Abstract
Background
Ischemia/reperfusion (I/R) injury is a common cause of acute renal failure after kidney transplantation. This study was designed to analyze the role of type I IFN signaling downstream of TLR2/TLR4 activation in the mechanism of I/R-triggered kidney damage.
Methods
Local warm ischemia was induced in groups WT and type I IFN receptor (IFNAR)−/− mice (C57BL/6) by clamping both kidney pedicles for 45min. Mice were sacrificed at 5/24/72h following reperfusion for serum and kidney sampling.
Results
At 5h, serum creatinine (sCr) and blood urea nitrogen (BUN) levels were markedly reduced in IFNAR−/− mice as compared with WT. By 24h after reperfusion, both sCr/BUN in WT increased further, whereas those in IFNAR−/− mice remained comparable with sham controls. Histological analyses showed significantly higher percentage of tubules in the outer medulla displaying cell necrosis, loss of the brush border, cast formation and tubular dilatation in WT mice, as compared with IFNAR−/−. Immunohistology revealed increased neutrophil and macrophage infiltration in the outer medulla in WT mice. The expression of pro-inflammatory TNFα, IL-1, IL- 6, and CXCL-2 was markedly reduced selectively in IFNAR−/− mice. Finally, TUNEL analysis showed significantly decreased frequency of apoptotic tubular epithelial cells in IFNAR-deficient mice, as compared with WT.
Conclusion
This is the first report, which documents the key role of type I IFN signaling in the mechanism of kidney I/R injury. Type I IFN may thus serve as a novel target for the therapy against renal I/R injury.
Keywords: Kidney ischemia/reperfusion injury, Innate immunity, Type I interferon, Inflammation
Introduction
Ischemia and reperfusion (I/R) injury is the major cause of acute renal failure after kidney transplantation, shock or renal artery stenosis. The I/R injury, a known prognostic factor of kidney allograft survival, associates with delayed organ function, and is one of the major risk factors for acute rejection and the development of chronic allograft nephropathy (1-3). The mechanisms of ischemic renal failure are complex and involve multiple mediators, such as reactive oxygen species (ROS), activation of adhesion molecules/chemokines, leukocyte recruitment, ultimately leading to tubular injury, endothelial dysfunction and inflammation. Due to prolonged warm ischemia times, utilization of kidneys from non-heart beating donors has increased the rate of primary non-function and delayed graft function. This makes warm ischemia experiments even more relevant for kidney transplantation.
Recombinant IFNs have been tested in clinical trials, including hepatitis B and C, multiple sclerosis, non-renal cell carcinoma, malignant melanoma, and others. Type I IFNs (IFN-α/IFN-β) are pleiotropic cytokines produced mainly by activated lymphocytes (NK cells, B- and T-cells), macrophages, fibroblasts, DCs and virus-infected cells. By recruiting/activating macrophages and NK cells, promoting the differentiation/activation of DCs, and inducing Th1-type cytokines, type I IFNs may act as a bridge system linking innate and adaptive immunity (4). They may also directly affect target cells by preventing virus replication, arresting cell cycle, and inducing apoptotic cell death (5). Type I IFNs bind to a common cell surface receptor complex, type I IFN receptor (IFNAR), consisting of IFNAR1 and IFNAR2 subunits (6), which signal through Tyk2 and Jak1 kinases. This results in the formation of STAT1-STAT2 heterodimers that migrate into the nucleus and activate the transcription of IFN-inducible genes. IFNAR can also activate and translocate STAT1 homodimers that bind to IFN-γ–activated sequences in IFN-γ–induced gene promoters (7).
Our group was the first to document that type I IFN pathway plays a key role in TLR4-triggered inflammation response and the development of liver IRI (8). We speculate that type I IFN might function as a novel target for prevention of renal dysfunction after ischemic kidney injury. Using IFNAR deficient mice in “warm” renal I/R injury model, we demonstrate that disruption of type I IFN signaling protects renal function, ameliorates local inflammation and attenuates the number of apoptotic cells within the ischemic kidney, resulting in significantly less histological evidence of acute tubular necrosis (ATN).
Results
Disruption of IFNAR signaling protects kidneys against IRI
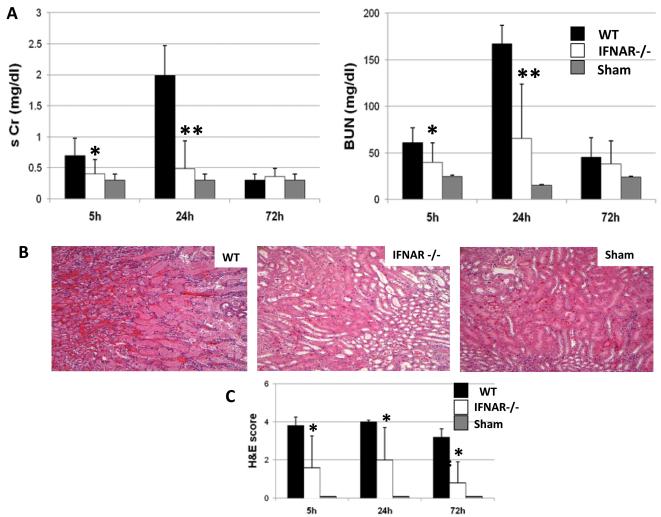
Forty-five minutes of bilateral kidney warm ischemia resulted in severe renal dysfunction in WT mice, consistent with significant elevation of serum creatinine (sCr) and blood urea nitrogen (BUN) levels (mg/dl) after reperfusion, as compared with sham controls (Fig. 1A). In marked contrast, renal function was largely preserved in IFNAR KO recipients, with sCr and BUN levels markedly lower than in WT counterparts (Fig. 1A, sCr: 0.4±0.2 vs. 0.7±0.2; p<0.05 and 0.4±0.4 vs. 1.9±0.4; p<0.001, at 5h and 24h respectively; BUN: 39±21 vs. 61±15; p<0.05 and 65±58 vs. 167±19; p<0.001, at 5h and 24h respectively). One hundred percent of mice survived throughout the 72h observation period, at which time the renal function normalized in both WT and KO groups. In addition, IFNAR deficiency afforded significant renal protection against I/R, as shown by histology (Fig. 1B&C). Hence, WT mice incurred severe tubular damage evidenced by widespread ATN, loss of the brush border, cast formation, and tubular dilation at the outer medulla. In contrast, the cardinal features of renal I/R damage were markedly attenuated in IFNAR KO recipients (Fig. 1C; score: 1.6±1.6 vs. 3.8±0.4; p<0.05; 2.0±1.6 vs. 4.0±0.1; p<0.05; 0.8±1 vs. 3.2±0.4; p<0.05, at 5h, 24h and 72h after reperfusion, respectively). Sham-operated mice incurred no tubular injury.
Figure 1. Renal function and histology after 45min of warm ischemia.
(A) Renal function at 5h, 24h and 72h of reperfusion. Serum creatinine (sCr) and blood urea nitrogen (BUN) levels were significantly reduced in IFNAR KO kidneys, as compared with WT controls (*p<0.05, **p<0.005; n=6-8/group). Means and SD are shown. (B) Representative renal histology of the outer medulla of the kidney at 24h of reperfusion (H&E staining; magnification ×200). (C) Renal histopathology score at 5h, 24h, and 72h. The tubular injury score was calculated according to the following criteria: tubular dilatation, cast deposition, brush border loss, and necrosis in 10 randomly chosen, non-overlapping fields. The tubular injury was based on the percentage of damaged tubules in the outer medulla using a scale from 0-4: 0=<10%; 1=moderate, 10-25%; 2=severe, 25-50%; 3=very severe, 50-75%; 4=extensive damage, >75%, *p<0.05, n=6-8/group.
IFNAR deficiency reduces kidney neutrophil infiltration/activity
We performed immunohistochemical staining for kidney infiltrating neutrophils at 5h, 24h and 72h of reperfusion following 45min of warm ischemia (Fig. 2A). Substantial infiltration of neutrophils, readily detectable in untreated WT group, was attenuated in IFNAR KO mice (Fig. 2B: 11.2±1.2 vs. 1.4±0.4; p<0.05; 24.6±6.5 vs. 2.3±1.5; p<0.05; 0.4±0.01 vs. 0.2±0.01; p<0.05; at 5h, 24h and 72h respectively).
Figure 2. Renal neutrophil infiltration/activity.
(A) Representative immunoperoxidase staining of neutrophils (Ly-6G), in the outer medulla of WT and IFNAR KO kidneys harvested at 5h, 24h, and 72h of reperfusion after 45min of warm ischemia. (B) Quantitation of I/R neutrophil infiltration by immunohistochemistry (*p <0.05; n=4/group) (×200 magnification). (C) Renal MPO activity in WT and IFNAR KO kidneys following I/R injury (*p<0.05; n=6-8/group). Means and SD are shown.
Myeloperoxidase (MPO), the most abundant protein in neutrophils (9) has been used as a quantitative measure of neutrophil infiltration (10). To confirm our finding that IFNAR deficiency protected IR-kidneys from neutrophil infiltration, we performed MPO assay. Indeed, MPO activity (U/g) was significantly depressed in IFNAR KO at 24h after reperfusion as compared with WT mice (Fig. 2C: 7.1±2.1 vs. 10.7±1.4; p<0.05). MPO levels in sham-operated controls were consistently lower (1±0.1).
IFNAR deficiency ameliorates renal macrophage infiltration
Disruption of the type I IFN signal significantly decreased the number of infiltrating macrophages compared to control mice at 5h and 24h (Fig. 3A&B: 3.8±2 vs. 14.8±2.6; p<0.05; 17.1±18.7 vs. 40.8±4.2; p<0.05; respectively).
Figure 3. Renal macrophage infiltration.
(A) Representative immunoperoxidase staining of macrophages (Mac-1) in the outer medulla of WT and IFNAR KO kidneys harvested at 5h, 24h and 72h of reperfusion after 45min of warm ischemia. (B) Quantitation of macrophage infiltration by immunohistochemistry (*p<0.05; n=4/group) (×200 magnification). Means and SD are shown.
Disruption of IFNAR signaling depresses IR-triggered pro-inflammatory genes
To investigate the effects of IFNAR deficiency on kidney cytokine/chemokine programs, we used qRT-PCR to analyze local expression of the pro-inflammatory cytokines (TNFα, IL-1β, IL-6), and MIP-2 (CXCL-2), a chemokine associated with neutrophil activation, and calculated the ratio between post-IR and constitutive mRNA levels in each animal. WT kidneys subjected to I/R demonstrated marked upregulation of TNFα, IL-1 β, IL-6 and CXCL-2 at 5h of reperfusion, as compared with sham-operated or native control kidneys, which did not express altered cytokine levels (Fig. 4). Indeed, kidneys in IFNAR KO mice were characterized by significant reduction of their mRNA levels, in comparison with WT (5h - TNFα: 0.5±0.05 vs. 0.9±0.3; p<0.05; IL-6: 0.4±0.4 vs. 1.2±0.7; p<0.05; IL-1β: 0.2±0.1 vs. 0.5±0.08; p<0.05; CXCL-2: 0.3±0.3 vs. 0.9±0.7; p<0.05). At 24h of reperfusion IL-6 and CXCL-2 mRNA levels still remained significantly elevated in WT, compared to IFNAR-deficient kidneys (IL-6: 0.01±0.01 vs. 0.06±0.03; p<0.05; CXCL-2: 0.06±0.04 vs. 0.16±0.10; p<0.05), confirming at the molecular level the apparent differences in renal function.
Figure 4. Renal cytokine/chemokine expression.
WT and IFNAR KO kidneys were analyzed by real-time RT-PCR for cytokine/chemokine gene expression at 5h, 24h and 72h of reperfusion following 45min of warm ischemia. Data were normalized to HPRT gene expression (*p <0.05, n =6/group). Means and SD are shown.
IFNAR deficiency protects kidneys from apoptosis
I/R-injury is associated with local apoptosis, which might contribute to renal dysfunction. To determine the impact of type I IFN signaling on the development of apoptosis in ischemic kidneys we scored the outer medulla of the kidneys, where the injury after ischemia is maximal for the presence of apoptotic bodies by deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay. As shown in Figure 5A&B, TUNEL assay has revealed a significant decrease in the number of apoptotic tubular epithelial cells (TEC) in IFNAR-deficient kidneys as compared with WT (0.7±0.6 vs. 2.6±0.4; p<0.05 and 1.2±1.7 vs. 4.6±2.3; p<0.05, at 5h and 24h, respectively). No TUNEL positive cells were seen in non-ischemic kidneys of sham-operated mice.
Figure 5. Renal tubular epithelial cell (TEC) apoptosis.
(A) Representative TUNEL-assisted detection of apoptosis in the outer medulla of WT and IFNAR KO kidneys, harvested at 24h of reperfusion after 45min of warm ischemia. Dark-brown dots correspond to representative TUNEL-positive nuclei. (H&E stain; ×400 magnification). (B) Quantitation of TEC apoptosis by TUNEL staining at 5h, 24h and 72h. WT controls had a higher frequency of TUNEL-positive cells than the IFNAR KO group at 5h and 24h. (*p <0.05; n=5/group). Means and SD are shown.
Discussion
This is the first study to our knowledge establishing the in vivo functional relevance of type I IFN pathway in the pathophysiology of kidney I/R injury. The disruption of IFNAR signaling in deficient mice protected renal function (decreased sCr and BUN levels) and ameliorated the cardinal histological features of I/R injury (diminished regional ATN scoring) after 45min of warm ischemia. Indeed, IFNAR KO mice had markedly reduced local inflammation characterized by a decreased recruitment of neutrophils and macrophages, along with reduced production of pro-inflammatory cytokines. In agreement with these findings, we have detected local cytoprotection, as evidenced by attenuated tubular epithelial cell (TEC) apoptosis within the ischemic kidney.
The mammalian sentinel Toll like-receptor (TLR) system plays a critical role in the development of organ IRI (11, 12). Both, TLR2 and TLR4 innate activation have been implicated in the induction of inflammation in warm kidney I/R injury in mice (13,14). TLR4 activation triggers two distinct signaling pathways. The MyD88-dependent pathway causes early phase NFκB activation, resulting in the production of pro-inflammatory cytokines; the MyD88-independent pathway activates interferon-regulatory factor 3 (IRF3) and causes the late-phase NFκB activation, both of which lead to the production of IFN-β and IFN-inducible genes. We have shown that IRF3-dependent pathway is essential in the development of liver IRI (12). The present results are in agreement with the significant role of type I IFN in the mechanism of organ I/R injury.
Since several different mechanisms contribute to renal I/R injury, there are likely multiple different pathways for TLR cross-talk in the kidney. There is some controversy as to the putative role of TLRs in renal I/R injury. The McKay group was the first to show that TLR2-dependent/MyD88-independent pathways contributed to, and TLR2 deficiency protected from the ischemic kidney damage (13). In agreement with the latter, targeted deletion of TLR2 or down-regulation of TLR2 with antisense oligonucleotides exerted local cytoprotection (11). However, others identified TLR4 as a cellular sentinel for acute renal damage that coordinates innate immune-driven local response (15). Recently, increased expression of TLR4 on endothelial cells in the outer kidney medulla, implied endothelial TLR4-triggered inflammation through stimulation of endothelial adhesion molecules to allow leukocyte diapedesis from the blood into the injured renal tissue (16). In accordance with the animal data, it has been confirmed that the pathogenesis of I/R injury following kidney transplantation in humans involves signaling through TLR4 expressed in donor kidney cells (17). Our novel findings highlight the role of type I IFN signaling, a MyD88-independent pathway downstream of TLR2 and TLR4, in the pathogenesis of renal I/R injury in mice.
Neutrophils are the major players in the mechanism of renal I/R injury (18). The reperfusion of ischemic transplanted kidney associates with massive neutrophil infiltration and accumulation, predominantly in the outer medulla/cortex (19). The involvement of both renal epithelial cells and bone marrow–derived cells in CXCL2 expression and neutrophil recruitment was recently shown by employing bone marrow TLR4 chimeric mice in uropathogenic E. coli pyelonephritis mouse model (20). However, defining which renal cell express type I IFN is not an easy task. Indeed, although different TLR signaling pathways can induce type I IFN, it remains unclear which renal parenchymal cells are actually involved in the process (21). We observed a marked increase in Ly-6G neutrophil infiltration in the outer medulla of WT kidneys throughout 72h of reperfusion, as compared to controls. Moreover, unlike in WT, kidneys in IFNAR KO mice revealed decreased neutrophil sequestration, along with diminished CXCL2 (MIP-2) levels, the chemokine that attracts PMNs to the inflammation site. Furthermore, MPO, the most abundant protein in neutrophils (9) and a quantitative measure of neutrophil infiltration (10) was increased in WT but depressed in INFAR KO kidneys by 24h of reperfusion. Hence, type I IFN signaling contributes to neutrophil recruitment and function in kidney I/R injury model.
In this study, disruption of IFNAR signaling reduced the number of macrophages sequestered in the kidney and their pro-inflammatory gene expression program, as compared with WT controls. Of the pro-inflammatory cytokines, TNFα, IL-1 and IL-6 were all markedly increased at 5h of reperfusion. TNF-α expression not only increases during the first hours of kidney I/R (22), but may also induce renal cell apoptosis, glomerular endothelial damage, neutrophil infiltration and renal failure (23). IL-1 released during the early reperfusion phase (24) can promote inflammatory and apoptotic processes (25). In a rat model of kidney warm I/R, the use of IL-1 receptor antagonist attenuated inflammatory response and reduced number of local apoptotic cells (26). We observed a significant decrease in TNFα and IL-1β expression in the kidneys at 5h of reperfusion, compared to WT controls. The urinary excretion of IL-6 is a known predictor for acute kidney injury in transplant recipients (27). In fact, IL-6 KO mice are resistant to acute ischemic renal injury (28,29). In the former study (26), WT mice showed increased production of macrophage-derived IL-6 in the ischemic kidney, whereas reconstitution of IL-6 KO mice with WT bone marrow reduced the resistance of IL-6 KO mice to ischemic injury. In the latter study (29), IL-6 Ab pretreatment reduced renal ischemic injury in WT mice. In our series, IL-6 mRNA levels were uniformly increased in WT at 5h, compared with sham-operated controls. Furthermore, IFNAR KO mice had significantly decreased IL-6 expression in the kidneys at 5h and 24h of reperfusion, compared with WT.
Although type I IFNs have been applied therapeutically to treat cancers, viral infections, and autoimmune diseases (30), the use of high-dose IFN therapy is limited by serious side effects. A common side effect is renal impairment, with analysis of renal tissue revealing tubular cell dysfunction and/or tubular cell death (31). In fact, addition of IFNα induced apoptosis in renal proximal tubular cell cultures, characterized by activation of caspase-3, -8, -9, DNA fragmentation, and nuclear condensation (32). Our TUNEL assay has revealed renal TEC apoptosis in WT kidneys as early as at 5h and peaking at 24h of reperfusion. In marked contrast, IFNAR KO mice showed significantly decreased number of apoptotic TEC as compared to WT mice, suggesting that type I IFN pathway is involved in IR-triggered pro-apoptotic pathway in the ischemic kidney. Thus, disruption of type I IFN signaling may limit the local injury/inflammation leading to a decreased recruitment of neutrophils/macrophages and reduced activation of adaptive immune mechanisms.
The impact of type I IFNs on organ I/R injury might amplify alloreactive T cell effector functions in transplant recipients (33). Type I IFNs enhance expression of MHC class I antigens, increase T-cell cytotoxicity, and NK cell activity, all of which contribute to allograft rejection. In fact, selective and lasting immunosuppression in Cynomologus recipients of allogeneic bone marrow cells was obtained following adjunctive treatment with anti-IFNAR mAb and sub-effective CsA (33). Similarly, treatment with polyclonal IFN α/β Ab markedly prolonged cardiac allograft survival in rat recipients (34). The inhibitors of the mammalian target of rapamycin (mTOR) may also regulate type I IFN production, and the expression of chemokine receptors. Indeed, mTOR activates IFN-regulated factor (IRF)-5 and IRF-7 to enable the production of type I IFNs (35). mTOR signaling was also shown crucial in TLR-mediated IFN-α/β responses by plasmocytoid DCs (36).
In summary, renal I/R injury triggers type I IFN signaling to activate local leukocytes, which in turn have downstream effect on later inflammation and organ dysfunction. Disruption of the pathway protects renal function by limiting local injury/inflammation, leading to decreased recruitment of neutrophils/macrophages, attenuation of local apoptosis, and reduced activation of adaptive immune mechanisms. This study provides evidence for a novel mechanism by which type I IFN signaling affects innate immunity-driven inflammation during the course of renal I/R injury. Indeed, type I IFN may serve as a novel target for the therapy against renal ischemia/reperfusion injury.
Material and Methods
Animals
Male wild-type (WT; C57BL/6) mice (8-12 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME); mice deficient in type I IFN receptor (IFNAR; C57BL/6) were bred at UCLA. Animals were housed in the UCLA facility under specific pathogen-free conditions, and received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of sciences and published by NIH (publication 86-23 revised 1985).
Renal I/R injury model
We used an established mouse model of “warm” renal I/R injury (37). Briefly, mice were anesthetized with pentobarbital sodium (50mg/kg i.p.), injected with heparin (100U/kg), a midline incision was made, and using an atraumatic clamp (Roboz Surgical Instrument Co., Inc., Gaithersburg, MD, USA) both renal pedicles were cross-clamped. To maintain fluid balance mice received 1 ml of sterile 0.9% NaCl i.p. After 45min of warm ischemia, clamps were removed initiating renal reperfusion. Mice were sacrificed at pre-determined time points (5-24-72h) after reperfusion for serum/kidney sampling. Sham WT controls underwent the same procedure but without vascular occlusion.
Assessment of renal function
Serum creatinine (sCr) and blood urea nitrogen (BUN) levels were measured after kidney reperfusion, using an auto analyzer (ANTECH Diagnostics, Los Angeles, CA).
Histopathology
Kidney specimens were fixed in a 10% buffered formalin solution, embedded in paraffin, and sections (5-μm) were stained with hematoxylin and eosin (H&E). The histopathology scoring was performed blindly by a renal pathologist to evaluate the degree of tubular injury based on the percentage of damaged tubules in the outer medulla, using a scale from 0 to 4: 0 = <10%; 1 = moderate, 10-25%; 2 = severe, 25 to 50%; 3 = very severe, 50-75%; 4 = extensive damage, >75%, as described (38). The tubular injury score was calculated according to the criteria: tubular dilatation, cast deposition, brush border loss, and necrosis in 10 randomly chosen, non overlapping fields (×400 magnification).
Immunohistochemistry
Kidney specimens were embedded in optimal cutting temperature (OCT) compound (Tissue-Tec, Sakura Finetek, Inc, CA), snap frozen, and cryostat sections (5μm) were fixed in acetone. Endogenous peroxidase activity was inhibited with 0.3% hydrogen peroxidase. Sections were then blocked with 10% normal goat serum. Primary rat Abs (BD Biosciences) against mouse neutrophil Ly-6G (1A8), macrophage CD11b (Mac-1, M/70), and T CD3 (17A2) were diluted (1/50; 1/200; 1/50 in 3% normal goat serum, respectively), and 100μL was added to each section. The primary Ab was incubated for 1h. The secondary Ab, a biotinylated goat anti-rat IgG (Vector; diluted 1:200), was incubated for 40min. Sections were then mixed incubated with immunoperoxidase (ABC Kit, Vector), washed, and developed with a 3,3′ -diaminobenzidine kit (Vector). Slides were counterstained with hematoxylin. Negative control was prepared by omission of primary Ab. Sections were evaluated blindly by counting labeled cells in 10 high-power fields (HPFs), and results expressed as average number of positive cells/HPF.
Myeloperoxidase activity assay
Myeloperoxidase (MPO) activity was evaluated as a quantitative measure of neutrophil infiltration (10). Frozen tissue was homogenized in an iced solution of 0.5% hexadecyltrimethyl-ammonium (Sigma, St. Louis, MO) and 50mmol/L of potassium phosphate buffer solution (Sigma) with the pH adjusted to 5.0. The quantity of enzyme degrading in 1 μmol/L of peroxide per minute at 25°C per gram of tissue was defined as 1 U of MPO activity.
Quantitative RT-PCR
RNA was extracted from liver tissue or cultured cells using Trizol Reagent (Invitrogen, Carlsbad, CA). Total RNA (5μg) was reverse transcribed to complementary DNA using Super-ScriptTM III First-Strand Synthesis System (Invitrogen). Quantitative PCR was performed using DNA Engine with Chromo 4Detector (MJ Research, Waltham, MA). In a final reaction of 20μL, the following were added: 1×SuperMix (Platinum SYBRGreen qPCR Kit, Invitrogen, Carlsbad, CA), complementary DNA, and 0.1μM of each primer. Amplification conditions were: 50°C (2min), 95°C (5min), followed by 45 cycles of 95°C (15sec), 60°C (30sec). Primers used to amplify specific mouse gene fragments have been published (39,40). Target gene expressions were calculated by their ratios to the housekeeping gene HPRT.
TUNEL assay
Kidneys sections were fixed in 10% formalin, dehydrated, and embedded in paraffin. FragEL™ DNA Fragmentation Detection kit (Calbiochem®) was used on 5-μm paraffin sections. TUNEL-positive cells were detected under light microscopy. Terminal Deoxynucleotidyl Transferase (Tdt) was omitted as a negative control. Positive controls were generated by treatment with DNase 1. Sections were evaluated blindly by counting labeled cells in triplicates in 10 HPFs; results are expressed as average number of positive cells/HPF
Statistical analysis
All values are expressed as mean ± standard deviation (SD). Data were analyzed with an unpaired two-tailed Student t-test. P<0.05 was considered statistically significant.
Acknowledgments
Grant support: NIH RO1 DK062357 and The Dumont Research Foundation. M.C.S.F. was supported by CAPES (Coordencao de Aperfeicoamento de Pessoal de Nivel Superior) and a Fellowship Grant from ISN (International Society of Nephrology).
Abbreviations
- ATN
acute tubular necrosis
- BUN
blood urea nitrogen
- HPF
high-power field
- IFN
interferon
- IFNAR
type I IFN receptor
- I/R
Ischemia/reperfusion
- IRF3
interferon-regulatory factor 3
- ROS
reactive oxygen species
- sCr
serum creatinine
- TEC
tubular epithelial cells
- TLR
Toll-like receptor
- TUNEL
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
Footnotes
Authors’ contribution: M.C.S.F. participated in research design, performance of the research, data anaysis and writing the manuscript; Y.U. performed microsurgery procedures and participated in the data anaysis; C.L. participated in the data anaysis; G.M.D. participated in discussion and provided partial funding; R.W.B. participated in discussion and provided partial funding; J.W.K.-W. participated in experimental design, writing manuscript and sponsored the project.
The authors declare no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rouschop KMA, Leemans JC. Ischemia–reperfusion treatment: opportunities point to modulation of the inflammatory response. Kidney Int. 2008;73:1364–1373. doi: 10.1038/ki.2008.156. [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Chapman JR. Chronic allograft nephropathy: current concepts and future directions. Transplantation. 2006;81:643–654. doi: 10.1097/01.tp.0000190423.82154.01. [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 4.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 5.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colamonici OR, Domanski P. Identification of a novel subunit of the Type I interferon receptor localized to chromosome 21. J. Biol. Chem. 1994;268:10895–10899. [PubMed] [Google Scholar]
- 7.Decker T, Müller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 8.Zhai Y, Qiao B, Gao F, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff SJ. Myeloperoxidase. Proc Assoc Am Physicians. 1999;111:383–389. doi: 10.1111/paa.1999.111.5.383. [DOI] [PubMed] [Google Scholar]
- 10.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 11.Leemans JC, Stokman G, Claessen N, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai Y, Shen XD, O’Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 13.Shigeoka AA, Holscher TD, King AJ, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and - independent pathways. J Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulskens WP, Teske GJ, Butter LM, et al. Toll-Like receptor-4 coordinates the Innate Immune Response of the Kidney to Renal Ischemia/Reperfusion Injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, John R, Richardson JA, et al. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011;79(3):288–299. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krüger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002;90:133–138. doi: 10.1159/000049032. [DOI] [PubMed] [Google Scholar]
- 19.Willinger CC, Schramek H, Pfaller K, et al. Tissue distribution of neutrophils in postischemic acute renal failure. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:237–243. doi: 10.1007/BF02899687. [DOI] [PubMed] [Google Scholar]
- 20.Patole PS, Schubert S, Hildinger K, et al. Toll-like receptor-4: renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 2005;68:2582–2587. doi: 10.1111/j.1523-1755.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 21.Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77:848–854. doi: 10.1038/ki.2010.71. [DOI] [PubMed] [Google Scholar]
- 22.Donnahoo KK, Meldrum DR, Shenkar R, et al. Early renal ischemia, with or without reperfusion, activates NFkappaB and increases TNF-alpha bioactivity in the kidney. J Urol. 2000;163:1328–1332. [PubMed] [Google Scholar]
- 23.Donnahoo KK, Meng X, Ayala A, et al. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999;277:R922–929. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- 24.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 25.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 26.Rusai K, Huang H, Sayed N, et al. Administration of interleukin-1 receptor antagonist ameliorates renal ischemia-reperfusion injury. Transpl Int. 2008;21:572–580. doi: 10.1111/j.1432-2277.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 27.Kwon O, Molitoris BA, Pescovitz M, et al. Urinary actin, interleukin-6, and interleukin-8 may predict sustained ARF after ischemic injury in renal allografts. Am J Kidney Dis. 2003;41:1074–1087. doi: 10.1016/s0272-6386(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 28.Song M, Kellum JA. Interleukin-6. Crit Care Med. 2005;33:S463–S465. doi: 10.1097/01.ccm.0000186784.62662.a1. [DOI] [PubMed] [Google Scholar]
- 29.Pittock ST, Norby SM, Grande JP, et al. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int. 2005;68:611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 30.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804–817. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Lechner J, Malloth N, Seppi T, et al. IFN-alpha induces barrier destabilization and apoptosis in renal proximal tubular epithelium. Am J Physiol Cell Physiol. 2008;294:C153–160. doi: 10.1152/ajpcell.00120.2007. [DOI] [PubMed] [Google Scholar]
- 33.Tovey MG, Benizri E, Gugenheim J, et al. Role of the type I interferons in allograft rejection. J Leukoc Biol. 1996;59(4):512–517. doi: 10.1002/jlb.59.4.512. [DOI] [PubMed] [Google Scholar]
- 34.Gugenheim J, Tovey M, Gigou M, et al. Prolongation of heart allograft survival in rats by interferon-specific antibodies and low dose cyclosporin A. Transplant int. 1992;5:460–465. doi: 10.1007/978-3-642-77423-2_134. [DOI] [PubMed] [Google Scholar]
- 35.Säemann MD, Haidinger M, Hecking M, Hörl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9(12):2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 36.Cao W, Manicassamy S, Tang H, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9(10):1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly KJ, Williams WW, Colvin RB, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savransky V, Molls RR, Burne-Taney M, et al. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 2006;69:233–238. doi: 10.1038/sj.ki.5000038. [DOI] [PubMed] [Google Scholar]
- 39.Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1671. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida Y, Freitas MC, Zhao D, et al. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2009;15:939–947. doi: 10.1002/lt.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]