Abstract
Concerned about the safety of conventional estrogen replacement therapy, women are using botanical dietary supplements as alternatives for the management of menopausal symptoms such as hot flashes. Before botanical dietary supplements can be evaluated clinically for safety and efficacy, botanically authenticated and standardized forms are required. To address the demand for a standardized, estrogenic botanical dietary supplement, an extract of hops (Humulus lupulus, L.) was developed. Although valued in the brewing of beer, hop extracts are used as anxiolytics and hypnotics and have well established estrogenic constituents. Starting with a hop cultivar used in the brewing industry, spent hops (the residue remaining after extraction of bitter acids) were formulated into a botanical dietary supplement that was then chemically and biologically standardized. Biological standardization utilized the estrogen dependent induction of alkaline phosphatase in the Ishikawa cell line. Chemical standardization was based on the prenylated phenols in hops that included estrogenic 8-prenylnaringenin (8-PN), its isomer 6-prenylnaringenin (6-PN), and pro-estrogenic isoxanthohumol (IX) and its isomeric chalcone xanthohumol (XN), all of which were measured using high performance liquid chromatography-tandem mass spectrometry (LC/MS-MS). The product of this process was a reproducible botanical extract suitable for subsequent investigations of safety and efficacy.
Keywords: Hops, botanical dietary supplements, standardization, 8-prenylnaringenin, xanthohumol, estrogen replacement, LC/MS-MS, Ishikawa cells
Introduction
Between 1995 and 2006, U.S. sales of botanical dietary supplements increased by 86%, and by 2012, the U.S. market for these supplements was estimated at $5.5 billion with a world-wide market expected to reach $93 billion by 2015 (Lindstrom, et al., 2013; Dennis, 2013; Cavaliere, et al., 2008). Unlike pharmaceuticals that must be chemically standardized and evaluated for efficacy, botanical dietary supplements in the U.S. market need only be prepared using good manufacturing practice (FDA, 2013). Although good manufacturing practice can protect consumers by ensuring botanical authentication and by minimizing contamination and adulteration, there is still no FDA requirement for standardization or testing for efficacy.
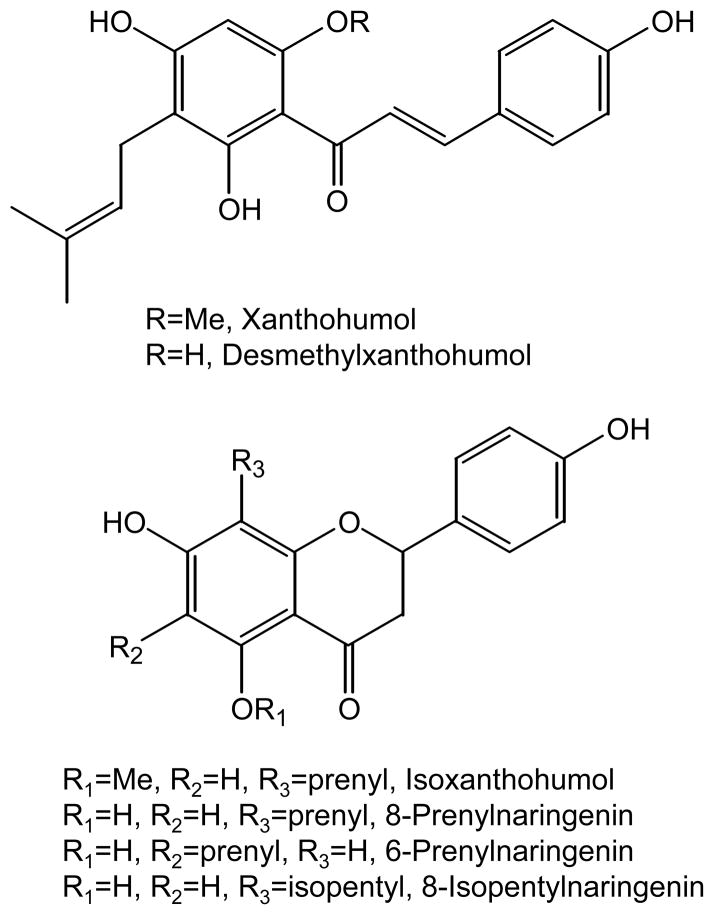
As part of our on-going investigations of the safety and efficacy of botanical dietary supplements used by menopausal women as alternatives to estrogen replacement therapy, we have been evaluating preparations of Humulus lupulus L. (hops) (Piersen, et al., 2004; van Breemen, et al., 2010). Used traditionally in the brewing of beer for taste and aroma as well as a preservative, hop preparations also are used as dietary supplements for their hypnotic and anxiolytic properties, and recently, for the management of menopausal hot flashes in women (Heyerick, et al., 2006). Hop preparations were prescribed by physicians as early as the 1930s for women’s menstrual complaints. By that time, the structure of estrogen had already been identified, but the first estrogen replacement product, Premarin, would not be approved by the FDA for the treatment of hot flashes until 1942. Many years later, the estrogenic constituent of hops was identified as 8-prenylnaringenin (8-PN) and its isomer 6-prenylnaringenin (6-PN) as well as precursors xanthohumol (XH), isoxanthohumol (IX) and desmethylxanthohumol (DMX) (Figure 1) were known (Stevens and Page, 2004; Overk, et al., 2005).
Figure 1.
Chemical structures of hop prenylated phenols and the internal standard, 8-isopentylnaringenin.
Among botanical estrogens, 8-PN shows the highest affinity to the estrogen receptor (ER) with similar binding affinities to both ERα and ERβ that are approximately one-tenth as strong as that of the natural ligand 17β-estradiol (Overk, et al., 2005; Milligan, et al., 1999; Schaefer, et al., 2003). The structurally similar prenylated flavonols 6-PN and IX (Figure 1) are less estrogenic than 8-PN. Although 8-PN is much less abundant in hop products than 6-PN, IX, XN, and DMX, XN can cyclize in vivo to form IX which can then be metabolized in the liver or by intestinal microbiota to form 8-PN (Guo, et al., 2006; Possemiers, et al., 2008). 8-PN has the chemoprevention property of blocking estrogen oxidative metabolism, and XN induces the antioxidant response element which codes for cytoprotective enzymes such as quinone reductase-1 (NQO1) (Hemachandra, et al., 2012; Dietz, et al., 2005; Dietz, et al., 2013; Liu, et al., 2005). The chalcone DMXis unstable and readily cyclizes to form 8-PN or 6-PN. Therefore, 8-PN, 6-PN, IX, and XN but not DMX are appropriate active constituents for the chemical standardization of hop dietary supplements for use by menopausal women.
When investigating the pharmacological properties of complex botanical dietary supplements during animal studies or clinical trials, the plant material should be botanically authenticated and then chemically and biologically standardized to ensure reproducibility (Farnsworth, et al., 2008). Standardization is essential for the evaluation of botanical dietary supplements for safety and efficacy. This paper describes the research process of developing a hop dietary supplement suitable for use during in vivo studies. This process begins with the acquisition of raw material and extends to the formulation of a biologically and chemically standardized extract.
Materials and Methods
Materials
Bitter acids and essential oils are often extracted from hop cones for use in beer using supercritical fluid carbon dioxide. The residual material is known as spent hops. Various spent hop products from Hopsteiner (New York, NY, USA) were assayed for prenylated flavonoids using liquid chromatography-tandem mass spectrometry (LC/MS-MS) as described below. The spent hops found to be highest in prenylated flavonoids were extracted with ethanol using good manufacturing practices by Hopsteiner. The resulting extract was provided as a dry, pale yellow powder. For comparison, 35 New Mexican Humulus lupulus cultivars were acquired from New Mexico Native Plant Recyclers (Embudo, NM, USA) and extracted using methanol.
Prenylated phenols for use as standards during quantitative analysis were prepared from hops. XN was isolated from hops as described previously, and the purity was >99.5% by quantitative proton NMR (Chadwick, et al., 2004). IX (>99% pure by qNMR) was prepared by cyclization of XN as described previously. 8-PN (purity >95% by HPLC) was chemically synthesized, and 6-PN (purity >95% by HPLC) was purified as previously reported (Chadwick, et al., 2004; Overk, et al., 2008).
All solvents were HPLC grade and were purchased from Thermo Fisher (Fair Lawn, NJ, USA). Purified water was prepared by using a Millipore Milli-Q purification system (Millipore, Billerica, MA, USA). All other chemicals were ACS reagent grade.
Biological Standardization
The Ishikawa cell line was used for the biological standardization. Ishikawa cells were provided by D. R. B. Hochberg (Yale University, New Haven, CT, USA) and were maintained in Dulbecco’s Modified Eagle Medium (DMEM/F12) containing 1% sodium pyruvate, 1% nonessential amino acids, 1% glutamax-1, 0.05% insulin, and 10% heat-inactivated fetal bovine serum (Hajirahimkhan, et al., 2013). The Ishikawa cell line is a well-established ERα (+) endometrial cancer cell line for the evaluation of estrogens and antiestrogens (Littlefield, et al., 1990). Two days before treating the cells, the medium was replaced with phenol red-free DMEM/F12 medium containing charcoal/dextran-stripped FBS and supplements. Authentication of this cell line, via determination of the short tandem repeat profile, revealed its similarity with the Ishikawa cells according to the Health Protection Agency Culture Collection in the UK (HPA, Porton Down, UK) and was recently confirmed by the American Tissue Culture Collection, ATCC database (Manassas, VA, USA).
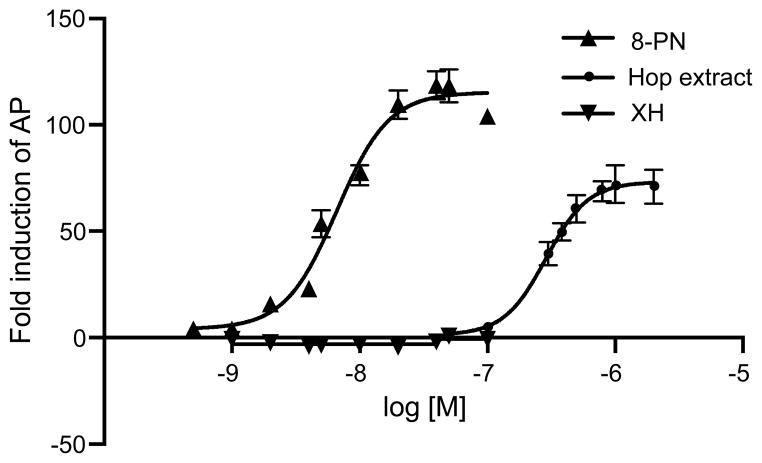
The estrogenic potential of the hop extract was biologically evaluated by measuring the activity of an estrogen responsive alkaline phosphatase (AP) enzyme in the Ishikawa cell line as reported previously (Overk, et al., 2005). 17β-Estradiol was used as a positive control for estrogenicity, and 4-hydroxytamoxifen was used to determine antiestrogenic activity. The relative AP activity of the hop extract was measured, as were those of isolated XH and 8-PN. The values were expressed as the mean ± standard deviation of at least three independent determinations.
Chemical Standardization
Standards of IX, XN, 8-PN, and 6-PN were dissolved in methanol at a concentration of 1 mg/mL each to form a stock solution, which was diluted to form calibration solutions of 950, 600, 450, 300, 150, 10, and 5 ng/mL using 50% aqueous methanol. Quality control (QC) samples were prepared similarly to the calibration standards at low (30 ng/mL), medium (200 ng/mL) and high (800 ng/mL) concentrations. A structural analogue of 8-PN not occurring in hops, 8-isopentylnaringenin (Figure 1), was spiked into each calibration solution, each QC sample, and each unknown to yield a final concentration of 60 ng/mL for use as an internal standard. A stock solution of the hop extract (256 μg/mL) was prepared in methanol and diluted with 50% aqueous methanol to 0.64, 3.2, 6.4, 12.8, and 25.6 μg/mL for analysis using liquid chromatography-tandem mass spectrometry (LC/MS-MS).
An Applied Biosystems (Foster City, CA, USA) API 4000 triple quadrupole mass spectrometer interfaced with a Shimadzu (Kyoto, Japan) LC-20AD HPLC system was used for sample analysis. A Waters (Milford, MA, USA) reversed-phase C18 column YM-AQ (3.5 μm, 2.1 × 100 mm) was used for the chromatographic separations with a solvent system consisting of a 10-min linear gradient from 35–90% acetonitrile in water (both solvents containing 0.1% formic acid). The mobile phase flow rate was 0.25 mL/min, and the column was equilibrated for 10 min between analyses. The column oven temperature was 35°C, and the injection volume was 10 μL.
Negative ion electrospray was used with an ion source temperature of 350°C, spray voltage of −4200 V, nitrogen curtain gas flow rate of 10 L/min, a nitrogen gas I flow rite of 20 L/min, a declustering potential of −66 V, and an exit potential of −10 V.
Nitrogen was also used as the collision gas at a collision energy of 31 eV for tandem mass spectrometry. Selected reaction monitoring (SRM) of two transitions (quantifier and qualifier) were used for each analyte as follows: m/z 353 to m/z 119 (quantifier) and m/z 353 to m/z 233 (qualifier) for IX and XN; and m/z 339 to m/z 119 (quantifier) and m/z 339 to m/z 219 (qualifier) for 8-PN and 6-PN. The SRM transition of m/z 341 to m/z 119 was monitored for the internal standard, 8-isopentylnaringenin. The ions of m/z 353 and m/z 339 are the deprotonated molecules of isomeric XN and IX and of isomeric 8-PN and 6-PN, respectively. The product ion of m/z 119 is common to all these compounds and corresponds to the B-ring after retro-Diels-Alder fragmentation (Nikolic and van Breemen, 2013). The ions of m/z 233 and m/z 219 correspond to the product ions with the negative charge retained on the A-rings of XN or IX and 8-PN or 6-PN, respectively, following retro-Diels-Alder fragmentation (Nikolic and van Breemen, 2013). The SRM dwell time was 150 ms/ion. Calibration curves were constructed over the range of 5 – 950 ng/mL for all analytes, with analyte to internal peak area ratio on the y-axis and concentration on the x-axis. A weighting factor of 1/x was applied to the calibration curves.
Results
Biological Standardization
The AP activity in Ishikawa cells indicated that the hop extract prepared from spent hops showed greater estrogenicity than the hop cultivars from New Mexico. Although extracts of all of the 35 hop cultivars from New Mexico were active, all subsequent studies were carried out using the extract of spent hops. Based on comparisons with isolated compounds, most of the activity of the spent hop extract could be attributed to the known phytoestrogen 8-PN (Table I and Figure 2). 8-PN yielded the highest AP induction rate relative to estradiol, IX exhibited some estrogenic properties, and the chalcone xanthohumol, as well as 6-PN, had no estrogenic activity in the AP induction assay. Although containing 8-PN (see Chemical Standardization below), the hop extract could only induce 52% as much AP activity as 17β-estradiol, whereas 8-PN could maximally induce AP 86% as effectively as 17β-estradiol (Table 1). This indicated that the hop extract was only a partial agonist of the estrogen receptor whereas 8-PN was nearly a full agonist.
Table 1.
Estrogenicities of the hop extract and its constituents, 8-PN, 6-PN, XH, and IX, determined using an alkaline phosphatase (AP) induction assay in Ishikawa cells.
| Compounds/Extract | AP induction Ishikawa cells EC50 | Maximum AP fold induction Ishikawa cells |
|---|---|---|
| hop extract [ng/mL] a | 296.6 ± 8.1 | 72 ± 8.8 |
| 8-PN [nM] | 6.65 ± 1.40 b | 118.0 ± 6.0 b |
| 6-PN | Not activec | Not activec |
| XH | Not active | Not active |
| IX [nM] | 634 ± 175 | 76.8 ± 6.4 |
| 17β-estradiol [nM] | 0.19 ± 0.05 b | 137.0 ± 2.5 b |
Values are expressed as the mean ± SD of at least three independent determinations. Estradiol (0.5 nM) was used as a positive control, and its AP induction was defined as 100-fold.
From Overk, et al. (2005).
Figure 2.
Alkaline phosphatase (AP) induction in Ishikawa cells after 96 h incubation with the hop extract. AP induction by 17β-estradiol, 8-PN and XH are shown for comparison.29 The data are averages of at least three independent determinations carried out in triplicate ± SD.
Chemical Standardization
Since the molecular masses and tandem mass spectra were nearly identical for the isomeric pair XN/IX and for the isomers 6-PN/8-PN (Yuan, et al., 2012), chromatographic separation was essential for their accurate quantitative analysis. All analytes and the internal standard (retention time 9.8 min) were chromatographically resolved to base line within 12 min (Figure 3). Isomeric IX and XN were separated by more than 4 min; 8-PN (retention time 9.1 min) and 6-PN (10.7 min) were resolved from each other from their unstable chalcone precursor DMX, which eluted at 10.1 min (Figure 3).
Figure 3.
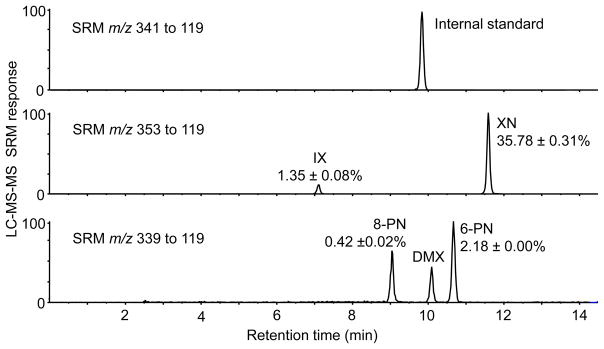
LC/MS-MS chromatograms of an ethanolic extract of spent hops obtained using negative ion electrospray, collision-induced dissociation and selected reaction monitoring (SRM).
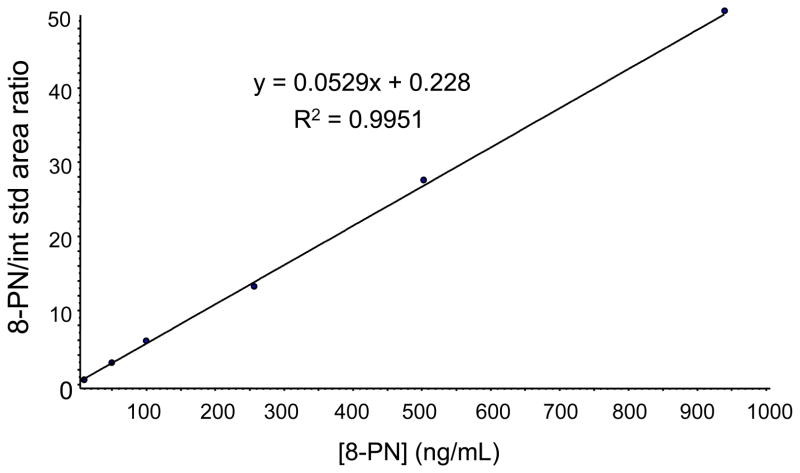
The calibration curves for all 4 prenylated phenols were linear from 10 ng/mL to 950 ng/mL for all 4 analytes. For example, the calibration curve for 8-PN is shown in Figure 4. The calibration curve for each analyte showed a good coefficient of determination (R2) >0.99 with less than ±15% bias. The LC/MS-MS assay showed excellent accuracy with bias <15% for the QC samples evaluated at low (30 ng/mL), medium (200 ng/mL) and high concentration (800 ng/mL) (see accuracy and precision in Table II).
Figure 4.
Calibration curve for the most estrogenic hop flavonoid, 8-PN, obtained using LC/MS-MS.
Table II.
Accuracy and precision of the LC/MS-MS quantitative analysis assay of 8-PN, 6-PN, XN, and IX (N=3)
| Analyte | Nominal value (ng/mL) | Measured value (ng/mL) | CV% | Accuracy |
|---|---|---|---|---|
| 8-PN | ||||
| Low | 30 | 33.2 | 8.2 | 110 |
| Med | 200 | 215 | 7.5 | 107 |
| High | 800 | 831 | 9.7 | 104 |
| 6-PN | ||||
| Low | 30 | 31.1 | 11.5 | 103 |
| Med | 200 | 204 | 8.4 | 102 |
| High | 800 | 758 | 7.3 | 95 |
| IX | ||||
| Low | 30 | 28.5 | 14.5 | 95 |
| Med | 200 | 186 | 5.8 | 93 |
| High | 800 | 842 | 6.7 | 105 |
| XN | ||||
| Low | 30 | 26.4 | 8.4 | 88 |
| Med | 200 | 224 | 7.2 | 112 |
| High | 800 | 830 | 11.1 | 103 |
The hop extract was tested using LC/MS-MS at 5 concentrations to ensure that each analyte was detected on-scale relative to the standard curves. Expressed as weight/percent of the spent hop extract, the levels of each analyte in the standardized extract were determined to be as follows: 8-PN, 0.42 ± 0.02; 6-PN, 2.18 ± 0.0; IX, 1.35 ± 0.08; and XN, 35.78 ± 0.31.
Discussion
Since the late 1990s, there has been growing consumer interest in natural products intended to maintain health and well-being which is reflected in yearly increases in the sales of botanical dietary supplements. By the early 2000s, women and many health care providers were seeking alternatives to conventional estrogen replacement therapy to address hot flashes and other somatic complaints accompanying the onset of menopause. This interest in non-steroidal alternatives became acute as reports from the Women’s Health Initiative (WHI) challenged long-held beliefs about the benefits of hormone therapy (Rossouw, et al., 2002; Anderson, et al., 2004). Since those results were first published, many of the conclusions drawn from the initial WHI trial analysis have withstood further analyses (Manson, et al., 2013), and women continue to seek alternatives to hormone therapy.
To meet the demand for safe and effective alternatives to conventional estrogen replacement therapy, natural products investigators have focused their research on botanicals. In particular, there has been renewed interest in the phytoestrogen properties of hops that were first recognized by the medical establishment in the 1930s. Since hop production is well established for the brewing industry, botanically authenticated hop material is readily available from fields that are cultivated using good agricultural practice. This is not always the case for botanicals used in dietary supplements that must sometimes be collected from the wild with widely varying levels of chemical constituents. Although still subject to environmental pressure, as a cultivated crop hops have a much more predictable chemical constituent profile. This is especially true for those hop cultivars that have been selected to satisfy specific standards for beer processing. Such plant predictability is important for achieving a consistent end product – a botanical dietary supplement in which targeted compounds are standardized to consistent levels of active constituents and biological activity.
There have been several small human trials of hop extracts examining the benefits of hops for hot flashes (Heyerick, et al., 2006; Bolca, et al., 2010; Erkkola, et al, 2010). In addition, several pharmacokinetics investigations of constituents from hops have been reported including studies of XN (Legette, et al., 2013) and 8-PN (Rad, et al. 2006). Currently, several hop botanical dietary supplements are being marketed for the relief of menopausal symptoms either as a single botanical or in combination with other botanicals.
Although estrogenic, 8-PN has only a small fraction of the estrogenic potency of 17β-estradiol (Table I). Previous clinical studies of hop dietary supplements used extracts containing 8-PN doses of 0.1 mg to 0.25 mg per day (Heyerick, et al., 2006; Bolca, et al., 2010; Erkkola, et al., 2010). In a single-dose clinical study of synthetic 8-PN, women received far greater doses, of 50 mg, 250 mg or 750 mg, and exhibited only mild adverse effects (Rad, et al., 2006). Even if administered at lower amounts in a hop extract containing XN and IX, 8-PN can be formed from IX by O-demethylation, and IX can be formed from XN via intramolecular cyclization, all of which might prolong in vivo exposure to 8-PN and its estrogenic activity (Nikolic, et al., 2005). The alkaline phosphatase bioassay showed weak estrogenic activity for IX, which is consistent with the moderate estrogenic properties that have been reported previously (Gerhauser, et al., 2002). However, future studies should investigate whether the observed estrogenic activity of IX in Ishikawa cells (Table 1) is due to metabolism of IX to 8-PN, or whether IX itself has moderate estrogenic properties in this bioassay. A follow-on paper will report on the UIC Botanical Center’s hops Phase I clinical trial to determine the safe dosing levels of this frequently consumed botanical.
To assess the safety and efficacy of botanical dietary supplements for human use, standardized and reproducible products are required. For the management of menopausal symptoms such as hot flashes, standardization to estrogenic activity is an appropriate approach. This paper describes both biological and chemical standardization of a hop product for subsequent evaluation in vivo, whether in preclinical animal models or in clinical trials of safety and efficacy.
Acknowledgments
This publication was supported by grants P50 AT000155 and P50 AT000155-12S1 from the Office of Dietary Supplements (ODS) and the National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (USA). We thank Harald Schwarz and Martin Bindel of Hopsteiner (New York, NY, USA) for their consultation and for providing the spent hop extract.
References
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Bolca S, Li J, Nikolic D, Roche N, Blondeel P, Possemiers S, De Keukeleire D, Bracke M, Heyerick A, van Breemen RB, Depypere H. Disposition of hop prenylflavonoids in human breast tissue. Mol Nutr Food Res. 2010;54(Suppl 2):S284–S294. doi: 10.1002/mnfr.200900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere C, Rea P, Blumenthal M. Herbal Supplement Sales in United States Show Growth in All Channels. HerbalGram. 2008;78:60–63. [Google Scholar]
- Chadwick LR, Nikolic D, Burdette JE, Overk CR, Bolton JL, van Breemen RB, Frohlich R, Fong HH, Farnsworth NR, Pauli GF. Estrogens and congeners from spent hops (Humulus lupulus) J Nat Prod. 2004;67:2024–2032. doi: 10.1021/np049783i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J. [accessed November 26, 2013];International perspectives on herbs and botanicals. http://www.nutraceuticalsworld.com/issues/2013-07/view_features/international-perspectives-on-herbs-botanicals/
- Dietz BM, Kang YH, Liu G, Eggler AL, Yao P, Chadwick LR, Pauli GF, Farnsworth NR, Mesecar AD, van Breemen RB, Bolton JL. Xanthohumol isolated from Humulus lupulus Inhibits menadione-induced DNA damage through induction of quinone reductase. Chem Res Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz BM, Hagos GK, Eskra JN, Wijewickrama GT, Anderson JR, Nikolic D, Guo J, Wright B, Chen SN, Pauli GF, van Breemen RB, Bolton JL. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol Nutr Food Res. 2013;57:1055–1066. doi: 10.1002/mnfr.201200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkola R, Vervarcke S, Vansteelandt S, Rompotti P, De Keukeleire D, Heyerick A. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine. 2010;17:389–396. doi: 10.1016/j.phymed.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Farnsworth NR, Krause EC, Bolton JL, Pauli GF, van Breemen RB, Graham JG. The University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements Research for Women’s Health: from plant to clinical use. Am J Clin Nutr. 2008;87:504s–508s. doi: 10.1093/ajcn/87.2.504S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. [accessed September 30, 2013];Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements. http://www.fda.gov/ohrms/dockets/98fr/cf0441.pdf. [PubMed]
- Gerhauser C, Alt A, Heiss E, Gamal-Eldeen A, Klimo K, Knauft J, Neumann I, Scherf HR, Frank N, Bartsch H, Becker H. Cancer chemopreventive activity of xanthohumol a natural product derived from hop. Mol Cancer Ther. 2002;1:959–969. [PubMed] [Google Scholar]
- Guo J, Nikolic D, Chadwick LR, Pauli GF, van Breemen RB. Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.) Drug Metab Dispos. 2006;34:1152–1159. doi: 10.1124/dmd.105.008250. [DOI] [PubMed] [Google Scholar]
- Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, Chen SN, Nikolić D, Dietz BM, Pauli GF, van Breemen RB, Bolton JL. PLoS One. 2013 Jul 12;8(7):e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyerick A, Vervarcke S, Depypere H, Bracke M, De Keukeleire D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54:164–175. doi: 10.1016/j.maturitas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Hemachandra LP, Madhubhani P, Chandrasena R, Esala P, Chen SN, Main M, Lankin DC, Scism RA, Dietz BM, Pauli GF, Thatcher GR, Bolton JL. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A) Cancer Prev Res (Phila) 2012;5:73–81. doi: 10.1158/1940-6207.CAPR-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legette L, Karnpracha C, Reed RL, Choi J, Bobe G, Christensen JM, Rodriguez-Proteau R, Purnell JQ, Stevens JF. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoids from hops. Mol Nutr Food Res. 2013 Aug 27; doi: 10.1002/mnfr.201300333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom A, Ooyen C, Lynch ME, Blumenthal M. Herb supplement sales increase 5. 5% in 2012. Herbalgram. 2013;99:60–65. [Google Scholar]
- Littlefield BA, Gurpide E, Markiewicz L, McKinley B, Hochberg RB. A simple and sensitive microtiter plate estrogen bioassay based on stimulation of alkaline phosphatase in Ishikawa cells: estrogenic action of delta 5 adrenal steroids. Endocrinology. 1990;127:2757–2762. doi: 10.1210/endo-127-6-2757. [DOI] [PubMed] [Google Scholar]
- Liu G, Eggler AL, Dietz BM, Mesecar AD, Bolton JL, Pezzuto JM, van Breemen RB. Screening method for the discovery of potential cancer chemoprevention agents based on mass spectrometric detection of alkylated Keap1. Anal Chem. 2005;77:6407–6414. doi: 10.1021/ac050892r. [DOI] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, Lacroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan SR, Kalita JC, Heyerick A, Rong H, De Cooman L, De Keukeleire D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab. 1999;84:2249–2252. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- Nikolic D, van Breemen RB. Analytical methods for quantitation of prenylated flavonoids from hops. Curr Anal Chem. 2013;9:71–85. [PMC free article] [PubMed] [Google Scholar]
- Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J Mass Spectrom. 2005;40:289–299. doi: 10.1002/jms.753. [DOI] [PubMed] [Google Scholar]
- Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, Bolton JL. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense) J Agric Food Chem. 2005;53:6246–6253. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overk CR, Guo J, Chadwick LR, Lantvit DD, Minassi A, Appendino G, Chen SN, Lankin DC, Farnsworth NR, Pauli GF, van Breemen RB, Bolton JL. In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. Chem Biol Interact. 2008;176:30–39. doi: 10.1016/j.cbi.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersen CE, Booth NL, Sun Y, Liang W, Burdette JE, van Breemen RB, Geller SE, Gu C, Banuvar S, Shulman LP, Bolton JL, Farnsworth NR. Chemical and biological characterization and clinical evaluation of botanical dietary supplements: a phase I red clover extract as a model. Curr Med Chem. 2004;11:1361–1374. doi: 10.2174/0929867043365134. [DOI] [PubMed] [Google Scholar]
- Possemiers S, Rabot S, Espin JC, Bruneau A, Philippe C, Gonzalez-Sarrias A, Heyerick A, Tomas-Barberan FA, De Keukeleire D, Verstraete W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J Nutr. 2008;138:1310–1316. doi: 10.1093/jn/138.7.1310. [DOI] [PubMed] [Google Scholar]
- Rad M, Humpel M, Schaefer O, Schoemaker RC, Schleuning WD, Cohen AF, Burggraaf J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br J Clin Pharmacol. 2006;62:288–296. doi: 10.1111/j.1365-2125.2006.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Schaefer O, Humpel M, Fritzemeier KH, Bohlmann R, Schleuning WD. 8-Prenyl naringenin is a potent ERα selective phytoestrogen present in hops and beer. J Steroid Biochem Mol Biol. 2003;84:359–360. doi: 10.1016/s0960-0760(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- van Breemen RB, Liang W, Banuvar S, Shulman LP, Pang Y, Tao Y, Nikolic D, Krock KM, Fabricant DS, Chen SN, Hedayat S, Bolton JL, Pauli GF, Piersen CE, Krause EC, Geller SE, Farnsworth NR. Pharmacokinetics of 23-epi-26-deoxyactein in women after oral administration of a standardized extract of black cohosh. Clin Pharmacol Ther. 2010;87:219–25. doi: 10.1038/clpt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Qiu X, Nikolić D, Dahl JH, van Breemen RB. Method development and validation for ultra-high-pressure LC/MS/MS determination of hop prenylflavonoids in human serum. J AOAC Int. 2012;95:1744–1749. doi: 10.5740/jaoacint.11-542. [DOI] [PMC free article] [PubMed] [Google Scholar]