INTRODUCTION
The definition of atherosclerosis as a chronic inflammatory disease [1] led to a critical shift in atherosclerosis research and identifying stimuli inducing vascular inflammation took center stage. Activated macrophages and T lymphocytes abound in atheromatous lesions at all stages of the disease [2, 3]. Their contribution to inflammation and tissue damage by releasing inflammatory mediators upon activation is accepted as an essential step in the evolution of atherosclerosis.
Hemodynamic stress, oxidation and other modifications of LDL, bacterial toxins, stress proteins, and infectious agents have been investigated as possible triggers of arterial wall inflammation, either through innate immunity pathways, activation of adaptive immunity, or combinations of the two [4–6]. Modified forms of LDL (mLDL) are immunogenic and elicit an autoimmune response in humans. The resulting autoantibodies have been shown to react with oxLDL, MDA-LDL and AGE-LDL [7, 8]. The body of evidence supporting the pathogenic significance of the antibody response to modified LDL has grown significantly, including in vitro data demonstrating the ability of oxLDL-immune complexes (IC) prepared with human reagents to activate human macrophages [9–11]. The detection in atherosclerotic lesions of oxLDL and IgG antibodies reacting with oxLDL by Yla-Herttüala is the best evidence pointing to the extravascular formation of IC containing modified LDL [12, 13]. Finally, recent clinical studies demonstrate that the levels of oxLDL and AGE-LDL in isolated IC strongly predict progression of coronary artery disease and coronary calcification in a large cohort of type 1 diabetes [7, 14, 15].
Our previous investigations demonstrated that high levels of oxLDL antibodies of the pro-inflammatory IgG1 and IgG3 isotypes, as well as high levels of oxLDL-IC, can be measured in patients with type 1 diabetes, as well as in non-diabetic patients and healthy controls [7, 16]. It is possible that patients with type 1 diabetes not only generate higher levels of mLDL through glyco-oxidative processes, but given the complex constellation of genetic factors associated with their autoimmune disease they may have an enhanced autoimmune response to modified lipoproteins. It is therefore quite important to investigate whether the same high predictive value of the levels of mLDL in circulating IC for CVD events is also present in type 2 diabetes and in the general population. In this article we report that the levels of MDA-LDL in circulating IC predict future myocardial infarction (MI) in patients with type 2 diabetes.
MATERIALS AND METHODS
The VADT design and population
The study design of the VADT study has been previously reported [17]. Briefly, 1791 veterans with type 2 diabetes and suboptimal glucose control were randomized in 20 participating sites to receive either intensive or standard glucose control. The goal for HbA1c levels was an absolute reduction of 1.5% in the intensive-therapy group, as compared with the standard-therapy group. A unique feature of the study was that other modifiable cardiovascular risk factors were treated aggressively and uniformly in both arms of the study. All patients were treated to guidelines according to the American Diabetes Association for blood pressure, hypertension, diet, exercise and diabetes education [18]. All patients were prescribed aspirin and all patients with elevated lipid levels were prescribed statins, unless contraindicated. The study was approved by the IRB at each of the participating sites. All patients provided written informed consent.
Of the 1791 VADT study participants, 995 patients from 17 of the participating sites, approximately half from the standard arm and half from the intensive treatment arm, agreed to participate in a sub-study focused on determining the association between specific biomarkers and macrovascular disease. The biochemical, physical, and demographic profiles of the 995 patients in the substudy do not differ significantly from the 796 not included in the substudy with the exception of slightly lower age and LDL-cholesterol and slightly higher triglyceride levels as well as a higher prevalence of aspirin use at baseline in substudy participants when compared to non substudy participants (see online supplementary Table 1). The study population for the current report consists of 907 of the 995 participants enrolled in the substudy on whom serum was available to measure mLDL in circulating IC. In 88 patients not enough serum was collected to perform the measurements.
Enrollment for the VADT study occurred from December 2000 to May 2003. Measurement of MDA-LDL, oxLDL and AGE-LDL was performed on IC isolated from serum samples collected during a routine follow-up between August 2002 and March 2006, a median of 2 years (range: 0 to 5 years) after participants' baseline examination. Serum samples were obtained after an overnight fast and stored at −80°C until assayed. Patients were followed until lost to follow-up, death or May 2008. The average follow-up time following measurement of modified LDL in circulating IC was 3.7 years (95% CI: 3.6, 3.8). All endpoints for the current analysis occurred after samples to perform the measurement of modified forms of LDL in IC were collected.
The baseline VADT cohort examination was standardized and included interviews, blood pressure measurements, anthropometric measurements and fasting venipuncture [17].
Measurement of MDA-LDL, oxLDL and AGE-LDL in circulating IC
We measured oxLDL, MDA-LDL and AGE-LDL in IC precipitated from serum and fractionated by protein G affinity chromatography to separate the IgG antibody from the modified LDL, as previously described [7, 14, 19]. The reactivity of mLDL separated from the IC with antibodies specific for oxLDL, MDA-LDL and AGE-LDL was then assayed by capture assays developed in our laboratory [20]. Coefficients of variation for 50 samples measured in two separate assays were 5.2% for oxLDL, 0.5% for MDA-LDL, and 8.3% for AGE-LDL. The levels of the different LDL modifications in LDL-IC were expressed as a function of the amount of apolipoprotein-B contained in the IC and the final values given as concentration in mg per L of serum.
Endpoints
The primary endpoint for the VADT study was the time to the first occurrence of any one of a composite of cardiovascular events. Each VADT event was adjudicated by an end-point committee that employed strict algorithms to define and document each event. The composite endpoint included documented MI; stroke; death from cardiovascular disease (CVD); new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease (CAD); and amputation for ischemic gangrene. Secondary outcomes included MI, CAD, death from CVD, and death from any cause. CAD included MI, coronary revascularization procedures and clinically identified inoperable CAD. Cardiovascular death included sudden death as defined by the Framingham study, CAD, cerebrovascular accident, and other cardiovascular events (i.e., cardiomyopathy).
Statistical analyses
Prospective analyses were carried out in which the levels of different mLDLs in circulating IC functioned as biomarkers and cardiovascular endpoints including MI and the “composite endpoint” were the outcomes of interest. Modified LDL values were log transformed due to their skewed, non-normal distribution. Spearman correlation coefficients were determined for the association between individual mLDL-IC levels and baseline VADT variables of interest.
Baseline clinical and demographic characteristics of the cohort are shown in table 1. Differences across quartiles of MDA-LDL-IC were tested using chi-square for categorical characteristics while means, adjusted for age, ethnic minority status and study treatment arm were determined for continuous variables using linear regression.
Table 1.
Baseline clinical and demographic characteristics of the VADT cohort
| MDAIC Quartiles (cut-points) | |||||
|---|---|---|---|---|---|
| 1st (4.7–52) | 2nd (52–83) | 3rd (83–128) | 4th (128–607) | ||
| n = 226 | n = 227 | n = 227 | n = 227 | P‡ | |
| Age* (years) | 60.4 (59.3, 61.4) | 60.8 (59.7, 61.9) | 60.2 (59.1, 61.4) | 57.8 (56.7, 58.9) | 0.0011 |
| Male* (%) | 96.46 | 96.48 | 98.68 | 95.59 | 0.2822 |
| Non-Hispanic White* (%) | 65.04 | 61.67 | 56.83 | 52.86 | 0.0447 |
| Intensive Treatment* (%) | 46.02 | 52.86 | 49.34 | 50.66 | 0.5261 |
| Prior Event* (%) | 33.19 | 37.44 | 44.05 | 38.33 | 0.1243 |
| Current Smoker* (%) | 15.93 | 20.26 | 11.56 | 19.38 | 0.0554 |
| Hypertension* (%) | 82.74 | 89.43 | 88.11 | 85.90 | 0.1732 |
| Albuminuria | |||||
| Micro * (%) | 29.33 | 31.28 | 24.78 | 32.74 | 0.4275 |
| Macro * (%) | 6.67 | 8.81 | 8.41 | 9.73 | |
| Exercise* (%) | 46.46 | 48.23 | 45.78 | 39.21 | 0.2329 |
| Statin* (%) | 56.64 | 59.91 | 58.15 | 62.11 | 0.6675 |
| ACE* (%) | 61.50 | 70.93 | 66.52 | 65.64 | 0.2096 |
| Aspirin* (%) | 80.09 | 81.70 | 79.45 | 80.18 | 0.9438 |
| Diabetes Duration (years) | 11.8 (10.8, 12.7) | 11.2 (10.3, 12.2) | 11.3 (10.3, 12.2) | 11.2 (10.3, 12.2) | 0.4414 |
| Hemoglobin A1c(%) | 9.4 (9.2, 9.6) | 9.4 (9.2, 9. 6) | 9.5 (9.3, 9.6) | 9.4 (9.3, 9.6) | 0.4457 |
| Body Mass Index (kg/m2) | 31.5 (30.9, 32.0) | 31.2 (30.7, 31.8) | 31.3 (30.7, 31.8) | 31.5 (30.9, 32.1) | 0.9204 |
| SB Pressure (mmHg) | 130 (128, 133) | 131 (129, 133) | 132 (130, 134) | 133 (131, 136) | 0.0581 |
| DB Pressure (mmHg) | 76 (75, 78) | 77 (76, 78) | 75 (74, 77) | 76 (75, 78) | 0.7714 |
| HDL–Cholesterol (mg/dl) | 36 (35, 37) | 36 (35, 37) | 35 (34, 36) | 36 (35, 38) | 0.9863 |
| LDL–Cholesterol (mg/dl) | 104 (100, 108) | 106 (101, 110) | 103 (99, 107) | 112 (108, 116) | 0.0337 |
| Triglycerides† (mg/dl) | 166 (153, 178) | 167 (155, 181) | 168 (156, 181) | 177 (164, 191) | 0.2387 |
Continuous and ordinal characteristics are shown as means or proportions with associated 95% confidence intervals while categorical characteristics are shown as percentages. The characteristics of the study population (n=907) were stratified by MDA IC category and adjusted for age, minority status and treatment arm of the study.
Unadiusted;
Due to non-normal distributions geometric means are presented;
Chi-square for categorical and P for trend for continuous variables.
For time to event outcomes, Cox proportional hazard models were used to calculate hazard ratios for endpoints of interest in relation to quartiles of MDA-LDL, oxLDL and AGE-LDL in IC. Because mLDL-IC levels were not measured at the baseline VADT examination, but a median of 2 years later, left-truncation was used to account for differences in time at risk; hence, a participant was considered at risk for a given event between measurement of mLDL-IC levels and the end of VADT follow-up. For regression analysis, each mLDL-IC was categorized into quartiles. The association between mLDL-IC quartiles and each cardiovascular event of interest was assessed separately for each mLDL-IC after controlling for age, ethnic minority, treatment arm, prior CVD event at the time of randomization as well as systolic blood pressure (SBP), LDL-cholesterol and statin use at time of IC measurement. These covariates were chosen a priori since they represent either study design variables or established cardiovascular risk factors. Additional covariates considered but excluded from the models because of two-sided p-values > 0.20 for MI and the composite endpoint included HDL–cholesterol level, triglycerides, smoking status and use of ACE inhibitors. For each mLDL-IC studied appropriate interaction terms were used to determine whether treatment arm, HbA1c level, ethnic minority status, or prior CVD event modified the relationship between each mLDL-IC and outcomes of interest. Potential effect modifiers were chosen a priori since they represent either study design variables or established cardiovascular risk factors. For the model testing whether HbA1c measured at the time of IC measurement was an effect modifier, HbA1c was included in the model as a main effect but the “treatment arm” was excluded from the model, due to its concordance with HbA1c. Using median splits to define high and low levels of each mLDL-IC, we examined the joint effect of mLDL-IC combinations and their ability to predict outcomes of interest. Specifically, we examined the joint effects of high/low levels of oxLDL-IC and MDA-LDL-IC as well as the joint effect of high/low levels of AGE-LDL-IC and MDA-LDL-IC. The assumption of proportional hazards was evaluated by testing for interaction between the three mLDL-IC index variables (i.e., defining the four IC quartiles) and continuous time variables. Reported p-values are two-sided with a type-I error rate significance level of α = 0.05. Hazard ratios and their 95% confidence intervals are displayed with the format HR, (95% confidence interval, CI). All analyses were performed using SAS v. 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
At VADT baseline, mean age of the study population was 59.8 ± 8.4 years, mean duration of diabetes was 11.4 ± 7.5 years, of the 907 participants studied 96.8% were males, 59.1% were non-Hispanic white and 49.7% were assigned to the VADT intensive treatment group. The percentage of non-Hispanic white participants and age decreased with increasing quartiles of MDA-LDL IC (p=0.0447 and p=0.0011, respectively) (Table 1). LDL-cholesterol levels increased across increasing quartiles of MDA-LDL-IC (p=0.0337). Duration of diabetes, HbA1c levels, BMI, blood pressure, HDL-cholesterol, triglycerides and ACR remained similar across quartiles of MDA-LDL-IC after adjusting for age, ethnic minority status and treatment arm of the study. Also there was no association of MDA-LDL-IC with current smoking status, adherence to diet, or treatment with statins or ACE inhibitors.
MDA-LDL-IC and oxLDL-IC but not AGE-LDL-IC levels had a significant negative correlation with age (rho=−0.11, rho=−0.12 and rho=−0.05). The levels of MDA-LDL, AGE-LDL and oxLDL in IC had a moderate and significant positive association with LDL-cholesterol levels (rho=0.09 to 0.16, p<0.007 to p<0.001), but were not correlated with HbA1c. Additionally, oxLDL-IC had a significant negative correlation with HDL cholesterol (rho=−0.08). The levels of the different mLDLs in IC were all highly inter-correlated (r=0.56 to 0.74, all p<0.001). During the 3.7 year follow-up, 16.8% of participants had one of the primary composite endpoints, 4.7% had a MI, 10.6% had a MI, coronary procedure or were diagnosed with inoperable CAD, 6.8% had non-fatal MI or died of cardiovascular death and 6.4% died of either cardiovascular or non-cardiovascular causes (online supplementary Table 2).
Table 2 shows that individuals in the highest quartile of MDA-LDL-IC as compared to individuals in the lowest quartile were at higher risk of MI [HR=2.44 (1.03, 5.77)] and the composite endpoint [HR=1.71 (1.04, 2.80)], but at similar risk of all-cause mortality [HR=1.15 (0.52, 2.52)]. Individuals in the second and third quartiles of MDA-LDL-IC did not have statistically significant elevation in risk of MI or composite endpoint relative to individuals in the lowest quartile of MDA-LDL-IC. Also as shown in table 2 individuals in the highest quartile of oxLDL-IC and AGE-LDL-IC levels versus those in the lowest quartiles were at similar risk for MI, the composite endpoint and death from any cause. There was no evidence that study treatment arm, ethnic minority status, prior CVD event, or HbA1c level modified the association between MDA-LDL, oxLDL or AGE-LDL-IC quartile and any of the cardiovascular endpoints examined.
Table 2.
Adjusted hazard ratios (and 95% confidence intervals) from Cox proportional hazard regression models for quartile of MDA, AGE and oxLDL IC in relation to various outcomes.
| MDA IC | oxLDL IC | AGE IC | |
|---|---|---|---|
| MI | |||
| IC Lowest Quartile | 1.00 | 1.00 | 1.00 |
| IC Quartile 2 | 0.86 (0.31, 2.39) | 1.47 (0.65, 3.32) | 1.01 (0.42, 2.43) |
| IC Quartile 3 | 1.46 (0.581,3.67) | 0.99 (0.40, 2.47) | 1.17 (0.49, 2.79) |
| IC Quartile 4 | 2.44 (1.03, 5.77) | 1.08 (0.44, 2.62) | 1.31 (0.56, 3.05) |
| MI, procedure or inoperable disease | |||
| IC Lowest Quartile | 1.00 | 1.00 | 1.00 |
| IC Quartile 2 | 1.01 (0.55, 1.88) | 1.35 (0.78, 2.36) | 1.42 (0.78, 2.59) |
| IC Quartile 3 | 1.20 (0.66, 2.19) | 1.42 (0.80, 2.51) | 1.61 (0.90, 2.89) |
| IC Quartile 4 | 1.61 (0.91, 2.87) | 0.87 (0.46, 1.64) | 1.35 (0.72, 2.51) |
| MI or CV Death | |||
| IC Lowest Quartile | 1.00 | 1.00 | 1.00 |
| IC Quartile 2 | 0.78 (0.35, 1.76) | 1.25 (0.64, 2.43) | 1.10 (0.53, 2.28) |
| IC Quartile 3 | 1.22 (0.58, 2.56) | 0.94 (0.45, 1.98) | 1.12 (0.53, 2.33) |
| IC Quartile 4 | 1.81 (0.89, 3.68) | 0.93 (0.44, 1.95) | 1.40 (0.68, 2.86) |
| Composite Endpoint | |||
| IC Lowest Quartile | 1.00 | 1.00 | 1.00 |
| IC Quartile 2 | 1.47 (0.90, 2.38) | 1.40 (0.91, 2.16) | 1.41 (0.90, 2.21) |
| IC Quartile 3 | 1.37 (0.83, 2.25) | 1.27 (0.80, 2.02) | 1.17 (0.73, 1.86) |
| IC Quartile 4 | 1.71 (1.04, 2.80) | 0.91 (0.56, 1.49) | 1.24 (0.78, 1.99) |
| All Death | |||
| IC Lowest Quartile | 1.00 | 1.00 | 1.00 |
| IC Quartile 2 | 0.98 (0.46, 2.09) | 1.54 (0.76, 3.12) | 1.79 (0.84, 3.81) |
| IC Quartile 3 | 1.20 (0.58, 2.50) | 1.33 (0.63, 2.83) | 1.53 (0.70, 3.33) |
| IC Quartile 4 | 1.15 (0.52, 2.52) | 1.07 (0.48, 2.39) | 1.29 (0.57, 2.95) |
Adjusted for age, minority, treatment arm, whether an individual had an event prior to randomization into VADT, systolic blood pressure, LDL and statin use at time of immune complex measurement.
Subsequently, we examined the interaction between joint effects of oxLDL-IC and MDALDL-IC, as well as the joint effects between AGE-LDL-IC and MDA-LDL-IC in relationship to outcomes of interest (online supplementary Table 3). Individuals with high MDA-LDL-IC, but low oxLDL-IC were at higher risk of MI [HR=3.46 (1.52, 7.87)] and the composite endpoint [HR=1.66 (1.08, 2.56)] but similar risk of all-cause mortality [HR=1.06 (0.50, 2.22)] as compared to individuals with low MDA-LDL-IC and low oxLDL-IC. Moreover, individuals with high MDA-LDL-IC, but low AGE-LDL IC were at higher risk of MI [HR=2.56 (1.06, 6.20)], the composite endpoint [HR=1.60 (1.01, 2.53)] and all-cause mortality [HR=2.16 (1.04, 4.51)] as compared to individuals with low MDA-LDL-IC and low AGE-LDL-IC.
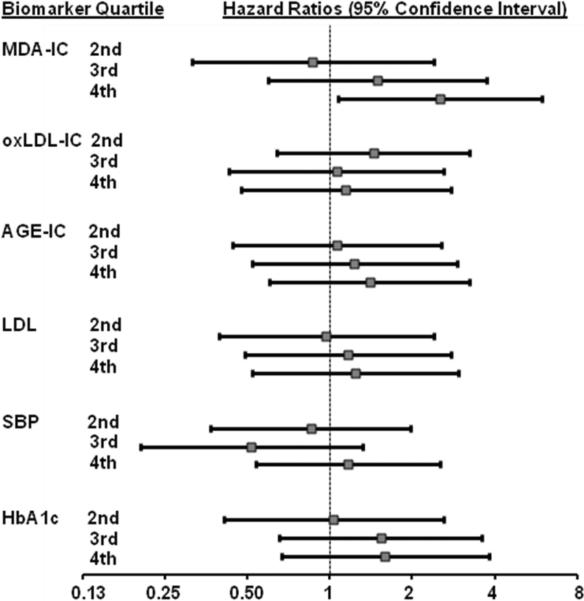
Finally, comparing the discriminatory power of MDA, oxLDL and AGE-LDL concentrations in IC to that of LDL-cholesterol, SBP and HbA1c, the adjusted hazard ratios for having a MI, comparing those in the highest versus lowest quartile of LDL-cholesterol, SBP, and HbA1c were 1.24 (0.52, 2.95), 1.17 (0.54, 2.53) and 1.59 (0.66, 3.82), respectively (Figure 1). Corresponding hazard ratios for MDA-LDL, oxLDL and AGE-LDL in IC were 2.52 (1.08, 5.92), 1.14 (0.47, 2.76), and 1.39 (0.60, 3.25).
Figure 1.
Adjusted* hazard ratios with 95% CI (calculated from Cox proportional hazard models) for given levels of MDA, oxLDL, and AGE-LDL in isolated IC, LDL cholesterol, systolic blood pressure (SBP) and HbA1c (levels in the 2nd, 3rd and 4th quartiles relative to quartile 1) to predict MI. MDA-IC categories are 5–52, 52–83, 83–128 and 128–607 mg/L; oxLDL-IC categories are 13.5–172, 172–257, 258–389 and 389–1712 mg/L; AGE-LDL-IC categories are 0.6–6.2, 6.2–10.8, 10.8–19.8, 19.8–103 mg/L; LDL categories are 20–74, 75–92, 93–111 and 112–234 mg/dL; SBP categories are 82–117, 118–126, 127–135 and 136–198 mmHg; HbA1c categories are 5.2–6.8, 6.9–7.7, 7.8–8.8 and 8.9–15.0%.
* Adjusted for age, ethnic minority, treatment arm and prior event with the exception of HbA1c which was not adjusted for treatment arm
DISCUSSION
We have previously reported that in patients with type 1 diabetes (DCCT/EDIC cohort) oxLDL and AGE-LDL levels in IC were strong predictors of increased progression of carotid IMT over time [7] and high oxLDL-IC levels were also indicative of increased coronary calcification [15]. Levels of MDA-LDL-IC showed a significant but weaker correlation with increased carotid IMT [7]. In contrast, in the VADT cohort, the levels of oxLDL-IC and AGELDL-IC, although considerably higher than those in the DCCT/EDIC cohort, were not significantly associated with the occurrence of acute CVD events. However in the same VADT cohort of type 2 diabetes with well-controlled lipids, blood pressure and HbA1c levels, high levels of MDA-LDL in circulating IC are predictive of acute MI and the VADT composite endpoint over a 3.7-year follow-up period.
A major difference between the VADT and DCCT/EDIC cohorts besides the different type of diabetes (type 1 and type 2), is the lack of established CVD disease at the time the IC were measured in the DCCT/EDIC cohort, and the presence of established CVD disease with 38.3% having had prior CVD events in the VADT cohort. Therefore, our clinical data strongly suggest that the relative composition of mLDL in LDL-IC is associated with different physiopathological effects on the vessel wall. While IC containing high levels of MDA-LDL and low levels of oxLDL and AGE-LDL are predictive of outcomes associated with plaque destabilization, IC containing high levels of oxLDL and AGE-LDL (and to a much lesser extent high levels of MDA-LDL) are associated with outcomes associated with plaque progression [9, 16, 17]. The unique role of MDA-LDL as a factor contributing to plaque instability is supported by two separate studies by Holvoet et al. showing a link between elevated plasma MDA-LDL levels and acute coronary syndromes which was not found with elevated plasma levels of oxLDL [21, 22].
As clearly shown for oxLDL [9] and oxLDL-IC, modified LDL-IC are more potent activators of human macrophages than modified LDL and lead to a much greater accumulation of cholesterol in these cells. This is not surprising because while free modified forms of LDL are taken up by scavenger receptors, the uptake of mLDL-IC is mediated by Fcγ receptors, specially FcγRI [23].
Recently, evidence supporting the concept that the nature of the IC influences the degree of macrophage activation has emerged. We have shown that oxLDL-IC induce the release of higher cytokine levels by macrophages than identical concentrations of keyhole limpet hemocyanin-IC [9]. We, and others, have also shown that oxLDL-IC induce macrophage survival[10, 24]. In contrast, we have found that MDA-LDL-IC induce macrophage apoptosis and increased expression of matrix metalloproteinases relative to oxLDL-IC, without an increase of TIMPs, (unpublished data), thus favoring plaque instability.
The circulating IC in our patient population are not likely to be constituted by “pure” discrete populations of different forms of modified LDL, but rather by LDL molecules with different modifications in different proportions. Therefore, the relative proportions of ox, MDA and AGE-LDL in a given patient reflect the predominance of one or another one of these LDL modifications. Our clinical data shows that high levels of MDA-LDL in isolated IC are more strongly associated with the occurrence of acute events in the presence of low levels of oxLDLIC or AGE-LDL-IC. In contrast, when oxLDL and AGE-LDL are the predominant modifications, or when all modifications are measured at high levels, the association of high levels of MDA-LDL-IC with acute events is no longer detected. Therefore, the data obtained with clinical samples agrees with the in vitro data obtained with laboratory-prepared oxLDL and MDA-LDL, suggesting that the composition of mLDL isolated from IC has a modulating activity on macrophage activation and survival. The modulation of macrophage activity by the antigen moiety of an IC is an entirely novel concept that will be the object of additional investigation in our laboratory.
Very few reports have been published about the possible predictive significance of oxLDL or MDA-LDL levels measured in unfractionated serum or plasma [21, 22, 25–27]. Although the data has suggested that modified LDL levels may correlate with progression of atherosclerosis and with the incidence of CVD, in general the results have been inconclusive. A major factor contributing to the limited number of reports on the significance of the levels of circulating forms of mLDL is the fact that over 90% of oxLDL-enriched and MDA-LDL-enriched LDL molecules circulate as IC [20]. Most methods proposed for their measurement in serum or plasma do not include steps designed to separate the antigens from the antibodies in order to measure the levels of mLDL present in circulation more accurately. Therefore the standard techniques measure only a small and variable fraction of circulating oxLDL or MDALDL. Many groups have also tried to measure antibodies to mLDLs as a way to indirectly assess their levels, but the results have been equally inconclusive [28]. Furthermore, the measurement of mLDLs involved in IC formation is physiopathologically more relevant than the measurement of “free” mLDL, because the IC containing mLDL are more strongly pro-inflammatory and proatherogenic than modified LDL [9, 10]. Our assay, while more complex, can accurately measure the levels of different mLDLs in isolated IC.
In recent years there has been considerable interest in identifying circulating biomarkers indicative of plaque instability, including metalloproteinases, C-reactive protein, cytokines (IL-6, IL-18), lipoprotein-associated phospholipase A-2, myeloperoxidase, monocyte chemotactic protein-1, and modified lipoproteins [29]. However, most studies were carried out in small patient populations, and their results are inconsistent. Our study suggests that the MDA-LDL content of circulating IC is a stronger biomarker candidate for plaque instability.
Supplementary Material
Highlights
Modified LDLs in IC were measured in 907 subjects of the VADT type 2 diabetes cohort
High levels of MDA-LDL in IC predict future acute CV events in the VADT cohort
MDA-LDL in IC is potentially a strong biomarker candidate for plaque instability
ACKNOWLEDGEMENTS
This work was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. This work was also supported by a program project funded by the National Institutes of Health (NIH) National Heart, Lung and Blood Institute Grant PO1-HL55782 and by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK081352 and R01-DK088778. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. M. L.-V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES None
REFERENCES
- [1].Ross R. Atherosclerosis-An inflammatory disease. N Eng J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- [2].De Boer OJ, van der Wal AC, Verhagen CE, et al. Cytokin secretion profiles of cloned T cells from human aortic atherosclerotic plaques. J Pathol. 1999;188:174–9. doi: 10.1002/(SICI)1096-9896(199906)188:2<174::AID-PATH333>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [3].Hansson GK, Jonasson L, Lojsthed B, et al. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988;72:135–41. doi: 10.1016/0021-9150(88)90074-3. [DOI] [PubMed] [Google Scholar]
- [4].Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- [5].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- [6].Virella G, Lopes-Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis. 2008;200:239–46. doi: 10.1016/j.atherosclerosis.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lopes-Virella MF, Hunt KJ, Baker NL, et al. The Levels of Oxidized LDL and AGE- LDL in Circulating Immune Complexes are strongly associated with increased levels of Carotid Intima-Media Thickness and its progression in Type 1 Diabetes. Diabetes. 2011;60:582–9. doi: 10.2337/db10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Virella G, Thorpe S, Alderson NL, et al. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Research. 2004;45:1859–67. doi: 10.1194/jlr.M400095-JLR200. [DOI] [PubMed] [Google Scholar]
- [9].Saad AF, Virella G, Chassereau C, et al. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47:1975–83. doi: 10.1194/jlr.M600064-JLR200. [DOI] [PubMed] [Google Scholar]
- [10].Hammad SM, Twal WO, Barth JL, et al. Oxidized LDL immune complexes and oxidized LDL differentially affect the expression of genes involved with inflammation and survival in human U937 monocytic cells. Atherosclerosis. 2009;202:394–404. doi: 10.1016/j.atherosclerosis.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kiener PA, Rankin BM, Davis PM, et al. Immune complexes of LDL induce atherogenic responses in human monocytic cells. Arterioscler Thromb Vasc Biol. 1995;15:990–9. doi: 10.1161/01.atv.15.7.990. [DOI] [PubMed] [Google Scholar]
- [12].Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–95. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Lipoproteins in normal and atherosclerotic aorta. European Heart J. 1990;11:88–9. doi: 10.1093/eurheartj/11.suppl_e.88. [DOI] [PubMed] [Google Scholar]
- [14].Lopes-Virella MF, McHenry MB, Lipsitz S, et al. Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis. 2007;190:359–69. doi: 10.1016/j.atherosclerosis.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [15].Lopes-Virella MF, Baker NL, Hunt KJ, et al. Oxidized LDL immune complexes and coronary artery calcification in type 1 diabetes. Atherosclerosis. 2011;214:462–7. doi: 10.1016/j.atherosclerosis.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mironova M, Virella G, Lopes-Virella MF. Isolation and characterization of human antioxidized LDL autoantibodies. Arterioscler Thromb Vasc Biol. 1996;16:222–9. doi: 10.1161/01.atv.16.2.222. [DOI] [PubMed] [Google Scholar]
- [17].Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003;17:314–22. doi: 10.1016/s1056-8727(02)00277-5. [DOI] [PubMed] [Google Scholar]
- [18].ADA Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2000;23:S32–S64. [PubMed] [Google Scholar]
- [19].Virella G, Carter RE, Saad A, et al. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin Immunol. 2008;127:394–400. doi: 10.1016/j.clim.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Virella G, Derrick MB, Pate V, et al. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol. 2005;12:68–75. doi: 10.1128/CDLI.12.1.68-75.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holvoet P, Perez G, Zhao Z, et al. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest. 1995;95:2611–9. doi: 10.1172/JCI117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holvoet P, Vanhaecke J, Janssens S, et al. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- [23].Lopes-Virella MF, Binzafar N, Rackley S, et al. The uptake of LDL-IC by human macrophages: predominant involvement of the Fc gamma RI receptor. Atherosclerosis. 1997;135:161–70. doi: 10.1016/s0021-9150(97)00157-3. [DOI] [PubMed] [Google Scholar]
- [24].Oksjoki R, Kovanen PT, Lindstedt KA, et al. OxLDL-IgG immune complexes induce survival of human monocytes. Arterioscler Thromb Vasc Biol. 2006;26:576–83. doi: 10.1161/01.ATV.0000201041.14438.8d. [DOI] [PubMed] [Google Scholar]
- [25].Holvoet P, Jenny NS, Schreiner PJ, et al. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;194:245–52. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [26].Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study) Arterioscler Thromb Vasc Biol. 2002;22:1162–7. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- [27].Yamazaki K, Bujo H, Taira K, et al. Increased circulating malondialdehyde-modified LDL in the patients with familial combined hyperlipidemia and its relation with the hepatic lipase activity. Atherosclerosis. 2004;172:181–7. doi: 10.1016/j.atherosclerosis.2003.05.001. [DOI] [PubMed] [Google Scholar]
- [28].Virella G, Lopes-Virella MF. Lipoprotein autoantibodies: measurement and significance. Clin Diag Lab Immunol. 2003;10:499–505. doi: 10.1128/CDLI.10.4.499-505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.