Abstract
Background
Although 5-fluorouracil (5-FU)-based combination chemotherapy (i.e. FOLFIRINOX) has demonstrated effectiveness against pancreatic cancer, novel therapeutic strategies must be developed to increase the therapeutic window of these cytotoxic agents. Genistein is a soy-derived isoflavone with pleiotropic biologic effects that can enhance the antitumor effect of chemotherapeutic agents.
Methods
To understand how genistein potentiates the antitumor effects of 5-FU, we examined apoptosis and autophagy in MIA PaCa-2 human pancreatic cancer cells or their derived xenografts. Apoptosis was evaluated using DNA fragmentation assays and Western blots of poly (ADP ribose) polymerase and caspase-3. Meanwhile, autophagy was evaluated using Western blots of microtubule-associated protein light chain 3 (LC3)-I/II, fluorescent microscopy observation of green fluorescent protein-LC3B puncta formation, and acidic vesicular organelle formation using acridine orange staining. Tumors from animal treatment studies were examined for apoptosis and autophagy using the TUNEL assay and immunohistochemical staining of LC3B, respectively.
Results
We observed that genistein increased 5-FU-induced cell death through increased apoptosis as well as autophagy. The increased apoptosis and autophagy was accompanied by decreased B-cell lymphoma 2 (bcl-2) and increased beclin-1 protein levels, respectively. Animal treatment studies supported these observations. The combination of 5-FU and genistein significantly decreased final xenograft tumor volume when compared to 5-FU alone by inducing apoptosis as well as autophagy.
Conclusions
Genistein can potentiate the antitumor effect of 5-FU by inducing apoptotic as well as autophagic cell death. These results demonstrate the potential of genistein as an adjuvant therapeutic agent against pancreatic cancer.
Keywords: Pancreatic ductal adenocarcinoma, Apoptosis, Autophagy, Cell death, Genistein, 5-Fluorouracil
Pancreatic ductal adenocarcinoma (PDAC) remains an unsolved health care dilemma in the United States and the world.1 One of the primary treatments of PDAC is chemotherapy, because more than 80% of patients present with unresectable or metastatic disease.2 Recently, FOLFIRINOX, the combination of 5-Fluorouracil (5-FU), leucovorin calcium, oxaliplatin, and irinotecan, has been applied in clinical settings as a first-line treatment for metastatic PDAC.3 While the anti-tumor effect of this regimen is promising, treatment-related toxicity remains a concern.3 5-FU is one of the principle agents responsible for both the therapeutic effect and the toxicity of the regimen.4, 5 Therefore, novel methods are needed to enhance drug effectiveness of 5-FU while limiting further toxicity.
Genistein, a soy-derived isoflavone, exhibits multiple biological effects against various cancers when studied using in vitro and in vivo models.6-10 Several reports have found that genistein can potentiate the antitumor effects of chemotherapeutic agents (e.g., gemcitabine, cisplatin, oxaliplatin) by modulating the apoptotic pathway.6, 7, 11 Furthermore, recent studies demonstrate that genistein stimulates autophagy.12, 13 Autophagy is a degradation process in which cytosolic proteins and organelles are sequestered into auto- phagosomes and degraded by lysosomes.14 Traditionally, autophagy has been considered to be a survival response during stressful conditions in which cancerous cells avoid apoptotic death through lysosomal degradation of damaged organelles.15, 16 Recent evidence, however, suggests that autophagy may also promote cell death through unintended degradation of essential cellular components and excessive self-digestion.17, 18 There is little data, however, on the influence of genistein on 5-FU based treatment of pancreatic cancer cells.
In this report, we describe how genistein modulates 5-FU-induced apoptosis and autophagy in human pancreatic cancer cells. Our results suggest that genistein potentiates the anti-cancer effects of 5-FU by promoting both apoptotic and autophagic cell death.
MATERIALS AND METHODS
2.1. Cell lines and reagents
The MIA PaCa-2 human pancreatic cancer cell line was obtained from American Type Culture Collection (ATCC) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were incubated in a 5% CO2 incubator at 37°C.
Genistein, 5-FU, acridine orange, thiazolyl blue tetrazolium bromide (MTT), and β-actin (used as protein loading control) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Chloroquine was purchased from Invitrogen (Grand Island, NY, USA) and z-VAD-fmk (a pan-caspase inhibitor) from Abcam (Cambridge, MA, USA). Antibodies against B-cell lymphoma (bcl-2), poly (ADP-ribose) polymerase (PARP), caspase-3, microtubule-associated protein light chain 3B (LC3B), and beclin-1 were purchased from Cell Signaling Technology (Boston, MA, USA).
2.2. MTT assay for cell proliferation
Cell viability was evaluated using the MTT assay as described previously.19 After treatment, MTT was added to each well, and the optical density (OD) of each well was measured at 570 nm by using a microplate reader (FLUOstar Omega, Cary, NC, USA). The OD570 in untreated cells control was taken as 100% viability. Each experiment was performed in triplicate.
2.3. Western blotting
Cells were rinsed twice with PBS and scraped with RIPA buffer (50 mM Tris HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Proteins were electrophoresed in sample buffer on acrylamide gels and were then transferred to a PVDF membrane (GE Healthcare, Piscataway, NJ, USA). After blocking with 0.5% TBST containing 5% non-fat milk, the membrane was incubated with antibodies (1:1000) overnight at 4°C, and subsequently incubated with horseradish peroxidase–linked anti-mouse or anti-rabbit antibodies (1:3000; Sigma-Aldrich). The blots were then visualized using the ECL Western Blotting Detection System (GE Healthcare). To provide a loading control, the membranes were probed with β-actin antibody according to standard protocols.20
2.4. Detection of LC3B puncta
MIA PaCa-2 cells were seeded onto eight-well chamber slides (2 × 104 cells/well) and infected with Premo Autophagy Sensors (LC3B-GFP, wild-type) BacMam2.0 (Invitrogen) to visualize green fluorescent protein (GFP)-LC3 puncta formation according to the manufacturer's instructions. After treatment, slides were observed with an Olympus IX71 inverted fluorescent microscope (Olympus America Inc., Center Valley, PA, USA), and images were recorded.
2.5. Detection of acidic vesicular organelles
Acridine orange staining was performed to detect acidic vesicular organelles as described previously.21 Briefly, cells were seeded onto eight-well chamber slides (2 × 104 cells/well) and incubated for 24 hours. After treatment, cells were stained with acridine orange (1 μg/mL) for 15 minutes at 37°C. Cells were washed with PBS and evaluated under the fluorescence microscope.
2.6. Apoptosis detection
After treatment, DNA fragmentation in cell lysates was analyzed by the Cell Death Detection ELISA kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer's instructions. Absorbance was measured at 405 nm using the plate reader. The apoptotic index was calculated as an enrichment factor, that is, the ratio of the result compared with the no-treatment control set arbitrarily at 1.0. Each experiment was performed in triplicate.
2.7. In vivo human pancreatic cancer xenograft study
We performed an animal experiment using MIA PaCa-2 human pancreatic cancer cells in a subcutaneous xenograft model. MIA PaCa-2 cells were resuspended in sterile 50% growth factor–reduced Matrigel (BD Biosciences, Bedford, MA, USA) in phosphate-buffered saline. Cells (2 × 106 cells/100 μL) were injected subcutaneously into the right flank of female nude mice aged 4-6 weeks. When the estimated tumor volume reached 100 mm3, mice were randomly assigned to four groups (four mice per group): no treatment, genistein, 5-FU, and the combination of 5-FU and genistein. In treatment groups, mice were given 5- FU (60 mg/kg, intraperitoneally) and/or genistein (1.3 mg, intraperitoneally) every 4 days for 21 days. Mice were sacrificed 29 days after initiation of treatment, and the final tumor volume was calculated using the formula 0.5 × [(length) × (width) × (thickness) of tumor].
All animal experiments were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and were conducted in accordance with institutional and national regulations (IACUC protocol # 01-11-00133).
2.8. Immunohistochemical staining and TUNEL assay
Tissues from subcutaneous xenograft tumors were embedded in paraffin and then cut into 4-μm-thick sections at the clinical core laboratory of MD Anderson. Apoptosis was determined by TUNEL assay with an ApopTag peroxidase in situ apoptosis detection kit (Chemicon Int., Temecula, CA, USA) according to the manufacturer's instruction. Three microscopic fields at ×400 magnification were evaluated from each section. Numbers of stained cells per visual field were recorded. Immunohistochemical staining for LC3B was performed using a Lab-Vision 480-2D immunostainer (Thermo Fisher Scientific, Fremont, CA, USA). All images were captured using an Olympus DP72 camera and its CellSens software through an Olympus BX51 microscope at a magnification of ×400 (Olympus America Inc.).
2.9. Statistical analysis
Data are presented as means ± standard deviation (SD). Statistical analyses were performed by using analysis of variance or Student's two-tailed t-test using GraphPad Prism 6.0 software. Differences with p < 0.05 were considered statistically significant.
RESULTS
3.1 Genistein enhances the antitumor effect of 5-FU in vitro
MIA PaCa-2 human pancreatic cancer cells were exposed to 5-FU (100 μM), genistein (100 μM), alone or in combination, for 72 hours. The combination of 5-FU and genistein resulted in a significant induction of cell death compared with the use of genistein or 5-FU alone (Figure 1a). We also observed an increase in apoptosis in MIA PaCa-2 cells after similar treatments. Relative to single agents, the combination significantly increased apoptosis as shown in the DNA fragmentation ELISA assay (Figure 1b). These results suggested that the increase in cell death induced when genistein was combined with 5-FU was due, in part, to a higher rate of apoptosis.
Fig. 1.
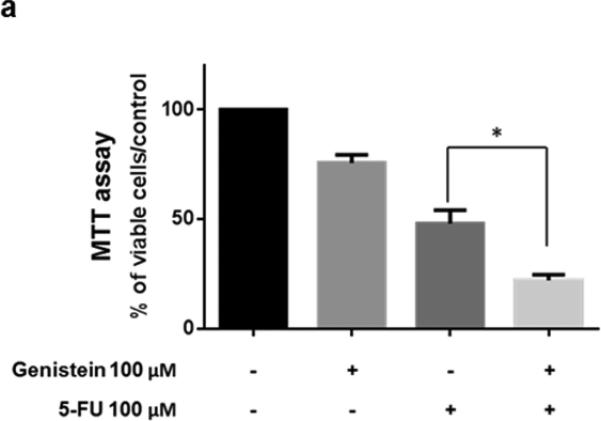
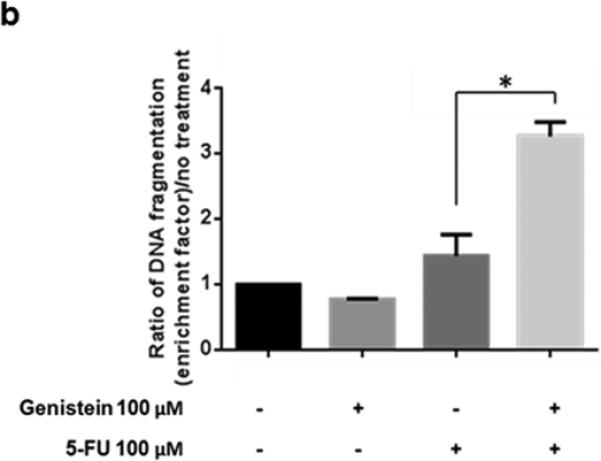
Genistein enhanced the antitumor effect of 5-FU in experiments using the MIA PaCa-2 human pancreatic cancer cell line. (a) Cells were treated with 100 μM 5-FU and/or 100 μM genistein. Cell viability was detected by the MTT assay, the optical density at 570 nm in untreated control cells was taken as 100% viability. (b) DNA fragmentation was detected using the Cell Death Detection ELISA kit. Each experiment was performed in triplicate and data are presented as means ± standard deviation. *p < 0.05
3.2 Genistein enhances 5-FU–induced apoptosis
To better understand the 5-FU–induced apoptosis, we treated MIA PaCa-2 cells with 5-FU (100 μM), genistein (100 μM), or the combination of them for 72 hours. Western blot detected decreased expression of the bcl-2 and increased expression of cleaved PARP and caspase-3 in the cells treated with the combination of 5-FU and genistein compared with cells treated with 5-FU alone (Figure 2a). To confirm whether apoptotic cell death was the main cause of overall cell death induced by the combination, we treated MIA PaCa-2 cells with the combination plus the pan-caspase inhibitor z-VAD-fmk (10 μM). This concentration of z-VAD-fmk (10 μM) had minimum cytotoxic effect when used alone but was sufficient to inhibit the combination treatment-induced apoptosis (Figure 2b – 2d). The addition of z-VAD- fmk prevented 47.1% of the cytotoxic effect of the treatment (75.1% vs 28% cytotoxicity), but it did not prevent 28% of the cytotoxic effect of the combination treatment (Figure 2b). These results suggested that the combination of 5-FU and genistein resulted in cell death through apoptosis as well as another mechanism. Based upon previous studies22, we hypothesized that autophagy was the method of non-apoptotic cell death in cells exposed to 5-FU and genistein.
Fig. 2.
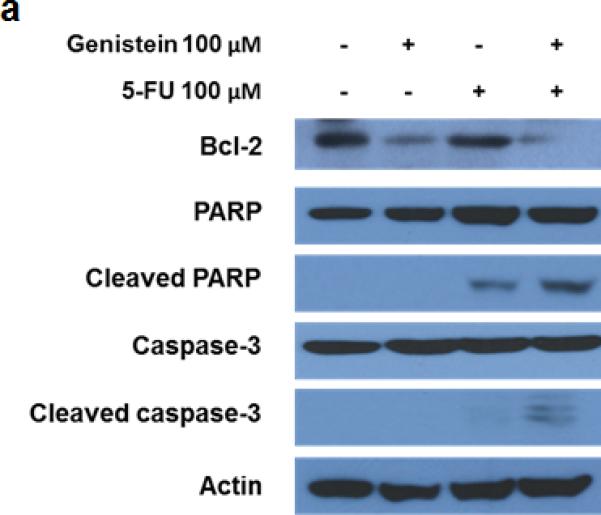
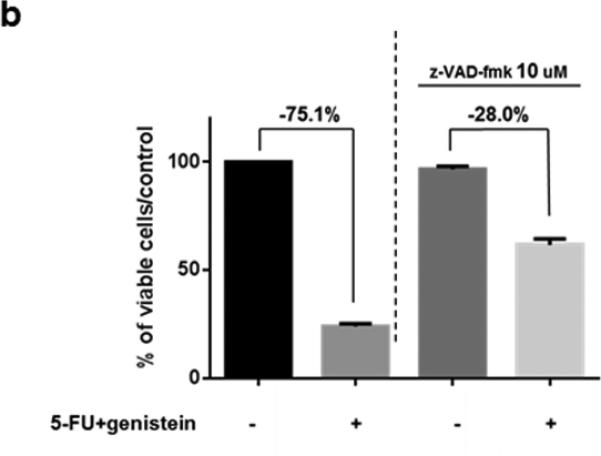
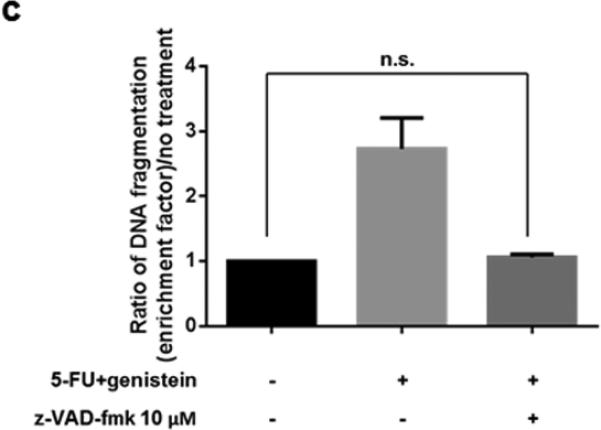
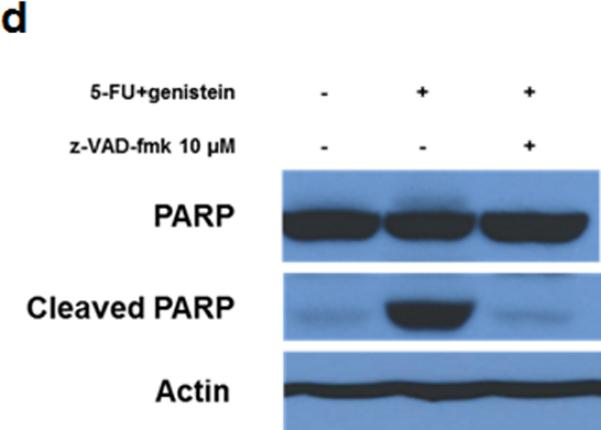
Downregulation of bcl-2 enhanced 5-FU–induced apoptosis by genistein. (a) The expressions of bcl-2, PARP, cleaved PARP, caspase-3, and cleaved caspase-3 were detected by the Western blot assay after 72 hours of treatment. β-actin was used as the loading control. (b) Cells were treated with the combination of 5-FU and genistein in the presence or absence of z-VAD-fmk (10 μM) for 72 hours. Cell viability was detected by the MTT assay, and the optical density at 570 nm in untreated control cells was taken as 100% viability. (c) DNA fragmentation was detected using the Cell Death Detection ELISA kit. (d) Expressions of PARP and cleaved-PARP were detected by the Western blot assay. Each experiment was performed in triplicate and data are presented as means ± standard deviation. *p < 0.05.
3.3 Genistein enhances 5-FU–induced autophagy
To investigate the role of autophagy, we exposed MIA PaCa-2 cells to 5-FU and genistein and examined the autophagy-related proteins, LC3-II and beclin-1. Western blots detected an increase in expression of both LC3-II and beclin-1 (supplemental figure 1a), which suggested autophagy. GFP-LC3 puncta and acidic vesicular organelle formations, a marker of autophagy, were increased after exposure to 5-FU or 5-FU combined with genistein (supplemental figure 1b and 1c). Together, these results demonstrated that genistein increased 5-FU–induced autophagy. As a next step, we investigated whether the enhanced autophagy after exposure was cytoprotective or cytotoxic. After first determining that 10 μM of the autophagy inhibitor chloroquine had minimal cytotoxic effects when used alone (supplemental figure 1d), we found that pancreatic cancer cells exposed to this concentration of chloroquine accumulated LC3-II, which indicated successful inhibition of autophagy (supplemental figure 1e). MTT assay was then performed after cells were exposed for 72 hours to 5-FU alone or in combination with genistein in the presence or absence of 10 μM chloroquine. This assay demonstrated that the cytotoxic effect of 5-FU alone was increased in the presence of chloroquine, which suggests that the 5-FU–induced autophagy was cytoprotective. Conversely, chloroquine reduced the cytotoxic effect of the 5-FU and genistein combination from 76% to 57% (supplemental figure 1f). This important finding indicated that the autophagy induced after cells were exposed to the combination of 5-FU and genistein was cytotoxic rather than cytoprotective.
3.3 Genistein potentiates in vivo therapeutic effect of 5-FU
To further test for genistein potentiation of the anti-cancer properties of 5-FU, we performed an animal experiment using the same cell line in a murine xenograft model (Figure 3a). The growth-inhibitory effects of 5-FU were significantly enhanced when combined with genistein, with significantly smaller tumors in this group compared with 5-FU or genistein alone (Figure 3b and 3c). Analysis of tumor lysates using TUNEL and DNA fragmentation assays demonstrated a significant increase in 5-FU–induced apoptosis when combined with genistein (supplemental figure 2a - 2c). Additionally, immunohistochemical staining demonstrated a qualitative increase in LC3B expression when 5-FU treatment was combined with genistein, which was consistent with our in vitro data.
Fig. 3.
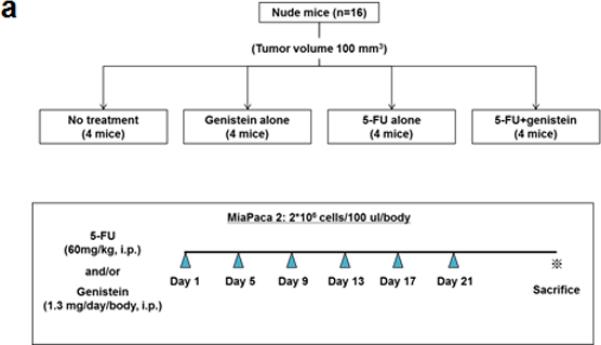
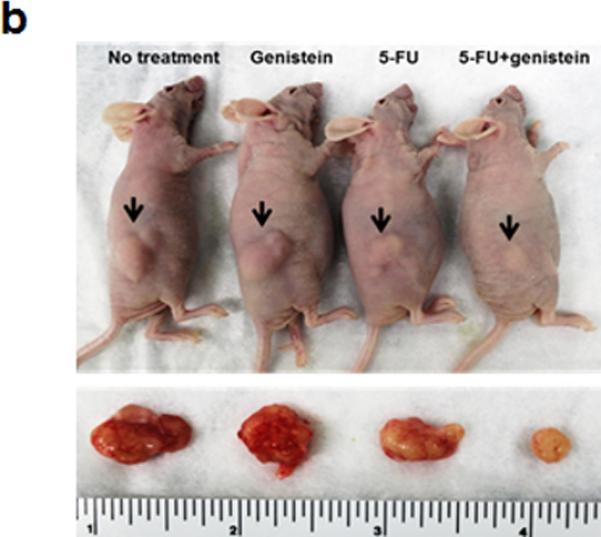
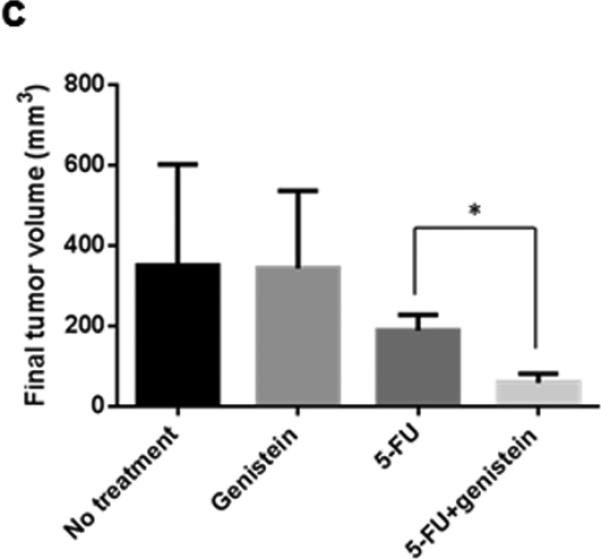
Genistein potentiates the therapeutic effect of 5-FU in vivo in a subcutaneous pancreatic tumor xenograft model. (a) Flowchart representation of in vivo experiment design and treatment schedule. (b) Mice were sacrificed at day 29 after initiation of treatment. (c) Final tumor volume was calculated with formula of 0.5 [(length) × (width) × (thickness) of tumor]. Data are presented as means ± standard deviation of four tumors in each treatment. Standard deviations are represented by error bars. *p < 0.05.
DISCUSSION
In the current study, in vitro and in vivo experiments both demonstrated that the combination of 5-FU and genistein showed superior antitumor effects against human pancreatic cancer cells when compared with either 5-FU or genistein alone. Although genistein itself did not induce apoptosis and autophagy, it significantly enhanced 5-FU–induced apoptosis and autophagy as evidenced by the associated decrease in bcl-2 and increase in beclin-1 protein levels. Experiments using chloroquine to inhibit autophagy support the concept that genistein enhanced 5-FU–induced autophagy and caused autophagic cell death. To our knowledge, this is the first study showing that genistein can enhance 5-FU -induced autophagy and trigger autophagic cell death in human pancreatic cancer cells.
We demonstrated that the combination of 5-FU and genistein induced autophagic cell death by significantly altering the expression of two important molecules, bcl-2 and beclin-1, that regulate autophagy. Bcl-2 is an anti-apoptotic protein overexpressed in various cancers, and it contributes to treatment resistance by inhibiting chemotherapy-induced apoptosis.23 Bcl-2 is also involved in autophagy inhibition by binding to the BH3 domain of beclin-1 and negatively regulating the autophagy-promoting beclin-1/VPS34 complex.24 It has been reported that mutant proteins of beclin-1 that do not bind to bcl-2 can trigger excessive cellular autophagy and death in the absence of autophagic stimuli.24 Therefore, the downregulation of bcl-2 and upregulation of beclin-1 by the combination of 5-FU and genistein shown here provides a reasonable mechanistic explanation of the observed autophagic cell death induction. As shown in the current study, both “cytoprotective” and “cytotoxic” autophagy could occur in the same experimental system in response to treatment, and “cytoprotective” autophagy induced by 5-FU can be converted to “cytotoxic” autophagy by the addition of genistein. This is similar to what Bristol et al. observed in breast cancer cell lines.22 The spectrum of autophagy in cancer cells is now recognized to include cytoprotective, cytostatic, cytotoxic, and nonprotective types, but the molecular mechanisms driving each type are relatively unexplored.25
In summary, we found that genistein potentiates the antitumor effect of 5-fluorouracil by inducing apoptotic as well as autophagic cell death in human pancreatic cancer cells. These results demonstrate the potential of genistein as an additional therapeutic agent. Future studies will focus on defining the molecular events after tumors are exposed to low toxicity agents like genistein in combination with cytotoxic therapies.
Supplementary Material
Supplemental fig. 1. Induction of cytotoxic autophagy with the combination of 5-FU and genistein. (a) Expression of LC3, beclin-1, and bcl-2 after treatment was detected by the Western blot assay. β-actin was used as the loading control. (b) Cells were transiently transfected with GFP-LC3 and treated with 5-FU, genistein, or both. Images were captured using fluorescence microscopy (magnification, ×200). (c) After treatment with the indicated concentrations of 5-FU, genistein, or the combination of them, cells were stained with acridine orange (1 μg/mL) for 15 minutes at 37°C, and images were captured using fluorescence microscopy (magnification ×200). (d) Cells were treated with 10 μM or 20 μM chloroquine (CQ) for 72 hours. Cell viability was detected by the MTT assay, and the optical density at 570 nm in untreated control cells was taken as 100% viability. (e) The change in expression of LC3 was detected by the Western blot assay. (f) Cells were treated with the combination of 5-FU and genistein with or without 10 μM CQ. Cell viability was detected by the MTT assay. *p < 0.05; n.s. = not significant.
Supplemental fig. 2. Induction of apoptosis and autophagy in a subcutaneous pancreatic tumor xenograft model. (a) TUNEL assay using the ApopTag peroxidase in situ apoptosis detection kit and immunohistochemical staining with anti-LC3B antibody were performed to show induction of apoptosis and autophagy. (b) Numbers of TUNEL-positive cells per visual field were recorded at ×400 magnification in 3 microscopic fields. (c) Apoptosis represented by DNA fragmentation was detected using tumor lysate. Each experiment was performed in triplicate and data are presented as means ± standard deviation. *p < 0.05.
Synopsis.
Genistein potentiates the antitumor effects of 5-fluorouracil against human pancreatic cancer cells by increasing apoptotic as well as autophagic cell death. Our data suggests the potential clinical utility of genistein as an adjuvant to enhance the effectiveness of cytotoxic chemotherapy against pancreatic cancer.
Acknowledgments
This research was supported in part by the NIH through MD Anderson's Cancer Center Support Grant CA016672. We acknowledge the Astellas Foundation for Research on Metabolic Disorders for financial support to RS and the Various Donors Pancreatic Research Fund supporting JBF.
Footnotes
Disclosure
Authors declare no Conflict of Interests for this article.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Gavoille C, Samalin E, et al. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep. 2013;15:182–9. doi: 10.1007/s11912-012-0290-4. [DOI] [PubMed] [Google Scholar]
- 5.Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP. 2012;13:497–501. doi: 10.6092/1590-8577/913. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S, Zhang Y, Ali S, et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–72. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Zhang Y, Wang Z, et al. In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer. Int J Cancer. 2007;120:906–17. doi: 10.1002/ijc.22332. [DOI] [PubMed] [Google Scholar]
- 8.Hwang KA, Kang NH, Yi BR, et al. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17beta-estradiol or bisphenol A via the inhibition of cell cycle progression. Int J Oncol. 2013;42:733–40. doi: 10.3892/ijo.2012.1719. [DOI] [PubMed] [Google Scholar]
- 9.Hwang KA, Park MA, Kang NH, et al. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 beta-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor alpha and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol. 2013;272:637–46. doi: 10.1016/j.taap.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang G, Yao L, Ruan K, et al. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int. 2009;33:1237–44. doi: 10.1016/j.cellbi.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Kong D, Azmi AS, et al. Restoring sensitivity to oxaliplatin by a novel approach in gemcitabine-resistant pancreatic cancer cells in vitro and in vivo. Int J Cancer. 2011;128:1240–50. doi: 10.1002/ijc.25658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Gossner G, Choi M, Tan L, et al. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol. 2007;105:23–30. doi: 10.1016/j.ygyno.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Yogosawa S, Izutani Y, et al. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer. 2009;8:100. doi: 10.1186/1476-4598-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao P, Bauvy C, Souquere S, et al. The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells. J Biol Chem. 2010;285:25570–81. doi: 10.1074/jbc.M110.118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto D, Blauer M, Hirota M, et al. Autophagy is needed for the growth of pancreatic adenocarcinoma and has a cytoprotective effect against anticancer drugs. Eur J Cancer. 2014 doi: 10.1016/j.ejca.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Puissant A, Auberger P. AMPK- and p62/SQSTM1-dependent autophagy mediate resveratrol-induced cell death in chronic myelogenous leukemia. Autophagy. 2010;6:655–7. doi: 10.4161/auto.6.5.12126. [DOI] [PubMed] [Google Scholar]
- 18.Mukubou H, Tsujimura T, Sasaki R, et al. The role of autophagy in the treatment of pancreatic cancer with gemcitabine and ionizing radiation. Int J Oncol. 2010;37:821–8. doi: 10.3892/ijo_00000732. [DOI] [PubMed] [Google Scholar]
- 19.Chang Z, Li Z, Wang X, et al. Deciphering the mechanisms of tumorigenesis in human pancreatic ductal epithelial cells. Clin Cancer Res. 2013;19:549–59. doi: 10.1158/1078-0432.CCR-12-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng X, Li L, Jiang H, et al. Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: involvement of apoptosis and autophagy. Biochem Biophys Res Commun. 2014;444:376–81. doi: 10.1016/j.bbrc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 21.Mohan N, Chakrabarti M, Banik NL, et al. Combination of LC3 shRNA Plasmid Transfection and Genistein Treatment Inhibited Autophagy and Increased Apoptosis in Malignant Neuroblastoma in Cell Culture and Animal Models. PLoS One. 2013;8:e78958. doi: 10.1371/journal.pone.0078958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristol ML, Di X, Beckman MJ, et al. Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy. 2012;8:739–53. doi: 10.4161/auto.19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan N, Wong M, Nannini MA, et al. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Mol Cancer Ther. 2013;12:853–64. doi: 10.1158/1535-7163.MCT-12-0949. [DOI] [PubMed] [Google Scholar]
- 24.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1- dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74:647–51. doi: 10.1158/0008-5472.CAN-13-2966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental fig. 1. Induction of cytotoxic autophagy with the combination of 5-FU and genistein. (a) Expression of LC3, beclin-1, and bcl-2 after treatment was detected by the Western blot assay. β-actin was used as the loading control. (b) Cells were transiently transfected with GFP-LC3 and treated with 5-FU, genistein, or both. Images were captured using fluorescence microscopy (magnification, ×200). (c) After treatment with the indicated concentrations of 5-FU, genistein, or the combination of them, cells were stained with acridine orange (1 μg/mL) for 15 minutes at 37°C, and images were captured using fluorescence microscopy (magnification ×200). (d) Cells were treated with 10 μM or 20 μM chloroquine (CQ) for 72 hours. Cell viability was detected by the MTT assay, and the optical density at 570 nm in untreated control cells was taken as 100% viability. (e) The change in expression of LC3 was detected by the Western blot assay. (f) Cells were treated with the combination of 5-FU and genistein with or without 10 μM CQ. Cell viability was detected by the MTT assay. *p < 0.05; n.s. = not significant.
Supplemental fig. 2. Induction of apoptosis and autophagy in a subcutaneous pancreatic tumor xenograft model. (a) TUNEL assay using the ApopTag peroxidase in situ apoptosis detection kit and immunohistochemical staining with anti-LC3B antibody were performed to show induction of apoptosis and autophagy. (b) Numbers of TUNEL-positive cells per visual field were recorded at ×400 magnification in 3 microscopic fields. (c) Apoptosis represented by DNA fragmentation was detected using tumor lysate. Each experiment was performed in triplicate and data are presented as means ± standard deviation. *p < 0.05.


