Abstract
Hypertrophic Cardiomyopathy (HCM) is the abnormal thickening of the ventricles and an increase in cardiac mass. Analyses of 108 rhesus macaque probands with pronounced HCM revealed a strong genetic predisposition to this disease. Macaques are ideal for investigating HCM because of their marked similarity to humans genetically, physiologically and anatomically.
Keywords: Genetic disease, heart disease, left ventricular hypertrophy, nonhuman primates
Introduction
Hypertrophic cardiomyopathy (HCM) results in abnormal thickening of the ventricular wall and an increase in cardiac mass [8]. Clinically, in the absence of other underlying causes such as hypertension or valvular disease, left ventricular hypertrophy (LVH) is likely a primary hypertrophic cardiomyopathy, i.e. thickening of the myocardium (muscle) of the left ventricle. In its most devastating form, individuals are frequently asymptomatic until sudden cardiac death and while HCM is the leading cause of sudden cardiac death in post puberty individuals, i.e., young adults [12], it affects 1 in 500 people [9]. Although abnormalities may be detected on an ECG, transthoracic echocardiography provides a definitive diagnosis, demonstrating increased wall thickening. Autopsy examinations confirm echocardigraphic findings of left ventricular hypertrophy that are usually associated with a small left ventricular chamber [4]. While HCM has been characterized as an inherited autosomal dominant condition in humans [5], up to ten different genes have been described as major risk factors suggesting a more complex genetic influence.
HCM has not previously been described in rhesus macaques (Macaca mulatta). It is estimated that only 1% of the entire CNPRC rhesus colony (i.e., 50 living animals) exhibits HCM albeit at varying degrees of severity. Interestingly, incidences of HCM have not been reported in any other National Primate Research Centers in the US. As a result, we tested the hypotheses that 1) founder effects may have in fact led to the occurrence of HCM among the colony animals at the CNPRC; and 2) rhesus macaques with pronounced HCM may exhibit the features of autosomal dominant inheritance in pedigrees as has been described in humans [5]. The analyses of pedigrees holds promise for studies in captive rhesus macaque owing to the availability of large numbers of half sibs in large breeding colonies, detailed colony records and genetic material from eight or more generations for phenotypic scoring and genotypic sampling. HCM pedigrees of rhesus macaques will provide initial justification of further exploration of genetic influences on this complex disease.
Methods
All procedures followed the Guidelines for Use and Care of Laboratory Animals of the National Research Council and the CNPRC Standard Operating Protocols (SOPs). The CNPRC is an accredited institution by the Association for Assessment and Accreditation of Laboratory Animal Care. Experimental protocols were approved by the University of California, Davis, Institutional Animal Care and Use Committees (IACUC) before implementation.
We used records from rhesus macaques diagnosed with gross evidence of HCM, including sudden cardiac death, or with clinical evidence of heart disease that have been documented at necropsy. We used the PEDSYS [2] program to construct multigenerational pedigrees that include the probands with complete genealogical information. Colony records of vital and demographic statistics of 10,814 rhesus macaques, including all relatives of the probands, some of which were themselves probands, were then searched for evidence of a familial history of HCM. Pedigrees were constructed connecting one or more probands by assembling ascending and/or descending genealogical trees of specified depth for each proband selected and by assembling family units (parents, full sibs, mates, and offspring) for a specified proband. A chi-squared test [1] was used to test the hypothesis that both sexes are equally susceptible to HCM.
Results
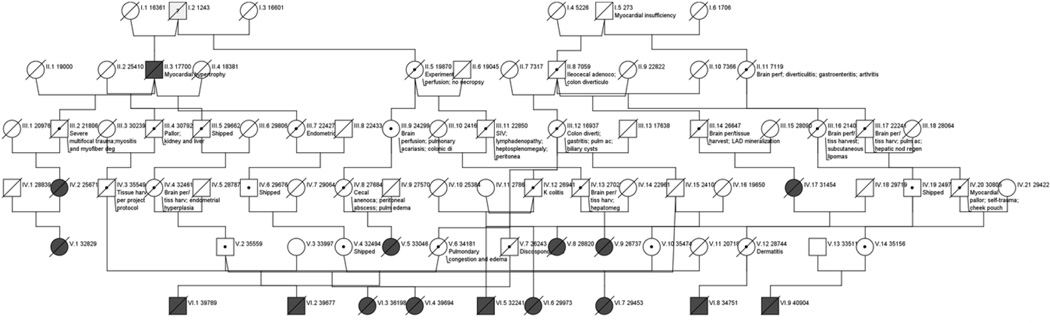
We identified a total of 108 animals (48 males and 60 females) with HCM. These 108 probands were half-sibs of 21 normal sisters and 27 normal brothers representing 106 individual families. Two sets of full sibs were among the 108 probands including a brother-sister pair and two full sisters. While many parents of offspring with HCM exhibited symptoms that may have resulted from HCM, provided in Figure 1, which would be consistent with an autosomal dominant mode of inheritance, only two of the probands had a parent diagnosed with the disease.
Figure 1.
Pedigree of rhesus macaques with known HCM (drawn using the online software package Pedigree Chart Designer by CeGaT GmbH (http://www.cegat.de)). For simplicity, only individuals directly involved in the transmission of the disease phenotype to the probands are displayed, many more unaffected individuals including close and distant relatives have been omitted from the pedigree. Health conditions that might represent symptoms of undiagnosed HCM are provided for family members. Clear squares and circles represent unaffected male and female individuals, respectively, and shaded squares and circles represent affected male and female individuals (or probands). Slash symbols represent deceased individuals, and dot symbols represent carriers but their absence does not mean an individual is not a carrier. Generations are labeled in Roman numerals while individuals within each generation are indicated in Arabic numerals. Additional pedigrees have been provided as supplementary documentation.
Table 1 presents the vital data of the 108 probands used in this study. The age range of affected animals with a common history of sudden death, occasionally following anesthesia was < 12 months to 30 of years age (average 7 years). Average reproductive success among the affected animals was approximately 4 offspring. An animal’s sex had no influence on the incidence of HCM (p > 0.05), but the probands exhibited high estimates (F=5.4% +/− 4.2%) of the inbreeding coefficient [11]. Figure 1 illustrates the largest of the four family pedigrees assembled based on the eight generations of animal records.
Table 1.
Vital statistics for the 108 HCM animals used in the pedigree analysis. Coefficients of inbreeding (F) were estimated using Wright’s [11] method.
| Field | Min | Max | Mean | Std. Dev. |
|---|---|---|---|---|
| Age at Death | <1 year | 30 years | 7 years | 6 years |
| Age at Death among Males | <1 year | 24 years | 6 years | 5 years |
| Age at Death among Females | <1 year | 30 years | 8 years | 6 years |
| Generation | 1 | 9 | 4.07 | 1.79 |
| F | 0.20% | 12.50% | 5.40% | 4.20% |
| Age Of Sire At Birth | 4 years | 26 years | 7 years | 3 years |
| Age Of Dam At Birth | 3 years | 17 years | 7 years | 3 years |
| Number of Offspring | 1 | 27 | 3.87 | 4.1 |
Inspection of all four pedigrees showed that affected animals all descended from a small number of founders, and while the disease may be passed on from an affected male or female that was not diagnosed with HCM, the pattern for HCM transmission in rhesus macaques is not ostensibly consistent with an autosomal dominant mode of inheritance hypothesized for human HCM [5]. The lack of a sufficient number of affected sibships with a parent exhibiting HCM precluded a formal segregation analysis to test its mode of inheritance.
Discussion
HCM’s etiology should be better understood to ensure the health and wellbeing of all colony animals. Although the current data does not present a compelling support for the dominant mode of inheritance observed in humans, the data suggests that the prevalence of HCM at the colony level is dominated by founder effects. Research into the pathogenesis, treatment and significance of underlying genetic heterogeneity in HCM should proceed. Clinical and pathologic features of colony-raised rhesus macaques contribute to the reliability and reproducibility of the results of experiments [3, 6, 7, 10]. Moreover, our study suggests that the disease represents a major challenge to captive breeding of rhesus macaque at the CNPRC as it impacts the supply of available rhesus macaques by diminishing the productivity of captive breeding stock.
From a rhesus macaque colony management perspective, clinical examinations may be useful for the identification of additional animals who are at increased risk for HCM. Moreover, these screening techniques may facilitate the identification of family members of probands who are at increased risk for HCM and may help address the need for a more detailed description of the families with affected members so that the autosomal dominant hypothesis as well as the heritability (h2) of the disease can be fully explored. Although HCM cannot be prevented, prior knowledge of risks for developing the condition based on clinical or biochemical tests may lead to early detection and a quicker response to the development of symptoms, as well as help in the detection of risks for more serious complications associated with HCM.
Acknowledgements
We would like to recognize the CNPRC husbandry, management, pathology, research, and veterinary staff who worked diligently to provide excellent care for the rhesus macaque colony with HCM. The authors wish to thank the staff in the veterinary and primate services units at the CNPRC for their contributions that have resulted in this report. This study was supported by the California National Primate Research Center base grant (No. RR000169-48), and by grants number RR16023-01, RR005090, RR025781 and RR18144-01 of the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH).
References
- 1.Agresti A. An Introduction to Categorical Data Analysis. 2nd ed. New York: Wiley-Interscience; 2007. [Google Scholar]
- 2.Dyke B. PEDSYS: A Pedigree Data Management System, version 2.0. User's Manual. 1999 [Google Scholar]
- 3.Hassimoto M, Harada T, Harada T. Changes in hematology, biochemical values, and restraint ECG of rhesus monkeys (Macaca mulatta) following 6-month laboratory acclimation. J. Med. Primatol. 2004;33:175–186. doi: 10.1111/j.1600-0684.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- 4.Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44:412–427. doi: 10.1111/j.1365-2559.2004.01835.x. [DOI] [PubMed] [Google Scholar]
- 5.ICIN working group on Hereditary Heart Diseases. Genetic diagnostics and genetic counseling in hypertrophic cardiomyopathy. Neth. Heart J. 2010;18(3):144–159. doi: 10.1007/BF03091753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korcarz CE, Padrid PA, Shroff SG, Weinert L, Lang RM. Doppler echocardiographic reference values for healthy rhesus monkeys under ketamine hydrochloride sedation. J. Med. Primatol. 1997;26(6):287–298. doi: 10.1111/j.1600-0684.1997.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 7.Losi M-A, Nistri St, Galderisi M, Betocchi S, Cecchi F, Olivotto I, Agricola E, Ballo P, Buralli S, D’Andrea A, D’Errico A, Mele D, Sciomer S, Mondilla S. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovascular Ultrasound. 2010;8:7–26. doi: 10.1186/1476-7120-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez JE, McCudden CR, Willis MS. Familial hypertrophic cardiomyopathy: Basic concepts and future molecular diagnostics. Clinical Biochemistry. 2009;42(9):755–765. doi: 10.1016/j.clinbiochem.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Tang H-L, Want L-L, Cheng G, Wang L, Song L. Evaluation of the cardiovascular function of older adult Rhesus monkeys by ultrasonography. J. Med. Primatol. 2008;37(2):101–108. doi: 10.1111/j.1600-0684.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright S. Coefficients of Inbreeding and Relationship. The American Naturalist. 1922;56(645):330–338. [Google Scholar]
- 12.Xu Q, Dewey S, Nguyen S, Gomes AV. Malignant and benign mutations in familial cardiomyopathies: insights into mutations linked to complex cardiovascular phenotypes. J. Mol. Cell Cardiol. 2010;48:899–909. doi: 10.1016/j.yjmcc.2010.03.005. [DOI] [PubMed] [Google Scholar]