Abstract
The mature cerebral cortex contains a staggering variety of projection neuron subtypes, and a number of complementary studies have recently begun to define their identity and embryonic origin. Among the different types of cortical projection neurons, subcerebral projection neurons, including corticospinal motor neurons (CSMN), have been extensively studied and some of the molecular controls over their differentiation have been elucidated. Here, we first provide an overview of the approaches used to purify and molecularly profile neuronal populations of the neocortex and, more broadly, of the central nervous system (CNS). Next, we specifically review recent progress in understanding the genes that define and control development of the CSMN population. Finally, we briefly discuss the relevance of this work to current questions regarding the mechanisms of the establishment of projection neuron subtype identity in the neocortex and its implications to direct the differentiation of CSMN for therapeutic benefit.
Keywords: Bhlhb5, Clim1, corticospinal motor neurons (CSMN), Crim1, Ctip2, Diap3, FACS, Fezf2, Mu-Crystallin, Satb2, Sox5, subcerebral projection neurons
Introduction
The beauty and complexity of the nervous system has fascinated scientists for centuries, as also evidenced by some extraordinary drawings of gross anatomical dissections detailing the human brain, spinal cord and nerves dating back to the 15th and 16th centuries (Fig. 1).(1) Some of the earliest, most detailed studies of the fine architecture of the central nervous system (CNS) were performed by the histologist Santiago Ramón y Cajal who, together with Camillo Golgi, was awarded the Nobel Prize in Physiology/Medicine in 1906 for examining the structure of the nervous system.(2) Through this pioneering work, and that of many others subsequently, a detailed description of the neuronal diversity of the CNS has evolved, and with it has come the realization of the heterogeneity of cell types of which the CNS is comprised. The mechanisms of origin, maintenance, and the functional relevance of such cellular diversity are currently topics of intense investigation.
Figure 1.
Plates illustrating dissections of the human brain, spinal cord and nerves from Anatomia del corpo humano by Juan Valverde de Amusco (1560).(1)
The neocortex is the region of the brain involved in high level functions including cognition, sensory perception, and generation of motor behavior. Within the six-layer rodent cerebral cortex, two main classes of neurons exist: the principal neurons, which are large, excitatory glutamatergic projection neurons (PN) that send axons to intracortical, subcortical, and subcerebral targets; and the smaller, inhibitory GABAergic interneurons (IN), which are mostly born from germinal zones of the ventral telencephalon and migrate into the cortex where they synapse with PNs of the different layers.(3–5) Both of these two broad populations are extremely heterogeneous, each containing different classes of neurons that can be classified not only according to morphology and anatomy, but also according to unique electrophysiological properties, their connections within the brain and subcerebral targets, and the expression of neuron type-specific genes.(4–11)
Recent work has provided new insight into some of the molecular mechanisms that govern the generation of such neuronal diversity, and has identified molecules that define individual neuronal types of the neocortex as they develop. The development of the cortical INs has been elegantly and extensively reviewed elsewhere.(4,9,12) In this paper, we mainly concentrate on the molecular development of the cortical principal neurons with a special emphasis on the population of corticospinal motor neurons (CSMN) and related subtypes of subcerebral PN. We briefly describe the development of PNs of the neocortex and broadly review approaches to purify individual neuron subtypes. Next, we cover recent progress in the identification of genes that together define CSMN and subcerebral PNs, and highlight those that control the development of these neuron types. We conclude by proposing a model for the establishment of PN subtype identity in the cortex, and discuss the therapeutic implications of this work.
Projection neuron development in the cerebral cortex
Development of the cortex in the rodent begins with the specification of the telencephalon from the most anterior region of the neural tube.(13) Several key determinants for the establishment of the anterior/posterior (A/P) and the dorsal/ ventral (D/V) axes have been identified and include, among others, members of the Wnt, Fgf, BMP, and Shh families (reviewed in Refs.(13–15)). Subsequently, the neocortical progenitor domain is specified within the dorsal telencephalon by important cell intrinsic determinants of cortical development.(13–18) Particularly important is the function of LIM homeobox 2 (Lhx2)(19–21) and forkhead box G1 (Foxg1)(22–24) to repress dorsal midline forebrain progenitor fates as well as that of empty spiracles homolog 2 (Emx2)(25) and paired box 6 (Pax6)(25–30) to repress ventral forebrain progenitor fates.
Upon specification of the cortical progenitor domain in the dorsal telencephalon, excitatory PNs are generated over the course of approximately 6 days (in the mouse) and sequentially migrate to their final position within appropriate layers(3) (Fig. 2). Initially, the ventricular zone (VZ), which lies immediately adjacent to the ventricles, exists as a single germinal zone of cortical progenitors. As development proceeds, a second proliferative zone, termed the subventricular zone (SVZ), appears dorsally to the VZ.(3,31,32) The neurons born first are those of a structure called the preplate (PP), which later splits into the superficially located marginal zone (MZ) and the deeply located subplate (SP). Between these two structures develops the cortical plate (CP), which contains successively generated layers of projection neuron types.(3) Elegant birth-dating studies using [3H]thymidine have shown that PNs of the different layers are generated in a specific temporal order, such that SP and deep layer VI and V neurons are born first (between approximately embryonic day (E) 10.5 and E13.5 in the mouse) while the neurons of the superficial layers IV and II/III are born later (approximately between E14.5 and E16.5).(3,5) The laminar cortex is thus generated in an inside-out manner, with late-born neurons bypassing layers of those born earlier and migrating radially and tangentially to their final location.(3,33–38) It is well known that cortical layers are heterogeneous, and contain many different subtypes of PN that, in addition to having a specific laminar address and birth date, can be anatomically classified according to their specific axonal targets, either within the cortex or to subcortical and subcerebral regions.(5) Specifically, PN whose axons project intracortically include associative PN that connect within the same hemisphere, and a broad class of commissural PN that connect to the opposite hemisphere by way of the corpus callosum or the anterior commissure.(5) Among the commissural neurons, callosal projection neurons (CPN) are a broad and anatomically diverse population of PN that are located primarily in layers II/ III, V, and VI and whose axons connect the two hemispheres via the corpus callosum. While all CPN extend axons through the corpus callosum, they can be further defined based on complex patterns of collateral projections to the ipsi- and contralateral striatum and the frontal cortex, as well as by the expression of selected combinations of molecular markers.(5,39) PN whose axons project to subcortical and subcerebral regions are broadly defined as corticofugal PN and are located in the deep cortical layers V and VI. These include, among others, corticothalamic PN of layer VI and a diverse variety of subcerebral PN located in different areas of layer Vb. CSMN are a distinct subtype of subcerebral PNs, which are classified by having axons that project from layer Vb of the cortex to different segmental levels of the spinal cord with collateral projections to the striatum and the pons.(5)
Figure 2.

Spatial and temporal aspects of development of the laminar cortex. A: Schematic representation of the region of interest (boxed). B: In the dorsal telencephalon, neural progenitors in the VZ and later in the SVZ, give rise to PN, which migrate to their appropriate layers. PN subtypes are born in a temporally distinct manner, with the deeper layer neurons born early in development, and the upper layer neurons born later. Each cortical layer contains multiple types of PN that connect to different intracortical, subcortical, and subcerebral targets. CP, cortical plate; Ctx, cortex; IZ, intermediate zone; LGE, lateral ganglionic eminence; LV, lateral ventricle; MGE, medial ganglionic eminence; MZ, marginal zone; PP, preplate; SP, subplate; SVZ, subventricular zone; VZ, ventricular zone; WM, white matter.
Isolation and purification of neuronal subtypes
The co-existence of multiple PN subtypes within each cerebral cortical layer and the diversity of cell types within the neocortex present serious challenges to the study of the mechanisms that govern the specification and development of individual types of neurons. In this regard, the field continues to face some of the same difficulties experienced by Ramón y Cajal over a century ago due to the diversity of neuronal types in the CNS and the fact that they are intermixed. The analysis of early neuron type-specific fate determination and differentiation are also negatively affected by the lack of molecular markers that might distinguish cortical progenitor subtypes, should such lineage-fate progenitors even exist. While the birth order, sequential cortical lamination, and connectivity of PN have been extensively studied, the molecular mechanisms that control these developmental events for individual types of PN have only recently begun to be elucidated.(5,10,40)
The recent development of experimental approaches to label and purify neuronal subtypes in the nervous system has, at least partially, overcome the issue of neuronal heterogeneity in the CNS and has enabled studies aimed at identifying genes that define and control the development of individual neuron types.(5,7,41,42) In the cortex, CSMN, CPN, and corticotectal PN have been retrogradely labeled and purified for the first time at different stages of late embryonic and early postnatal development using fluorescence-activated cell sorting (FACS).(41,43–45) This has enabled the gene expression comparison of these pure projection neuron populations as well as the identification of the first series of genes that, in specific combinations, define the CSMN population(41) and the callosal neuron population.(39) More recently, similar methods of FACS purification have also been applied to the isolation of other types of neurons in the nervous system, including adult retinal ganglion cells,(46) cortical IN,(47) and two populations of striatal PN.(48) Of particular note, a recently developed methodology, called translating ribosome affinity purification (TRAP), has used immunoaffinity purification as an alternative to FACS to isolate polysomal mRNA from two genetically labeled medium spiny neuron subclasses.(49,50) While this approach cannot be used to obtain intact purified neurons, it offers the distinct advantage of achieving purification of mRNA from a large number of cells, and it may be broadly applicable to mRNA profiling of neuronal populations isolated from the adult nervous system, which are notoriously more fragile cells. Similar immunopanning methods have also been successfully used to purify intact PN subpopulations labeled by retrograde axonal transport of the cell-surface epitope of cholera toxin B (CTB), followed by immunopurification with an anti-CTB antibody.(51) While these studies demonstrate that FACS and immunopanning can be successfully used to purify relatively large numbers of labeled neuron types, other methodologies have also been developed to obtain very pure, albeit fewer in number, populations of neurons. For example, in addition to the use of laser capture microdissection (LCM) methods,(52–54) transgenic reporter mice, in which GFP is expressed in different types of neurons in the adult brain, have been used to hand purify and transcriptionally profile neuronal populations of the cortex, hippocampus, amygdala, and thalamus.(55,56) While single-neuron hand-picking is useful to purify only a relatively small number of cells and, thus, is not applicable to certain downstream applications (i.e., large-scale mRNA screens, epigenetic, and proteomic analysis), it offers the distinct advantage of achieving high cell purity without the need for expensive FACS equipment and optimized FACS protocols. As methodologies to label and purify individual populations of neurons from the CNS evolve, some of the barriers related to the cellular complexity of the nervous system are falling. This is particularly evident in the cerebral cortex, given the high number of neuronal types that coexist within each layer, and the overall cellular diversity of the tissue.
Corticospinal motor neurons
One of the most studied subtypes of PN of the cortex are the CSMN.(57–59) These are prototypical PN that reside within layer Vb of the cerebral cortex, primarily within the area of motor cortex, and extend axons through the internal capsule to the midbrain, pons, and spinal cord, where they synapse with lower motor neurons (via intermediate IN in the mouse).(60) CSMN are part of a broader group of highly related subcerebral PN, which share largely common programs of early development with CSMN, including location in the same cortical layer, but which differ by their location within different cortical areas and by having primary axonal projections to different targets below the brain.(5) From a clinical perspective, CSMN have been extensively studied, because in humans, degeneration of or damage to this neuronal population results in compromised motor function. For example, individuals affected by amyotrophic lateral sclerosis (ALS) show degeneration of both CSMN in the cortex and lower motor neurons in the spinal cord,(61,62) while those affected by hereditary spastic paraplegia (HSP) and primary lateral sclerosis (PLS) experience primarily the degeneration of CSMN.(63) Similarly, in spinal cord injury (SCI), the interruption of the axonal connections made by CSMN to the spinal cord contributes to the loss of motor control of the parts of the body below the injury site.(64,65) Understanding the molecular pathways that govern neuron subtype-specific development of CSMN, including signals of specification, maturation, survival, and connectivity, may contribute critically to the development of protocols to instruct the differentiation of CSMN for therapeutic uses.
Molecular definition and development of corticospinal motor neurons
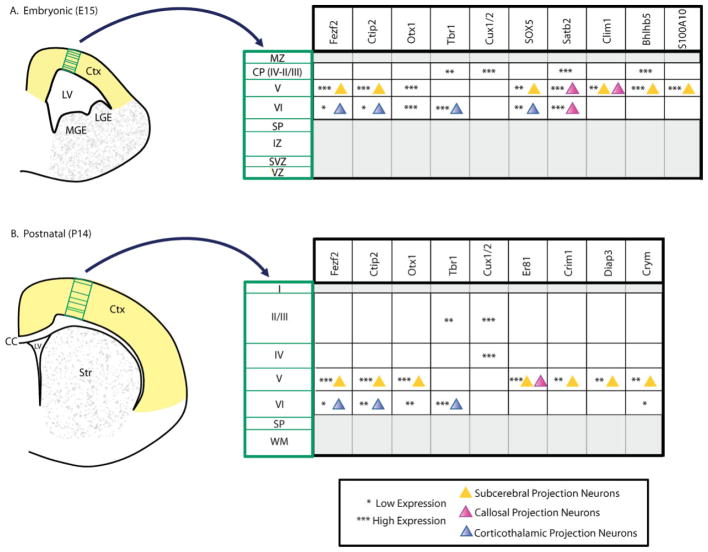
To identify genes that mark CSMN and to begin to understand the molecular pathways that control CSMN-specific development, CSMN have been previously retrogradely labeled at four developmental time points (E18, P3, P6, and P14), purified by FACS, and compared by microarray to two other subtypes of similarly purified PN – CPN and corticotectal PN.(39,41) This work led to the identification of several genes that together begin to define CSMN at the molecular level. These include transcription factors (e.g., Ctip2, Bcl6, Sox5, Fezf2), cell surface proteins (e.g., Encephalopsin, Itm2a, Daf1), calcium signaling proteins (e.g., Pcp4, S100a10), cell adhesion proteins (e.g., Cdh22, Cdh13, Cntn6), and axon guidance molecules (e.g., Neto1, Netrin-G1). Due to space restrictions, we refer the readers to Arlotta et al.,(41) and Molyneaux et al.(5) to obtain a full list of genes. In addition, Fig. 3 outlines the layer- and subtype-specific expression (where known) of several key genes during embryonic and postnatal development. Based on the developmental stage and neuron subtype-specific profile of expression, these genes can also be classified into categories that potentially reflect gene function. For example, genes like Ctip2, Sox5, Fezf2, Clim1, Cadherin13, and Cntn6 are expressed in the early stages of development of CSMN and subcerebral neurons, suggesting their possible involvement in events controlling CSMN birth, early specification and connectivity. Indeed, functional analysis performed so far on Ctip2,(41,66–68) Fezf2,(41,69–71) and Sox5(41,72,73) confirms this prediction. Fezf2, a zinc finger transcription factor expressed at high levels in all subcerebral neurons and at low levels in layer VI neurons, is centrally important for CSMN early specification in vivo.(41,69–71,74) In the absence of Fezf2 in null-mutant mice, CSMN and subcerebral PN are not specified and the corticospinal tract (together with cortical connections to the brainstem) does not form. In contrast, upper layer IV and II/III neurons appear to develop normally, and layer VI neurons, despite some morphological and molecular abnormalities are specified and correctly located in layer VI of the Fezf2−/ − cortex.(69–71) It is likely that Fezf2 acts by controlling the specification of subcerebral neurons at the expense of other PN types. This hypothesis is supported by the observation that the loss of subcerebral PN in the mutant is compensated for by the generation of other PN, which mostly locate in layer V.(69) While the exact identity of these neurons has not been fully elucidated, at least some of them express markers of layer VI corticofugal PN (e.g., Tbr1) and resemble corti-cothalamic neurons.(69) In addition, more recent data provides support for a model in which the absence of Fezf2 results in increased numbers of deep layer CPN, as defined by the expanded expression in layers V and VI of Satb2, a gene important for callosal neuron development, the high frequency of randomly recorded neurons with spike frequency adaptation to current injections (typical of callosal neurons), and the extension of callosal projections by Fezf2−/ − neurons grown in a wild-type environment.(67) These PN are likely a subset of the heterogeneous population of CPN that are normally present within the cortex, since contrary to Satb2 expansion, the expression of Cux1 (a marker of upper layer neurons, including CPN) and Plexin-D1 (a marker of layer Va) are not changed in the Fezf2 null-mutant cortex.(69) Together, these data suggest that Fezf2 is not necessary for the generation of layer V glutamatergic PN but, rather, is critical for instructing the specific development of the population of CSMN and other subcerebral PN, including the development of axonal projections to the brainstem and the spinal cord. This central role of Fezf2 is also supported by the finding that elevated levels of Fezf2 expression within progenitors of upper layer neurons is, at least in part, sufficient to instruct a switch of fate of progenitors and the birth of deep layer neurons that make connections to subcerebral targets and to the thalamus.(69) In the future, it would be interesting to elucidate the molecular pathways that mediate the specification of subcerebral neurons, including CSMN, downstream of Fezf2, as well as the signals that induce the cell type-specific and temporally restricted expression of Fezf2. One potential mediator of Fezf2 function, although probably not a direct target gene, is the transcription factor Ctip2. In the cortex, CTIP2 is expressed at high levels in postmitotic subcerebral PN of layer Vb and at lower levels in corticofugal neurons of layer VI.(41) In mice lacking Ctip2, the cortex appears normal but CSMN and other subcerebral neurons have abnormal axonal fasciculation and outgrowth, and, by postnatal day P0, axons have not innervated the spinal cord.(41) The critical role of Ctip2 as a necessary factor in controlling CSMN axonal connectivity is further supported by the finding that loss of Satb2, a gene important for maintaining callosal neuron projections, causes upper layer neurons to express CTIP2 and, consequently, to extend axons through the internal capsule.(66,68) Ctip2 likely acts earlier than genes involved in controlling axon guidance to targets in the brainstem and spinal cord,(45,75–77) and genes, including the transcription factor Otx1,(78) that refine the postnatal pruning of axon collaterals of different classes of subcerebral PN.
Figure 3.
Expression of selected PN genes in the embryonic and postnatal mouse brain. Boxed regions illustrate the area for which gene expression is described at A: E15 and B: P14. Asterisks describe relative expression levels, while colored triangles indicate gene expression in specific PN subtypes (where known). For more detailed information on the neuron subtype-specific expression of these genes, we refer to the text, as well as to the following references: Fezf2,(41,69–71,74) Ctip2,(41) Sox5,(41,72,73) Clim1,(41,93,94) Bhlhb5,(85,95,96) Otx1,(78,97) Satb2,(66,68) Cux1 and Cux2,(98,99) Tbr1,(100–104) S100a10,(41) Crim1,(41) Diap3,(41) Crym (Mu-Crystallin),(41) Er81.(102,105) CC, corpus callosum; CP, cortical plate; Ctx, cortex; IZ, intermediate zone; LGE, lateral ganglionic eminence; LV, lateral ventricle; MGE, medial ganglionic eminence; MZ, marginal zone; SP, subplate; Str, striatum; SVZ, subventricular zone; VZ, ventricular zone; WM, white matter.
Subcerebral PN belong to the broader class of corticofugal PN that include layer V subcerebral neurons (CSMN and related corticobrainstem neurons), layer VI corticothalamic neurons, and SP neurons.(5) The different subtypes of corticofugal neurons are generated in a specific order, locate in distinct laminar positions, and project to different subcortical and subcerebral targets. The transcription factor Sox5 has been recently found to be expressed in subcerebral PN and other corticofugal neurons, and controls the normal sequence of birth, migration, differentiation, and connectivity of these related neuronal subtypes.(41,72,73) In the absence of Sox5, subcerebral PN specification is disrupted, with failure by these neurons to reach their proper position in layer V and accumulation within the white matter and layer VI. In addition, early born SP neurons acquire some molecular and morphological features of subcerebral neurons, and, at least a subpopulation sends aberrant and exuberant projections to subcerebral targets. Ultimately, abnormal specification of SP neurons results in their incomplete separation from the PP, with some SP neurons remaining juxtaposed to MZ cells in the upper layers.(72,73) In its function in the broad control of corticofugal neuron generation and subtype delineation, Sox5 might act upstream of Fezf2 and Ctip2, possibly repressing the expression of these two transcription factors selectively in deep layer VI and SP neurons. This is supported by the identification of a conserved enhancer element on the proximal Fezf2 promoter that contains multiple Sox5 binding sites and that has been shown to mediate transcriptional repression by Sox5 in vitro.(72)
The discovery of genes that together delineate CSMN from other cortical PN populations and the understanding of their functional roles make it possible to determine the relationships amongst these genes toward assembling a functional multi-gene program that shapes CSMN specification and development in vivo. In particular, it would be interesting to determine the exact temporal sequence of expression of each gene, and define whether early transcription factors like Sox5, Fezf2, and Ctip2 directly or indirectly control other genes that are specific to CSMN at later stages of development. Of note, the recent transcriptome analysis of selected regions of the developing human brain, including the cerebral cortex, demonstrates that several of the same transcription factors that play important roles during cortical PN-type development in the mouse are also expressed in the fetal human cortex, suggesting evolutionarily conserved molecular mechanisms of cortical PN development.(79)
Generation of cortical projection neuron subtypes: Starting to put the pieces together
The variety of neuronal types and the complexity of neuronal circuitry present in the cerebral cortex bring about the central question of how this cellular diversity is generated during corticogenesis. This appears to be a multistep and sequential process that builds on the concerted action of both early controls over dividing progenitor fate specification, and later molecular programs that control the postmitotic establishment of neuronal identity with lineage specificity. At the earliest stages of PN specification in the cortex, neuroepithelial progenitors become restricted to a neocortical fate in response to extracellular patterning molecules (reviewed in Ref.(14)). Among others, they begin to express genes like Lhx2, Emx2, Pax6, and FoxG1, and differentiate themselves along the D/V axes from other forebrain progenitors of a different fate (reviewed in Ref.(16)). It is not known whether neocortical progenitors are additionally parcellated into subpopulations that could predict their differentiation into individual types of postmitotic PNs at this or later stages. However, population level analysis has shown that along the tangential A/P extension of the VZ, progenitors differ in their expression of transcription factors (i.e., COUP-TFI, Emx2, and Pax6), which are critical for proper adult cortical arealization (reviewed in Ref.(16)).
Cortical progenitors undergo specification along a third axis: time. Progenitors generate different types of postmitotic PN at different developmental time points (Fig. 2), and, similarly to progenitors in other parts of the nervous system (e.g., the retina, reviewed in Ref.(80)), they become progressively restricted in their fate choices.(81) In this framework, the critical steps of transitioning from dividing neocortical progenitors to a specific subtype of postmitotic cortical PN are largely not understood and several questions remain unanswered. Is commitment to a specific neuron-type fate determined within dividing progenitors, or postmitotic neurons or, potentially, is this regulated as a continuum of fate and specification decisions that occur at multiple stages of differentiation? What are the signals that instruct the generation of individual types of PN and when do neuronal lineages branch from each other? Is neuronal type-specific identity actively maintained throughout life, or, rather, irreversibly determined? These and many other questions lay at the core of our understanding of the mechanisms that control the generation of PN diversity in the cortex, and some answers are beginning to emerge.
Elegant studies by the Temple group have shown that isolated cortical progenitors that are cultured at clonal density can cell-autonomously control the generation of the correct temporal sequence of cortical PN subtypes.(82) This suggests that the order of generation of PN is at least in part determined at the progenitor stage. In support of this, the transcription factor AP2-gamma controls aspects of cortical progenitor laminar fate, as loss of AP2-gamma results in SVZ progenitor misspecification and a reduction of upper layer neurons within the occipital cortex.(83) Interestingly, exposure of progenitors in vivo to brain-derived neurotrophic factor can also cell-extrinsically change the layer-specific fate of the neurons generated,(84) further demonstrating that important layer-specific and neuron type-specific fate choices occur or can be modulated in dividing progenitors.
However, it is intriguing to note that some of the recently identified transcription factors that promote (Ctip2, Sox5) or repress (Satb2) CSMN and subcerebral PN specification and connectivity are expressed postmitotically, in newly generated neurons, and are excluded from cortical progenitors.(41,66,72) This suggests that at least some events of differentiation of CSMN (and likely those of other cortical neuron types) may be regulated after cell cycle exit, outside of the germinal zone. Interestingly, some aspects of acquisition of area identity by PN types in the neocortex are also controlled postmitotically. This is illustrated by the discovery that the transcription factor Bhlhb5, which is expressed in postmitotic PN in layers II/III-V (including CSMN in layer V), is important for the acquisition of arealization properties of PN within somatosensory and caudal motor cortices.(85) As dividing progenitors and early postmitotic neurons are lineage related, it is also easy to imagine that postmitotic neurons could directly affect the fate and commitment of neural progenitors using a feed-back loop that allows differentiated neuronal progeny to control the fate of their progenitors. A first example that this may be the case in the cerebral cortex, comes from the recent report that Sip1, a transcription factor expressed in postmitotic cortical neurons, controls progenitor layer-specific fate in a non-cell autonomous manner, likely via the production of neurotrophin-3 (Ntf3).(86) Together, these data suggest that the specification of distinct PN types in the cortex relies on the orchestrated action of signals that act within both cortical progenitors and their postmitotic neuronal progeny.
Diseased cortex and directed differentiation of CSMN
As the genes that instruct CSMN-specific development in the cortex are defined, these same instructive molecular cues might be used to directly guide the specification and differentiation of CSMN for future therapeutic applications. The recent advances in the field of stem cell biology, and in particular the reprogramming of mouse and human somatic cells into induced pluripotent stem (iPS) cells,(87–89) now enable work aimed at using iPS cells to possibly direct the differentiation of large numbers of CSMN in vitro. This is supported by the findings that embryonic stem (ES) cells, which share many common properties with iPS cells, have the potential to generate different subtypes of cortical PN in vitro.(90,91) It is conceivable that large numbers of CSMN can be selectively generated using the correct sequence and combination of cell-autonomous signals that shape this neuron type in the embryo and under the culture conditions that allow cortical PN generation from ES and iPS cells.(90,91) Availability of large numbers of relatively pure CSMN cultures would benefit downstream applications, including the therapeutic screening of small molecules that may control differentiation, survival, and connectivity of CSMN. This type of approach may also be useful to define compounds that activate or repress the expression of CSMN-specific genes, which may in turn be used to instruct CSMN-directed differentiation by compound administration in vitro. Of note, iPS lines have been recently developed from patients with ALS and directed to differentiate into lower motor neurons in vitro.(92) Since both lower motor neurons and CSMN degenerate in ALS, these same iPS lines might be used to generate patient-specific CSMN for further investigation.
Conclusions
The neocortex contains a great diversity of projection neurons and interneuron subtypes, which have been historically classified according to morphology, connectivity, and electro-physiological properties. Among the PN, distinct populations exist that connect to different distal targets within the cortex or to subcortical and subcerebral locations.(5) The timing of birth, the cellular events of migration into laminae, and the maturation and connectivity of cortical neuron types has been extensively investigated. The recent identification of genes that label and control the development of individual PN subtypes has advanced this prior work and refined PN classification to also include the expression of different combinations of genetic markers. This should facilitate studies aimed at defining the timing of progressive specification of PN subtypes during corticogenesis. For example, it will be interesting to understand when individual PN populations branch into distinct subtypes during development and whether related classes of PN share common progenitors, or go through the same postmitotic stages of early molecular development. Indeed, PN types that are more closely related (e.g., CSMN and corticotectal PN) share several early genes, which are instead excluded from more distally related neurons (e.g., CPN).(41) In some cases, the molecular distinction among neuron types occurs over time, as shown by the fact that CSMN and CPN both initially express Clim1 (a gene expressed in CSMN and other subcerebral neurons) and Lmo4 (a gene that in layer V labels CPN), and only later during differentiation do they each express one gene over the other.(93)
As the mechanisms that control the development of specific populations of PN during corticogenesis are unraveled, there is an opportunity to further our understanding of the molecular programs that first establish and later maintain neuron type-specific identity throughout the life of the organism. For example, it is intriguing to speculate that beyond developmental corticogenesis, the identity of distinct classes of PN may be reversible and that differentiated neurons (and glia) could possibly be instructed to reprogram into specific PN subtypes. The availability of molecular programs that govern the differentiation of distinct classes of PN may in the future help to define the boundaries of neuronal plasticity and reprogramming in the neocortex.
Acknowledgments
We would like to thank Zachary Trayes-Gibson, Simona Lodato, Caroline Rouaux, and Eiman Azim for their suggestions and critical reading. This work was partially supported by the NIH (NS062849), the Harvard Stem Cell Institute and the Spastic Paraplegia Foundation to P. A.; L. D. S. was partially supported by a William Randolph Hearst Fellowship.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- A/P
anterior/posterior
- CNS
central nervous system
- CP
cortical plate
- CPN
callosal projection neuron
- CSMN
corticospinal motor neurons
- CTB
cholera toxin B
- D/V
dorsal ventral
- ES
embryonic stem
- FACS
fluorescence-activated cell sorting
- HSP
hereditary spastic paraplegia
- IN
interneuron
- iPS
induced pluripotent stem
- LCM
laser capture microdissection
- MZ
marginal zone
- PLS
primary lateral sclerosis
- PN
projection neuron
- PP
preplate
- SCI
spinal cord injury
- SP
subplate
- SVZ
subventricular zone
- TRAP
translating ribosome affinity purification
- VZ
ventricular zone
References
- 1.Libraries UoT. Anatomia 1522–1867: Anatomical Plates from the Thomas Fisher Rare Book Library. 2003 http://www.library.utoronto.ca/fisher/
- 2.Sourkes TL. Nobel Prize Winners in Medicine and Physiology 1901–1965. Amsterdam, London, New York: Elsevier Publishing Co; 1967. [Google Scholar]
- 3.Bayer SA, Altman J. Neocortical Development. New York: Raven Press; 1991. [Google Scholar]
- 4.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 5.Molyneaux BJ, Arlotta P, Menezes JRL, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–37. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 6.Markram H, Toledo-Rodriguez M, Wang Y, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 7.Nelson SB, Hempel C, Sugino K. Probing the transcriptome of neuronal cell types. Curr Opin Neurobiol. 2006;16:571–6. doi: 10.1016/j.conb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Petilla Interneuron Nomenclature Group. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molnár Z, Cheung AFP. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–15. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Monyer H, Markram H. Interneuron diversity series: molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–7. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Flames N, Marín O. Developmental mechanisms underlying the generation of cortical interneuron diversity. Neuron. 2005;46:377–81. doi: 10.1016/j.neuron.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3:943–51. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- 14.Hébert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–85. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakic P, Ayouba AE, Breuniga JJ, et al. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Leary DDM, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 18.Mallamaci A, Stoykova A. Gene networks controlling early cerebral cortex arealization. Eur J Neurosci. 2006;23:847–56. doi: 10.1111/j.1460-9568.2006.04634.x. [DOI] [PubMed] [Google Scholar]
- 19.Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 20.Bulchand S, Grove EA, Porter FD, et al. LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev. 2001;100:165–75. doi: 10.1016/s0925-4773(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 21.Vyas A, Saha B, Lai E, et al. Paleocortex is specified in mice in which dorsal telencephalic patterning is severely disrupted. J Comp Neurol. 2003;466:545–53. doi: 10.1002/cne.10900. [DOI] [PubMed] [Google Scholar]
- 22.Dou CL, Li S, Lai E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb Cortex. 1999;9:543–50. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- 23.Muzio L, Mallamaci A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–41. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanashima C, Li SC, Shen L, et al. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–9. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- 25.Muzio L, DiBenedetto B, Stoykova A, et al. Conversion of cerebral cortex into basal ganglia in Emx2−/−Pax6Sey/Sey double-mutant mice. Nat Neurosci. 2002;5:737–45. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- 26.Schuurmans C, Armant O, Nieto M, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–71. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 28.Stoykova A, Treichel D, Hallonet M, et al. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–50. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 30.Kroll TT, O’Leary DDM. Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate. Proc Natl Acad Sci. 2005;102:7374–9. doi: 10.1073/pnas.0500819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smart IH. Proliferative characteristics of the ependymal layer during the early development of the mouse neocortex: a pilot study based on recording the number, location and plane of cleavage of mitotic figures. J Anat. 1973;116:67–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–90. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- 33.Noctor SC, Flint AC, Weissman TA, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 34.Britanova O, Alifragis P, Junek S, et al. A novel mode of tangential migration of cortical projection neurons. Dev Biol. 2006;298:299–311. doi: 10.1016/j.ydbio.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 35.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–9. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 36.Angevine JJ, Sidman R. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;25:766–8. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 37.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–7. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 38.Caviness V, Sidman R. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148:141–51. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 39.Molyneaux BJ, Arlotta P, Fame RM, et al. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–54. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leone DP, Srinivasan K, Chen B, et al. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arlotta P, Molyneaux BJ, Chen J, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–21. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 42.Nelson SB, Suginoa K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006;29:339–45. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Catapano LA, Arnold MW, Perez FA, et al. Specific neurotrophic factors support the survival of cortical projection neurons at distinct stages of development. J Neurosci. 2001;21:8863–72. doi: 10.1523/JNEUROSCI.21-22-08863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catapano L, Arlotta P, Cage T, et al. Stage-specific and opposing roles of BDNF, NT-3 and bFGF in differentiation of purified callosal projection neurons toward cellular repair of complex circuitry. Eur J Neurosci. 2004;19:2421–34. doi: 10.1111/j.0953-816X.2004.03303.x. [DOI] [PubMed] [Google Scholar]
- 45.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–81. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov D, Dvoriantchikova G, Barakat DJ, et al. Differential gene expression profiling of large and small retinal ganglion cells. J Neurosci Methods. 2008;174:10–7. doi: 10.1016/j.jneumeth.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batista-Brito R, Machold R, Klein C, et al. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–17. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lobo MK, Karsten SL, Gray M, et al. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–52. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 49.Doyle JP, Dougherty JD, Heiman M, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–62. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heiman M, Schaefer A, Gong S, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dugas JC, Mandemakers W, Rogers M, et al. A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 2008;28:8294–305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W-Z, Oeschger F, Lee S, et al. High quality RNA from multiple brain regions simultaneously acquired by laser capture micro-dissection. BMC Mol Biol. 2009:10. doi: 10.1186/1471-2199-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamme F, Salunga R, Yu J, et al. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J Neurosci. 2003;23:3607–15. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao F, Yu F, Gong L, et al. Microarray analysis of fluoro-gold labeled rat dopamine neurons harvested by laser capture microdissection. J Neurosci Methods. 2005;143:95–106. doi: 10.1016/j.jneumeth.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Sugino K, Hempel CM, Miller MN, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 56.Hempel CM, Sugino K, Nelson SB. A manual method for the purification of fluorescently labeled neurons from the mammalian brain. Nat Protoc. 2007;2:2924–29. doi: 10.1038/nprot.2007.416. [DOI] [PubMed] [Google Scholar]
- 57.Terashima T. Anatomy, development and lesion-induced plasticity of rodent corticospinal tract. Neurosci Res. 1995;22:139–61. doi: 10.1016/0168-0102(95)00895-9. [DOI] [PubMed] [Google Scholar]
- 58.Jones EG, Schreyer DJ, Wise SP. Growth and maturation of the rat corticospinal tract. Prog Brain Res. 1982;57:361–79. doi: 10.1016/S0079-6123(08)64137-0. [DOI] [PubMed] [Google Scholar]
- 59.O’Leary DDM, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 60.Cantya A, Murphy M. Molecular mechanisms of axon guidance in the developing corticospinal tract. Prog Neurobiol. 2008;85:214–35. doi: 10.1016/j.pneurobio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68:1571–75. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- 62.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–19. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 63.Strong M, Gordon P. Primary lateral sclerosis, hereditary spastic paraplegia and amyotrophic lateral sclerosis: discrete entities or spectrum? Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:8–16. doi: 10.1080/14660820410021267. [DOI] [PubMed] [Google Scholar]
- 64.Deumens R, Koopmans GC, Joosten EAJ. Regeneration of descending axon tracts after spinal cord injury. Prog Neurobiol. 2005;77:57–89. doi: 10.1016/j.pneurobio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Hains BC, Black JA, Waxman SG. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol. 2003;462:328–41. doi: 10.1002/cne.10733. [DOI] [PubMed] [Google Scholar]
- 66.Britanova O, de Juan Romero C, Cheung A, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neo-cortex. Neuron. 2008;57:378–92. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 67.Chen B, Wang SS, Hattox AM, et al. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci USA. 2008;105:11382–7. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alcamo EA, Chirivella L, Dautzenberg M, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–77. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Molyneaux BJ, Arlotta P, Hirata T, et al. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–31. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–9. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen JG, Rasin MR, Kwan KY, et al. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17792–7. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwan KY, Lam MMS, Krsnik Z, et al. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci USA. 2008;105:16021–6. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai T, Jabaudon D, Molyneaux BJ, et al. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–47. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 74.Hirata T, Suda Y, Nakao K, et al. Zinc finger gene fez-like functions in the formation of subplate neurons and thalamocortical axons. Dev Dyn. 2004;230:546–56. doi: 10.1002/dvdy.20068. [DOI] [PubMed] [Google Scholar]
- 75.Wegmeyer H, Egea J, Rabe N, et al. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-Chimaerin. Neuron. 2007;55:756–67. doi: 10.1016/j.neuron.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 76.Beg AA, Sommer JE, Martin JH, et al. Alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron. 2007;55:768–78. doi: 10.1016/j.neuron.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Shi J, Lu C-C, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–9. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 78.Weimann JM, Zhang YA, Levin ME, et al. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–31. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 79.Johnson MB, Kawasawa YI, Mason CE, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Livesey F, Cepko C. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–18. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 81.Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–72. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 82.Shen Q, Wang Y, Dimos JT, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–51. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 83.Pinto L, Drechsel D, Schmid M-T, et al. AP2gamma regulates basal progenitor fate in a region- and layer-specific manner in the developing cortex. Nat Neurosci. 2009;12:1229–37. doi: 10.1038/nn.2399. [DOI] [PubMed] [Google Scholar]
- 84.Fukumitsu H, Ohtsuka M, Murai R, et al. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26:13218–30. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joshi PS, Molyneaux BJ, Feng L, et al. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II-V of the developing neocortex. Neuron. 2008;60:258–72. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seuntjens E, Nityanandam A, Miquelajauregui A, et al. Sip1 regulates sequential fate decisions by feedback signaling from post-mitotic neurons to progenitors. Nat Neurosci. 2009;12:1373–80. doi: 10.1038/nn.2409. [DOI] [PubMed] [Google Scholar]
- 87.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 89.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 90.Gaspard N, Bouschet T, Hourez R, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–58. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 91.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–32. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 93.Azim E, Shnider SJ, Cederquist GY, et al. Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cereb Cortex. 2009;19:62–9. doi: 10.1093/cercor/bhp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226:460–9. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- 95.Mattar P, Langevin LM, Markham K, et al. Basic helix-loop-helix transcription factors cooperate to specify a cortical projection neuron identity. Mol Cell Biol. 2008;28:1456–69. doi: 10.1128/MCB.01510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brunelli S, Innocenzi A, Cossu G. Bhlhb5 is expressed in the CNS and sensory organs during mouse embryonic development. Gene Expr Patterns. 2003;3:755–9. doi: 10.1016/s1567-133x(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 97.Frantz GD, Weimann JM, Levin ME, et al. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci. 1994;14:5725–40. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nieto M, Monuki E, Tang H, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–80. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 99.Zimmer C, Tiveron M-C, Bodmer R, et al. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–20. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 100.Bulfone A, Smiga SM, Shimamura K, et al. T-Brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 101.Hevner RF, Shi L, Justice N, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–66. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 102.Hevner RF, Daza RAM, Rubenstein JLR, et al. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci. 2003;25:139–51. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- 103.Hevner H, Miyashita-Lin E, Rubenstein J. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: Evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- 104.Kolk SM, Whitman MC, Yun ME, et al. A unique subpopulation of Tbr1-expressing deep layer neurons in the developing cerebral cortex. Mol Cell Neurosci. 2006;32:200–14. doi: 10.1016/j.mcn.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 105.Yoneshima H, Yamasaki S, Voelker CCJ, et al. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience. 2006;137:401–12. doi: 10.1016/j.neuroscience.2005.08.075. [DOI] [PubMed] [Google Scholar]