 (1.11–2.60) | 0.014 |
| 2 components | 2.38 (1.53–3.71) | <0.001 |
| 3 components | 4.11 (2.42–6.98) | <0.001 |
| Noncritical group | ||
| 0 component (reference) | 1.00 | |
| 1 component | 1.11 (0.84–1.48) | 0.457 |
| 2 components | 0.89 (0.63–1.27) | 0.527 |
GFR, glomerular filtration rate; CI, confidence interval; OR, odds ratio.
Critical group included elevated blood pressure, reduced HDL cholesterol, or elevated plasma glucose. Noncritical group included elevated triglycerides, or obesity.
*Multivariable logistic regression models included critical group and noncritical group shown above and also adjusted for age and gender.
Discussion
The prevalence of decreased GFR was 0.4% in the present study, lower than the prevalence raised by Zhang et al [16] in a cross-sectional survey of China. Age was recognized as an independent risk factor for nephritic disease [1], [17]. Tanaka et al [18] indicated that the prevalence of reduced GFR increased markedly in Japanese participants aged 60 years or older. Our results consistently supported evidence for the notion that age played a significant role in renal dysfunction.
Substantial epidemiological studies, such as cohort studies [19]–[21], cross-sectional studies [22], and meta-analysis [23], on the association of MetS with CKD have yielded broadly consistent results that MetS was an independent risk factor for CKD, regardless of age, gender, and other potential confounders. Moreover, on histopathology examination, Singh et al [7] found that there was a steeper decline in renal function over time in patients with MetS. As expected, a strong association of MetS with decreased GFR was established in the present study, with an estimated OR of 1.43(1.13–1.83) after adjustment for age and gender. A similar study [9] performed on 19,012 Chinese populations aged 35 to 74 years revealed that the OR of the elevated serum creatinine with MetS was 1.36 (95% CI 1.07–1.73). Collectively, the apparent concerted result further indicated that MetS was a significant effective modifier in decreased GFR. To find measurements for reduced GFR control in public health practice, our in-depth analysis of the relationship between reduced GFR and various elements of MetS showed that even mildly elevated BP (≥130/85 mm Hg), FPG levels (≥6.1 mmol/L), or reduced HDL-c levels (<1.04 mmol/L) contributed to decreased GFR. Furthermore, the PARP of elevated BP, reduced HDL-c, and elevated FPG for decreased GFR was 27.55%, 19.67% and 14.31%, respectively. These findings illustrated that regulating BP to normal range (<130/85 mm Hg) could circumvent 27.55% clustering of risk factors. In the same vein, regulating HDL-c and FPG to normal range (≥1.04 mmol/L and <6.1 mmol/L, separately) could prevent 19.67% and 14.31% clustering of risk factors.
Studies have yielded mixed results for the association between various fractions of MetS and CKD. Chen et al [24], for example, reported a positive association between elevated BP, reduced HDL, elevated TG and CKD in a cross-sectional study of 6,217 U.S. adults. A Southeast Asian cohort study [10] included 3,195 participants and revealed that only high BP related to the prevalence of CKD. Recently, obesity, as an emerging threat, has also played an important role in the CKD [25]–[27]. The continuing debate over the deeply controversial issue of the association between individual components of MetS and CKD or reduced GFR revealed our lacking recognition of dynamic response of elements of MetS along with the developing process of renal dysfunction. Thus, ingredients of MetS and the cumulative number of Mets should not be considered as risk factors for decreased GFR indiscriminately.
Numerous studies reported a biologic gradient between the number of MetS ingredients and CKD [18]–[22]. At first glance, the current study was concordant with previous studies. However, an intriguing finding was presented after dividing all five components of MetS into critical arm (including elevated BP, reduced HDL-c, or elevated FPG) and noncritical arm (including elevated TG or obesity). The risk of reduced GFR was proportional to the cumulative number of critical components of MetS. Conversely, null association was found between decreased GFR and the cumulative number of noncritical components of MetS. To further validate this result, 50% of cases were randomly sampled from the established database, and the models were repeatedly reconstructed 20 times. Consistent results supported a paradigm-shaking hypothesis that decreased GFR was associated with the cumulative number of critical elements of MetS but not the non-critical elements. The impact of the number of MetS component on prevalence of CKD should therefore be interpreted judiciously. Future studies, of course, are needed to validate this possibility.
There are several strengths to our study. First, a large sample of healthy participants and early stage of patients were called to overcome information bias and prevalence-incidence bias. Second, we provided new insights to calculate the PARP of critical components of MetS. This may greatly help to find potential implications for controlling reduced GFR in public health practice. Third, to circumvent confounders, various MetS components were divided into critical arm and noncritical arm, the study of which, to the extent of our knowledge, was rare in this area.
This study also has some limitations. First, our population consisted mostly of urban workers who underwent an annual health checkup with a low prevalence of CKD. This study was therefore not nationally representative or geographically diverse. A completely random large sample to satisfy this study would be prohibitively expensive and impractical. Our cross-sectional analysis of a non-probability sample of the Chinese population decreases the accuracy of the prevalence, but not the mechanism of the study. Second, a cross-sectional design in the present study prevented us from drawing any definite conclusion unless a prospective follow-up study is performed. Third, the models in the current study were only adjusted for age and gender. Other associated factors such as marital status, income should also regard as potential confounders. However, relevant information was not obtained in this retrospective survey. Fourth, BMI was used instead of waist circumference in the definition of MetS due to a lack of corresponding data. This lack of data could have led to miss-estimation of the prevalence of MetS. However, we believe part of these potential limitations should be consistently attenuated by the large sample size of the study.
In conclusion, critical components of MetS including elevated BP, reduced HDL-c, and elevated FPG were significantly associated with decreased GFR with a PARP of 27.55%, 19.67%, and 14.31%, respectively. In addition, the prevalence of decreased GFR is solely linked to the cumulative number of critical elements with a biologic gradient present, but not the non-critical elements. Thus, the impact of MetS, its elements, and its number of elements on the prevalence of CKD should therefore be interpreted judiciously. Our study, to some extent, may facilitate the decision-making process of the public health, enabling early and appropriate critical elements recognition and elimination.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by grants from Postgraduate Research and Innovation Project of Colleges and Universities of Jiangsu Province (No. CXLX13_998), by Xuzhou science and Technology Project (NO. XZZD1239), and by Fund of Philosophy and Social Science of Jiangsu Province (No. 13SQB-058). The specific role of the funding organization or sponsor is as follows: design and conduct of the study, collection of the data, management of the data.
References
- 1. Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. 2013;382:273–283. [DOI] [PubMed] [Google Scholar]
- 4. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 5. Hanratty R, Chonchol M, Havranek EP, Powers JD, Dickinson LM, et al. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol. 2011;6:2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pyram R, Kansara A, Banerji MA, Loney-Hutchinson L. Chronic kidney disease and diabetes. Maturitas. 2012;71:94–103. [DOI] [PubMed] [Google Scholar]
- 7. Singh AK, Kari JA. Metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:198–203. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Chen Y, Liu X, Liang Y, Shao X, et al. Metabolic syndrome and chronic kidney disease in a Southern Chinese population. Nephrology (Carlton). 2014;19:325–331. [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Gu D, Chen CS, Wu X, Hamm LL, et al. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol Dial Transplant. 2007;22:1100–1106. [DOI] [PubMed] [Google Scholar]
- 10. Kitiyakara C, Yamwong S, Cheepudomwit S, Domrongkitchaiporn S, Unkurapinun N, et al. The metabolic syndrome and chronic kidney disease in a Southeast Asian cohort. Kidney Int. 2007;71:693–700. [DOI] [PubMed] [Google Scholar]
- 11. Cho JA, Lee SJ, Reid EA, Jee SH. Metabolic syndrome component combinations and chronic kidney disease: the severance cohort study. Maturitas. 2013;75:74–80. [DOI] [PubMed] [Google Scholar]
- 12. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel (2001) on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 13.WHO Western Pacific Region, IASO IOTF. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney, Australia, Health Communications Australia Pty Limit. 2000; Available: http://www.wpro.who.int/nutrition/documents/Redefining_obesity/en/. Accessed 2014 May 4.
- 14. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 15. Bruzzi P, Green SB, Byar DP, Briton LA, Schairer C. Estimating the populationattributable risk for multiple factors using case control data. Am J Epidemiol. 1985;122:904–914. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Wang F, Wang L, Wang W, Liu B, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. [DOI] [PubMed] [Google Scholar]
- 17. Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, et al. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71:159–166. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006;69:369–374. [DOI] [PubMed] [Google Scholar]
- 19. Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. [DOI] [PubMed] [Google Scholar]
- 20. Ryu S, Chang Y, Woo HY, Lee KB, Kim SG, et al. Time-dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am J Kidney Dis. 2009;53:59–69. [DOI] [PubMed] [Google Scholar]
- 21. Tozawa M, Iseki C, Tokashiki K, Chinen S, Kohagura K, et al. Metabolic syndrome and risk of developing chronic kidney disease in Japanese adults. Hypertens Res. 2007;30:937–943. [DOI] [PubMed] [Google Scholar]
- 22. Kang YU, Kim HY, Choi JS, Kim CS, Bae EH, et al. Metabolic syndrome and chronic kidney disease in an adult korean population: results from the korean national health screening. PLoS One. 2014;9:e93795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. [DOI] [PubMed] [Google Scholar]
- 25. Hollenberg NK. Obesity and the kidney: why is the kidney at risk? Kidney Int. 2007;71:187–188. [DOI] [PubMed] [Google Scholar]
- 26. Bandgar TR, Kalra S, Sahay M. Metabolic syndrome leading to chronic kidney disease: An emerging threat. Indian J Endocrinol Metab. 2012;16:151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoon YS, Park HS, Yun KE, Kim SB. Obesity and metabolic syndrome-related chronic kidney disease in nondiabetic, nonhypertensive adults. Metabolism. 2009;58:1737–1742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.