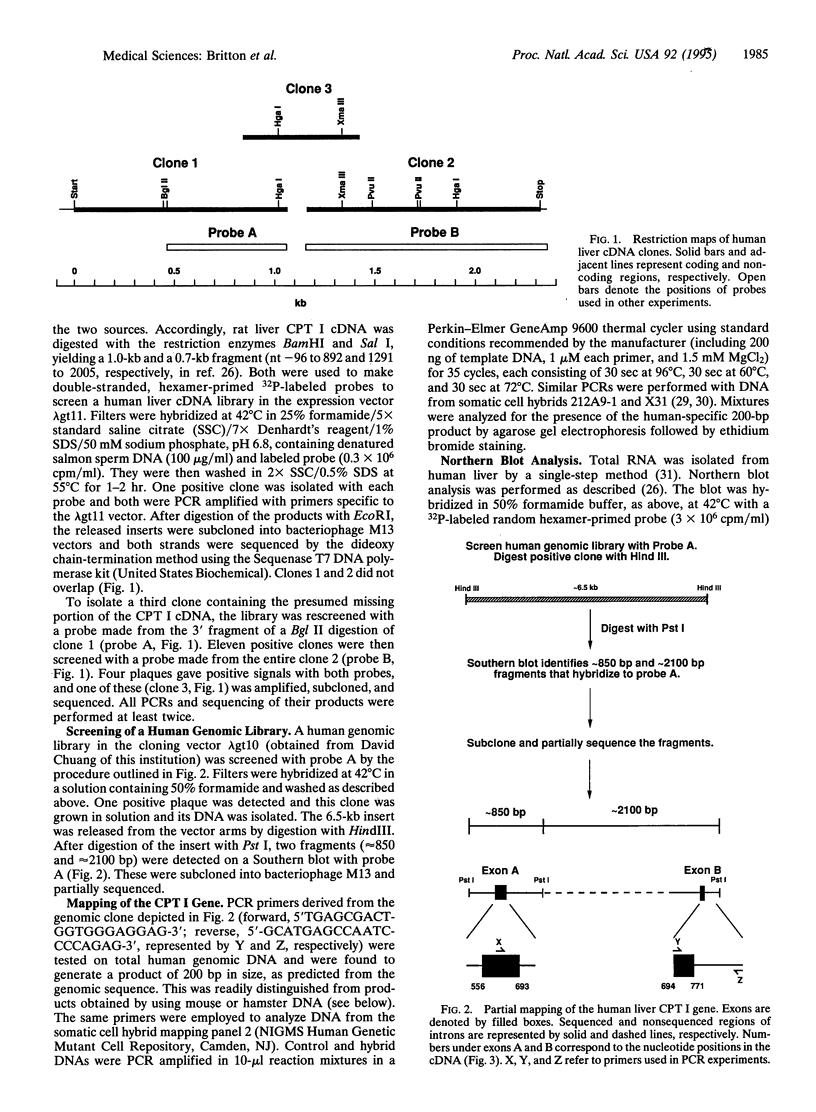
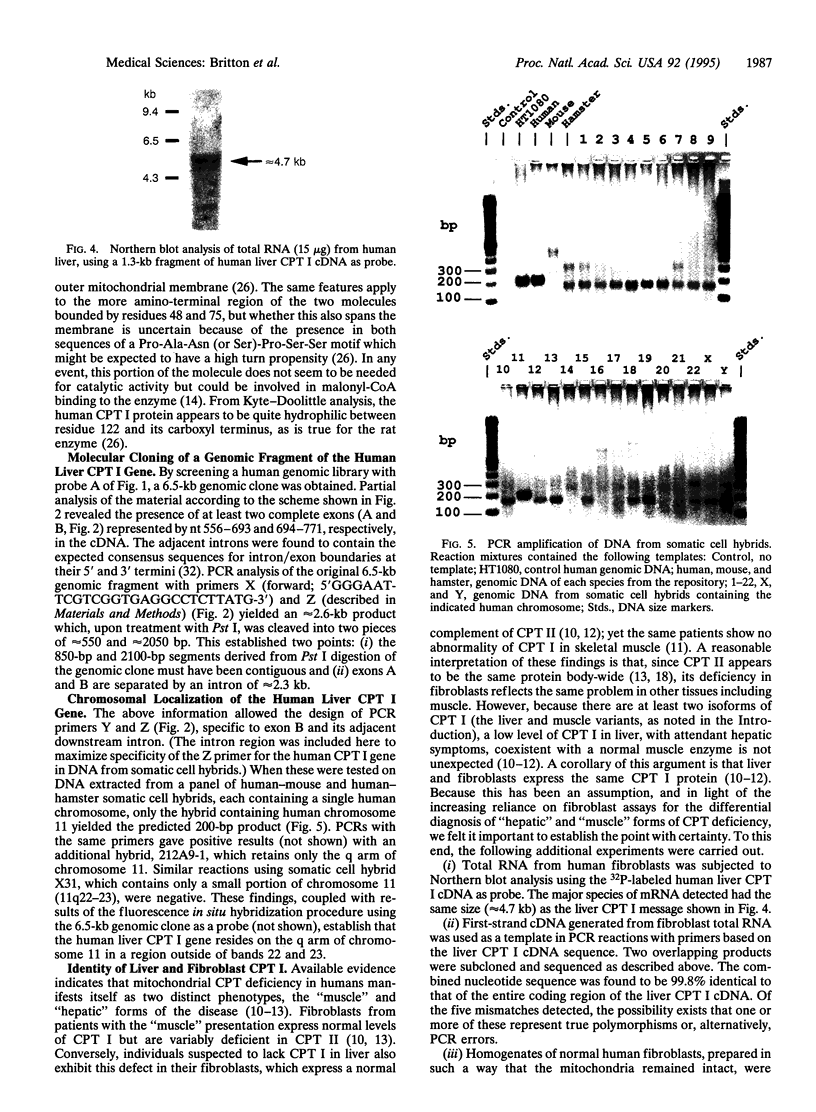
Abstract
Using the cDNA for rat liver mitochondrial carnitine palmitoyltransferase I (CPT I; EC 2.3.1.21) as a probe, we isolated its counterpart as three overlapping clones from a human liver cDNA library. Both the nucleotide sequence of the human cDNA and the predicted primary structure of the protein (773 aa) proved to be very similar to those of the rat enzyme (82% and 88% identity, respectively). The CPT I mRNA size was also found to be the same (approximately 4.7 kb) in both species. Screening of a human genomic library with the newly obtained cDNA yielded a positive clone of approximately 6.5 kb which, upon partial analysis, was found to contain at least two complete exons linked by a 2.3-kb intron. Oligonucleotide primers specific to upstream and downstream regions of one of the exon/intron junctions were tested in PCRs with DNA from a panel of somatic cell hybrids, each containing a single human chromosome. The results allowed unambiguous assignment of the human liver CPT I gene to the q (long) arm of chromosome 11. Additional experiments established that liver and fibroblasts express the same isoform of mitochondrial CPT I, legitimizing the use of fibroblast assays in the differential diagnosis of the "muscle" and "hepatic" forms of CPT deficiency. The data provide insights into the structure of a human CPT I isoform and its corresponding gene and establish unequivocally that CPT I and CPT II are distinct gene products. Availability of the human CPT I cDNA should open the way to an understanding of the genetic basis of inherited CPT I deficiency syndromes, how the liver CPT I gene is regulated, and which tissues other than liver express this particular variant of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brown N. F., Esser V., Foster D. W., McGarry J. D. Expression of a cDNA for rat liver carnitine palmitoyltransferase I in yeast establishes that catalytic activity and malonyl-CoA sensitivity reside in a single polypeptide. J Biol Chem. 1994 Oct 21;269(42):26438–26442. [PubMed] [Google Scholar]
- Chen S., Ogawa A., Ohneda M., Unger R. H., Foster D. W., McGarry J. D. More direct evidence for a malonyl-CoA-carnitine palmitoyltransferase I interaction as a key event in pancreatic beta-cell signaling. Diabetes. 1994 Jul;43(7):878–883. doi: 10.2337/diab.43.7.878. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Demaugre F., Bonnefont J. P., Mitchell G., Nguyen-Hoang N., Pelet A., Rimoldi M., Di Donato S., Saudubray J. M. Hepatic and muscular presentations of carnitine palmitoyl transferase deficiency: two distinct entities. Pediatr Res. 1988 Sep;24(3):308–311. doi: 10.1203/00006450-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J Biol Chem. 1993 Mar 15;268(8):5817–5822. [PubMed] [Google Scholar]
- Falik-Borenstein Z. C., Jordan S. C., Saudubray J. M., Brivet M., Demaugre F., Edmond J., Cederbaum S. D. Brief report: renal tubular acidosis in carnitine palmitoyltransferase type 1 deficiency. N Engl J Med. 1992 Jul 2;327(1):24–27. doi: 10.1056/NEJM199207023270105. [DOI] [PubMed] [Google Scholar]
- Finocchiaro G., Taroni F., Rocchi M., Martin A. L., Colombo I., Tarelli G. T., DiDonato S. cDNA cloning, sequence analysis, and chromosomal localization of the gene for human carnitine palmitoyltransferase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):661–665. doi: 10.1073/pnas.88.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E. Rationale and application of fatty acid oxidation inhibitors in treatment of diabetes mellitus. Diabetes Care. 1992 Jun;15(6):773–784. doi: 10.2337/diacare.15.6.773. [DOI] [PubMed] [Google Scholar]
- Gellera C., Verderio E., Floridia G., Finocchiaro G., Montermini L., Cavadini P., Zuffardi O., Taroni F. Assignment of the human carnitine palmitoyltransferase II gene (CPT1) to chromosome 1p32. Genomics. 1994 Nov 1;24(1):195–197. doi: 10.1006/geno.1994.1605. [DOI] [PubMed] [Google Scholar]
- Kerner J., Zaluzec E., Gage D., Bieber L. L. Characterization of the malonyl-CoA-sensitive carnitine palmitoyltransferase (CPTo) of a rat heart mitochondrial particle. Evidence that the catalytic unit is CPTi. J Biol Chem. 1994 Mar 18;269(11):8209–8219. [PubMed] [Google Scholar]
- Lambert C., Schultz R. A., Smith M., Wagner-McPherson C., McDaniel L. D., Donlon T., Stanbridge E. J., Friedberg E. C. Functional complementation of ataxia-telangiectasia group D (AT-D) cells by microcell-mediated chromosome transfer and mapping of the AT-D locus to the region 11q22-23. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5907–5911. doi: 10.1073/pnas.88.13.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk G. D., Witters L. A., Itoi T., Barr R., Barr A. Acetyl-CoA carboxylase involvement in the rapid maturation of fatty acid oxidation in the newborn rabbit heart. J Biol Chem. 1994 Oct 14;269(41):25871–25878. [PubMed] [Google Scholar]
- McGarry J. D. Disordered metabolism in diabetes: have we underemphasized the fat component? J Cell Biochem. 1994;55 (Suppl):29–38. doi: 10.1002/jcb.240550005. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- Montermini L., Wang H., Verderio E., Taroni F., DiDonato S., Finocchiaro G. Identification of 5' regulatory regions of the human carnitine palmitoyltransferase II gene. Biochim Biophys Acta. 1994 Sep 13;1219(1):237–240. doi: 10.1016/0167-4781(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA-sensitive and -insensitive carnitine palmitoyltransferase activities of microsomes are due to different proteins. J Biol Chem. 1994 Jul 15;269(28):18283–18286. [PubMed] [Google Scholar]
- Mynatt R. L., Park E. A., Thorngate F. E., Das H. K., Cook G. A. Changes in carnitine palmitoyltransferase-I mRNA abundance produced by hyperthyroidism and hypothyroidism parallel changes in activity. Biochem Biophys Res Commun. 1994 Jun 15;201(2):932–937. doi: 10.1006/bbrc.1994.1791. [DOI] [PubMed] [Google Scholar]
- Pande S. V. A mitochondrial carnitine acylcarnitine translocase system. Proc Natl Acad Sci U S A. 1975 Mar;72(3):883–887. doi: 10.1073/pnas.72.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Vischer S., Glennon M. C., Regazzi R., Deeney J. T., Corkey B. E. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem. 1992 Mar 25;267(9):5802–5810. [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Saha A. K., Kurowski T. G., Colca J. R., Ruderman N. B. Lipid abnormalities in tissues of the KKAy mouse: effects of pioglitazone on malonyl-CoA and diacylglycerol. Am J Physiol. 1994 Jul;267(1 Pt 1):E95–101. doi: 10.1152/ajpendo.1994.267.1.E95. [DOI] [PubMed] [Google Scholar]
- Schultz R. A., Saxon P. J., Glover T. W., Friedberg E. C. Microcell-mediated transfer of a single human chromosome complements xeroderma pigmentosum group A fibroblasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4176–4179. doi: 10.1073/pnas.84.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taroni F., Verderio E., Fiorucci S., Cavadini P., Finocchiaro G., Uziel G., Lamantea E., Gellera C., DiDonato S. Molecular characterization of inherited carnitine palmitoyltransferase II deficiency. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8429–8433. doi: 10.1073/pnas.89.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tein I., Demaugre F., Bonnefont J. P., Saudubray J. M. Normal muscle CPT1 and CPT2 activities in hepatic presentation patients with CPT1 deficiency in fibroblasts. Tissue specific isoforms of CPT1? J Neurol Sci. 1989 Sep;92(2-3):229–245. doi: 10.1016/0022-510x(89)90139-1. [DOI] [PubMed] [Google Scholar]
- Thumelin S., Esser V., Charvy D., Kolodziej M., Zammit V. A., McGarry D., Girard J., Pegorier J. P. Expression of liver carnitine palmitoyltransferase I and II genes during development in the rat. Biochem J. 1994 Jun 1;300(Pt 2):583–587. doi: 10.1042/bj3000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis B. C., Cowan A. T., Brown N., Foster D. W., McGarry J. D. Use of a selective inhibitor of liver carnitine palmitoyltransferase I (CPT I) allows quantification of its contribution to total CPT I activity in rat heart. Evidence that the dominant cardiac CPT I isoform is identical to the skeletal muscle enzyme. J Biol Chem. 1994 Oct 21;269(42):26443–26448. [PubMed] [Google Scholar]
- Weis B. C., Esser V., Foster D. W., McGarry J. D. Rat heart expresses two forms of mitochondrial carnitine palmitoyltransferase I. The minor component is identical to the liver enzyme. J Biol Chem. 1994 Jul 22;269(29):18712–18715. [PubMed] [Google Scholar]
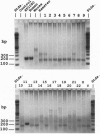
- Woeltje K. F., Esser V., Weis B. C., Cox W. F., Schroeder J. G., Liao S. T., Foster D. W., McGarry J. D. Inter-tissue and inter-species characteristics of the mitochondrial carnitine palmitoyltransferase enzyme system. J Biol Chem. 1990 Jun 25;265(18):10714–10719. [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Sen A., Cox W. F., McPhaul M. J., Slaughter C. A., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver mitochondrial carnitine palmitoyltransferase II. J Biol Chem. 1990 Jun 25;265(18):10720–10725. [PubMed] [Google Scholar]