Abstract
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in children and is responsible for as many as 199,000 childhood deaths annually worldwide. To support the development of viral therapeutics and vaccines for RSV, a human adult experimental infection model has been established. In this report, we describe the provenance and sequence of RSV Memphis-37, the low-passage clinical isolate used for the model's reproducible, safe, experimental infections of healthy, adult volunteers. The predicted amino acid sequences for major proteins of Memphis-37 are compared to nine other RSV A and B amino acid sequences to examine sites of vaccine, therapeutic, and pathophysiologic interest. Human T- cell epitope sequences previously defined by in vitro studies were observed to be closely matched between Memphis-37 and the laboratory strain RSV A2. Memphis-37 sequences provide baseline data with which to assess: (i) virus heterogeneity that may be evident following virus infection/transmission, (ii) the efficacy of candidate RSV vaccines and therapeutics in the experimental infection model, and (iii) the potential emergence of escape mutants as a consequence of experimental drug treatments. Memphis-37 is a valuable tool for pre-clinical research, and to expedite the clinical development of vaccines, therapeutic immunomodulatory agents, and other antiviral drug strategies for the protection of vulnerable populations against RSV disease.
Introduction
Respiratory syncytial virus (RSV) is a paramyxovirus that infects more than 60% of children during the first year of life [1]. This virus is associated with significant morbidity and mortality, particularly among young infants [2]. Globally, RSV infections were estimated to cause 66,000–199,000 deaths in 2005 in children under the age of 5 years, mostly occurring in the developing world, and no vaccine or effective antiviral treatment for RSV disease exists.
Prior to the clinical testing of new vaccines, antivirals, and other novel interventions in infants, safety and efficacy tests should be performed in and proven in consenting adults. However, RSV-directed drug efficacy is difficult to evaluate in healthy adult populations, because natural RSV infections severe enough to prompt a health care concern are relatively rare in adults, producing generally mild symptoms that are difficult to distinguish from those of the common cold. The development of an RSV human adult experimental infection model is therefore imperative to expedite drug paths to licensure and commercialization.
RSV Memphis-37 was isolated from a child with bronchiolitis, characterized, and manufactured for use as a challenge virus in the adult experimental infection model. It supports safe, reproducible, quantifiable, and transient RSV infection and respiratory disease manifestations in adult volunteers. The virus has been used for studies of human RSV disease [3], [4] and the clinical testing of disease inhibitory drugs including anti-inflammatory immunomodulators and passively-transferred antibodies (e.g. MEDI-557 by MedImmune LLC [ClinicalTrials.gov identifier NCT01475305], ALS-008176 by Alios Biopharma, Inc. [ClinicalTrials.gov identifier NCT02094365], ALN-RSV01 by Alnylam Pharmaceuticals [ClinicalTrials.gov identifier NCT00496821], GS-5806 by Gilead Sciences [5], and RV568 by Respivert Ltd. [ClinicalTrials.gov identifier NCT01230645]), as well as pre-clinical research [4], [6]–[10]. Based on results from human adult tests with RSV Memphis-37, antiviral drug products are gaining regulatory approval for testing in high-risk adult populations, infants and children.
In this report, we describe the provenance of Memphis-37. We also compare 11 predicted protein sequences of Memphis-37 to those of other RSV A and B isolates. This information serves as a baseline reference for evaluation of future tests with the experimental RSV infection model. In addition, when escape mutants appear after vaccine or therapeutic drug testing with Memphis-37, results will indicate viral sites that may be targeted when second-generation drugs are developed.
Results and Discussion
In order to select a challenge virus for a reliable and useful RSV human experimental infection model, children with RSV were first identified from an outpatient urgent care center, emergency department, or from the inpatient area of a large regional pediatric hospital in Memphis, TN. All investigations involving any human subject were approved by the University of Tennessee Health Science Center Internal Review Board. Written informed consent was obtained from parents or legal guardians of the subjects, all of whom were below the age of ascent. From the years 2000–2005, 288 patients without chronic underlying conditions under two years of age who tested positive for RSV by antigen detection (Binax Now and Directigen) were enrolled. Participants were excluded if they had a history of prematurity, cardiac disease, chronic lung disease, immune deficiencies, bacterial co-infections, treatment with corticosteroids, ribavirin, RSV-Ig (Respigam), or palivizumab, or any experimental RSV intervention. The initial nasal aspirates were collected quantitatively as described previously [11] using FDA-approved sterile normal saline for inhalation. The samples were then aliquoted into sterile-sealed polystyrene vials. One fresh aliquot was utilized to quantify RSV by quantitative plaque assay in HEp2 cells without freezing [11], while the remainder of the sample was snap-frozen on dry ice and stored at −80°C. All 288 virus samples were evaluated by RSV strain-specific antibodies, N-gene specific genotype PCR, and quantitative PCR [11], [12].
To manufacture a virus that caused infection and disease similar to natural RSV infection in humans, we kept the viral passage number as low as possible. This minimized nucleic acid mutations caused by RNA-dependent RNA polymerase infidelity and the consequent loss of primary virus features, including patterns of viral protein glycosylation defined by post-translational modifications in human respiratory tract epithelial host cells. Original aliquots of samples from the 288 patients with the six highest viral loads in nasal washes (that were RSV-A defined by both serotyping and genotyping) were taken into a manufacturing suite and processed following Current Good Manufacturing Practices (cGMP). Samples were thawed and plaqued in FDA-approved Vero cell cultures. Each of the six selected viruses produced visible cytopathic effects (CPE), and three individual plaques from each of the six viruses were selected and aliquoted. One aliquot from each of the 18 plaques was placed into a Vero cell serum-free culture to assess quantitative viral growth kinetics. Primary aliquots from cultures exhibiting the most optimal in vitro growth kinetics were then manufactured using cGMP guidelines by passage in Vero cell culture roller bottles. Memphis-37 was then selected for final production, and the fill-finish was aliquoted in sterile glass vials and stored at −80°C. The final test product was passaged only five times. Tests for purity and adventitious agents during cGMP production were negative as listed in the FDA guidance documents for live viral vaccine production for human use.
The patient from whom Memphis-37 was originally isolated was a four month old, 5.9 kg, non-breast fed, African-American male who was the 2.6 kg product of a 40 week full term gestation. He was previously healthy and without environmental tobacco smoke exposure. In 2001, he developed respiratory symptoms without fever. On the 5th day of symptoms, the child was hospitalized for bronchiolitis, at which time informed consent and repository consent were obtained, and the patient's first nasal aspirate (from which Memphis-37 strain was isolated) was collected. His mother's prenatal labs were negative for human immunodeficiency virus (HIV) and the surface antigen of the hepatitis B virus (HBsAg), and her standard pre-natal screening labs were unremarkable. She had no history of transfusion or sexually transmitted infection. The patient's initial viral load was >6.78 log plaque forming units (PFU)/ml by fresh quantitative culture on HEp2 cells. The patient's quantitative culture-based viral load declined typically over successive days indicating normal RSV clearance [13]. The patient's viral load by qPCR was initially 7.29 log PFU(e)/ml and declined similarly over successive days. The patient remained hospitalized for four days, did not require supplemental oxygen, never required intensive care or mechanical ventilation, and did not receive any antivirals, passive antibodies or antibiotics.
The Memphis 37 virus was plaqued onto HEp-2 cells to examine morphology. Virus plaquing was performed with the patient's initial nasal aspirate and with the virus that had been passaged five times on Vero cells. The latter sample was also examined by electron microscopy at Advanced Biotechnologies Inc. (Columbia, MD). Plaques were easily countable on both HEp-2 cells and Vero cells. No contaminating adventitious agents including other viruses, yeasts, molds or bacteria were found. Virions were pleomorphic, round or filamentous, and of size consistent with RSV. As expected, small spikes were observed on virion surfaces.
Memphis-37 was amplified once more in HEp2 cells (for a total of six passages) to obtain RNA for Sanger sequencing. The complete sequence was submitted to GenBank (Accession # KM360090). There were several base positions for which major and minor peaks were identified in Sanger electropherograms. Affected positions and major and minor bases in the GenBank sequence were: 4188 (G/A), 4734 (A/G), 6041 (C/T), 8990 (A/C), 10,101 (A/T), 10,102 (A/T), 10,103 (A/T), and 14,102 (G/T). Attention to these heterogeneous sequences may reveal genetic bottlenecks in future infection or transmission studies with the Memphis-37 challenge virus.
The predicted amino acid sequences of RSV Memphis-37 proteins were determined using CLC software (Genomics Workbench, Qiagen) and aligned with nine additional viral sequences from GenBank, including five RSV type A sequences and four RSV type B sequences. Only the major bases described above were used for amino acid predictions. Within the comparator sequences were four laboratory isolates, including RSV A2 and Long, which are routinely used in basic and translational research laboratories [14], [15].
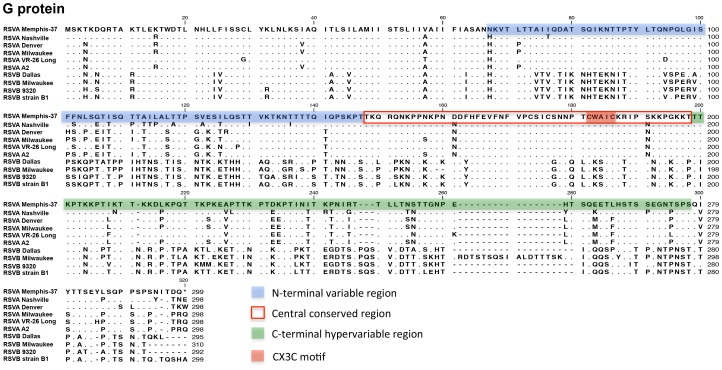
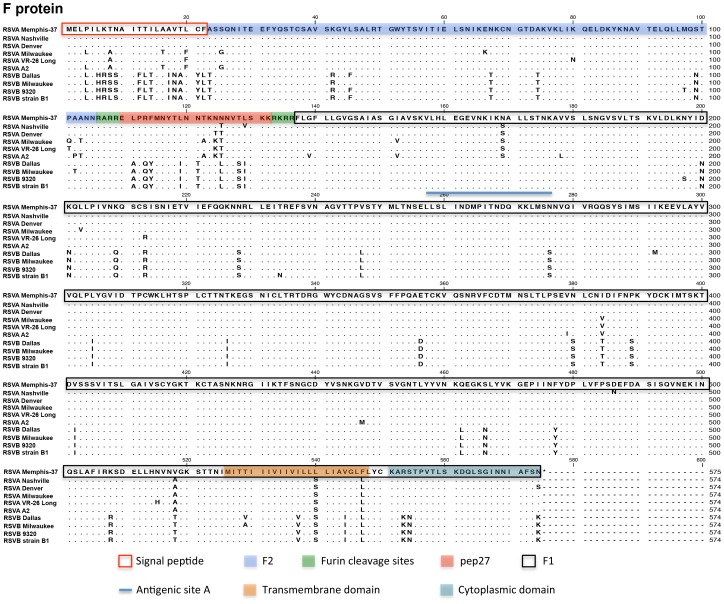
Results are shown for the RSV membrane glycoprotein (G) and fusion (F) protein in Figures 1 and 2, respectively, and for the nine additional proteins in Supplementary Figures (Figure S1. NS-1 protein; Figure S2. NS-2 Protein; Figure S3. N Protein; Figure S4. P protein; Figure S5. M Protein; Figure S6. SH Protein [16]; Figure S7. M2-1 Protein; Figure S8. M2-2 Protein; Figure S9. L protein [15], [17]). Memphis-37 was confirmed to be a type A virus and was subtyped as GA5 based on G protein C-terminal sequences (beginning at a.a. position 210 [18], [19]). GA5 was a common circulating genotype in 2001, the isolation year for Memphis-37 [18], [20].
Figure 1. Predicted amino acid sequence for RSV Memphis-37 G protein and alignments.
Predicted amino acid sequence is shown for the RSV Memphis-37 G protein. Sequence was aligned with five subtype A viruses (including two laboratory strains) and four subtype B viruses (including two laboratory strains). Virus nomenclature and GenBank Accession numbers used in the alignment are: RSVA Nashville (JX069801.1), RSVA Denver (GU591769.1 [42]), RSVA Milwaukee (JF920069.1 [43]), RSVA VR-26 Long (AY911262.1, Laboratory strain), RSVA A2 (M74568.1, Laboratory strain [15]), RSVB Dallas (JQ582843.1), RSVB Milwaukee (JN032117.1 [43]), RSVB 9320 (AY353550.1, B9320 Laboratory strain), RSV strain B1 (AF013254.1, B1 Laboratory strain [44]). N-terminal variable region: a.a. 67–147, central conserved region: a.a. 148–198, C-terminal hypervariable region: a.a. 199–298, CX3C motif: a.a. 182–186. RSV Memphis-37 sequencing methods: For sequencing purposes, Memphis-37 was taken after five passages and was amplified once more in a T25 flask of HEp-2 cells. Briefly, virus was added to cells in DMEM/0.1% BSA for 1.5 hours. Medium was removed and replaced with EMEM/5% FCS for four days. A lysate was prepared and viral RNA was extracted using a Qiagen viral RNA mini kit. PCR reactions were performed using the TaKaRa One Step RNA PCR Kit (AMV) using 5 µl (145 ng/µl) of viral RNA extracted with Qiagen QIAmp Viral RNA Mini Kit. Forward and reverse oligonucleotides were prepared at concentrations of 100 µM and 1 µl of each pair was used in each reaction. Incubations were at 50°C for 30 min. and 94°C for 2 min. Then 40 cycles were run at 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1.5 min. For each sequencing reaction, 40 ng PCR product were mixed with 3.2 pmoles oligonucleotide primer in a final volume of 12 µl and submitted to the Hartwell Center at St. Jude for Sanger sequencing. Sequences were edited and a contig was created in Vector NTI SeqMan. The consensus contig for Memphis-37 was imported into CLC Workbench and predicted amino acid sequences were aligned with other RSV sequences.
Figure 2. Predicted amino acid sequence for RSV Memphis-37 F protein and alignments.
Alignments are as described in Figure 1, but for the F protein. Features of interest are shaded or boxed as indicated below the sequence. The blue line marks an antigenic site A, which is targeted by the monoclonal antibody Palivizumab.
RSV G (Figure 1) is an attachment protein that adheres virus to its target mammalian cell, in part by binding glycosaminoglycans on the host cell surface [21]. A relatively well-conserved central cysteine loop region (a.a. 148–198) was shared in sequence between Memphis-37 and other RSV type A viruses. This region contains a CX3C motif (CWAIC at a.a. position 182–186) known to modify the host immune response during RSV infection by chemokine mimicry and alteration of leukocyte migration [22]. The CX3C motif binds the fractalkine receptor CX3CR1 and modifies CX3CR1-positive RSV-specific T-cell responses [23]. Antibodies generated toward this central conserved region of RSV G have some ability to cross-neutralize RSV A and B strains [24], [25].
Multiple potential N-linked glycosylation sites (N-X-T or N-X-S when X is not proline) were present in the G protein. The G protein alignment in Figure 1 illustrated the positioning of variable regions known to include sites for neutralizing antibody recognition and associated virus escape [26]. Alignments of predicted amino acid sequences for other RSV proteins (Figure 2 and Figures S1 to S9) showed additional variable regions including those in the C-terminal, extracellular region of SH (including a site of potential N-linked glycosylation, a.a. positions 52-54 in Figure S6), the central region of M2-2, and two regions in L [17].
The RSV F protein (Figure 2) is essential for RSV fusion and entry into its host cell target. The full-length protein F0 is cleaved to fragments F1 and F2 to form the mature F protein. Figure 2 illustrates important features of F including its signal peptide (a.a. 1–22), furin cleavage sites (a.a. 106–109 and 133–136 [27]), pep27 (a.a. 110–136, a small peptide released upon protein cleavage), the transmembrane domain (a.a. 526–548), and the cytoplasmic domain (a.a. 550–574). A key site for virus resistance to a monoclonal antibody that is used as RSV prophylaxis in children (Palivizumab [28]) exists between a.a. positions 257–276 (antigenic site A, indicated by the blue line in Figure 2). Amino acid substitutions in this site that have conferred resistance to Palivizumab in cell culture, animal models, or humans are N262Y/S, N268I, K272E/M/N/Q/T, S275F, and N276Y [29]–[31]. These substitutions were not identified in the Memphis-37 sequence. A more recently described D25 antibody also exhibits potent neutralization of RSV [32]. D25 inhibits F-mediated fusion. This antibody binds a quaternary epitope named antigenic site Ø at the membrane-distal apex of the pre-fusion F trimer. It interacts with the α4 helix (F1 residues 196–210) and forms intermolecular hydrogen bonds with F2 residues 63, 65, 66, and 68. Antibody contact points are shared between RSV Memphis 37 and A2, but not between RSV A and B isolates. Continued research studies with neutralizing antibodies and Memphis 37 are encouraged to unravel mechanisms of virus inhibition, while defining new strategies for RSV control.
RSV-specific CD4-positive and CD8-positive T lymphocytes partner with antibodies to inhibit virus infections by producing cytokines, killing virus infected targets, and regulating innate and adaptive effector functions. We examined human T-cell epitope sequences that had previously been described by in vitro studies, mapped onto the RSV A2 sequence [33]–[41]. Table 1 shows that these epitope sequences were almost entirely matched between Memphis-37 and RSV A2. All epitopes within the G, N, M, M2, and NS proteins showed 100% sequence homology between the two strains. A T-cell epitope sequence flanking the CXC3 motif in the RSV G protein was perfectly matched between RSV A2 and Memphis-37 (a.a. 162–175). Only a few epitope sequences within the F proteins, which are bolded in Table 1, were observed to be different. As studies with the experimental infection model progress, attention should be paid to variable sites and regions, which may mark positions of virus escape from candidate vaccines and therapeutic drugs.
Table 1. Comparison of human CD4 and CD8 T-lymphocyte epitope sequences between respiratory syncytial virus Memphis-37 and A2 strains.
| RSV protein | Amino acid position | Peptide sequence* | % Homology between peptides | ||||
| RSV-Memphis-37 | RSV-A2 | ||||||
| CD4 | G | 162−175 | DFHFEVFNFVPCSI | DFHFEVFNFVPCSI | 100 | ||
| F | 7−30 | KTNAITTILAAVTLCFASSQNITE | KANAITTILTAVTFCFASGQNITE | 83 | |||
| F | 25−42 | SQNITEEFYQSTCSAVSK | GQNITEEFYQSTCSAVSK | 94 | |||
| F | 43−60 | GYLSALRTGWYTSVITIE | GYLSALRTGWYTSVITIE | 100 | |||
| F | 49−72 | RTGWYTSVITIELSNIKENKCNGT | RTGWYTSVITIELSNIKENKCNGT | 100 | |||
| F | 55−72 | SVITIELSNIKENKCNGT | SVITIELSNIKENKCNGT | 100 | |||
| F | 73−90 | DAKVKLIKQELDKYKNAV | DAKVKLIKQELDKYKNAV | 100 | |||
| F | 85−102 | KYKNAVTELQLLMQSTPA | KYKNAVTELQLLMQSTPP | 94 | |||
| F | 109−132 | RELPRFMNYTLNNTKNNNVTLSKK | RELPRFMNYTLNNAKKTNVTLSKK | 88 | |||
| F | 175−192 | NKAVVSLSNGVSVLTSKV | NKAVVSLSNGVSVLTSKV | 100 | |||
| F | 193−210 | LDLKNYIDKQLLPIVNKQ | LDLKNYIDKQLLPIVNKQ | 100 | |||
| F | 229−252 | RLLEITREFSVNAGVTTPVSTYML | RLLEITREFSVNAGVTTPVSTYML | 100 | |||
| F | 265−288 | PITNDQKKLMSNNVQIVRQQSYSI | PITNDQKKLMSNNVQIVRQQSYSI | 100 | |||
| F | 295−318 | EVLAYVVQLPLYGVIDTPCWKLHT | EVLAYVVQLPLYGVIDTPCWKLHT | 100 | |||
| F | 337−360 | TDRGWYCDNAGSVSFFPQAETCKV | TDRGWYCDNAGSVSFFPQAETCKV | 100 | |||
| F | 391−408 | YDCKIMTSKTDVSSSVIT | YDCKIMTSKTDVSSSVIT | 100 | |||
| F | 409−426 | SLGAIVSCYGKTKCTASN | SLGAIVSCYGKTKCTASN | 100 | |||
| F | 427−444 | KNRGIIKTFSNGCDYVSN | KNRGIIKTFSNGCDYVSN | 100 | |||
| F | 457−486 | YYVNKQEGKSLYVKGEPIINFYDPLVFPSD | YYVNKQEGKSLYVKGEPIINFYDPLVFPSD | 100 | |||
| F | 493−516 | SQVNEKINQSLAFIRKSDELLHNV | SQVNEKINQSLAFIRKSDELLHNV | 100 | |||
| F | 517−534 | NVGKSTTNIMITTIIIVI | NAGKSTTNIMITTIIIVI | 94 | |||
| F | 541−558 | LIAVGLFLYCKARSTPVT | LIAVGLLLYCKARSTPVT | 94 | |||
| CD8 | F | 8−17 | TNAITTILAA | ANAITTILTA | 80 | ||
| F | 93−102 | LQLLMQSTPA | LQLLMQSTPA | 100 | |||
| F | 106–113 | RARRELPRF | RARRELPRF | 100 | |||
| F | 109–118 | RELPRFMNYT | RELPRFMNYT | 100 | |||
| F | 260–269 | LINDMPITND | LINDMPITND | 100 | |||
| F | 273–282 | LMSNNVQIVR | LMSNNVQIVR | 100 | |||
| F | 285–294 | SYSIMSIIKE | SYSIMSIIKE | 100 | |||
| F | 374–383 | TLPSEVNLCN | TLPSEVNLCN | 100 | |||
| F | 388–397 | NPKYDCKIMT | NPKYDCKIMT | 100 | |||
| F | 519–528 | GKSTTNIMIT | GKSTINIMIT | 90 | |||
| F | 521–530 | STTNIMITTI | STINIMITTI | 90 | |||
| F | 542–550 | IAVGLFLYC | IAVGLLLYC | 89 | |||
| N | 46–59 | KLCGMLLITEDANH | KLCGMLLITEDANH | 100 | |||
| N | 232–245 | STRGGSRVEGIFAG | STRGGSRVEGIFAG | 100 | |||
| N | 250–263 | AYGAGQVMLRWGVL | AYGAGQVMLRWGVL | 100 | |||
| N | 253–266 | AGQVMLRWGVLAKS | AGQVMLRWGVLAKS | 100 | |||
| N | 256–269 | VMLRWGVLAKSVKN | VMLRWGVLAKSVKN | 100 | |||
| N | 298–311 | AGFYHILNNPKASL | AGFYHILNNPKASL | 100 | |||
| N | 306–314 | NPKASLLSL | NPKASLLSL | 100 | |||
| M | 195–203 | IPYSGLLLV | IPYSGLLLV | 100 | |||
| M | 229–237 | YLEKESIYY | YLEKESIYY | 100 | |||
| M2-1 | 64–72 | AELDRTEEY | AELDRTEEY | 100 | |||
| M2-1 | 151–159 | RLPADVLKK | RLPADVLKK | 100 | |||
| NS1 | 41–49 | LAKAVIHTI | LAKAVIHTI | 100 | |||
Legend: *Differences between Memphis-37 and RSV A2 are bolded.
In conclusion, we have described the provenance, production, and sequences of Memphis-37. These data serve as valuable reference material for the evaluation of RSV vaccines and therapeutics in the adult human experimental infection model. Memphis 37 has already supported RSV research in vitro and in vivo, including studies in lambs, monkeys and man Clinical studies test numerous drug products, including candidates for RSV prophylaxes in children (e.g. monoclonal antibodies) and RSV treatments (e.g. small molecules and siRNAs). Future research will continue to examine the characteristics of Memphis 37 in challenge, transmission and serological studies while expediting the development of clinical vaccines and therapeutic drug strategies for the protection of vulnerable populations against RSV disease.
Supporting Information
Predicted amino acid sequence for RSV Memphis-37 NS1 protein and alignments. Alignments are as described for Figure 1, but for the NS1 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 NS2 protein and alignments. Alignments are as described for Figure 1, but for the NS2 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 N protein and alignments. Alignments are as described for Figure 1, but for the N protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 P protein and alignments. Alignments are as described for Figure 1, but for the P protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 M protein and alignments. Alignments are as described for Figure 1, but for the M protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 SH protein and alignments. Alignments are as described for Figure 1, but for the SH protein. Transmembrane domain: a.a. 23–41, Potential site for glycosylation: a.a. 52–54.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 M2-1 protein and alignments. Alignments are as described for Figure 1, but for the M2-1 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 M2-2 protein and alignments. Alignments are as described for Figure 1, but for the M2-2 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 L protein and alignments. Alignments are as described for Figure 1, but for the L protein.
(PDF)
Acknowledgments
We would like to thank Dave Konys (Alnylam), Akshay Vaishnaw (Alnylam), Preston Dorsett, (Viral Antigens) Robert Studholme (Viral Antigens/Meridian Life Science, Inc.), and Victor Van Cleave (Viral Antigens/Meridian Life Science, Inc.) for their assistance with Memphis-37 virus production. With profound gratitude, we acknowledge the numerous altruistic parents and caregivers of the many research subjects without which this work would be impossible. We recognize and applaud the humanitarian decision of John Maraganore and Barry Green of Alnylam Pharmaceuticals to fund the Memphis-37 development project while allowing the general commercial availability of Memphis-37 to be free of intellectual property, royalties, or other restrictions. The manufacturing of Memphis-37 and the development of the experimental infection model itself, using Memphis-37 was supported by Alnylam Pharmaceuticals.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. Data have also been submitted to Genbank as accession number KM360090.
Funding Statement
This study was supported by the National Institutes of Health (NIH) NIAID R01 AI088729, NIH P01 P30 CA21765 and the American Lebanese Syrian Associated Charities to JLH, NIH RR 16187-01 to JPD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Glezen WP, Taber LH, Frank AL, Kasel JA (1986) Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140:543–546. [DOI] [PubMed] [Google Scholar]
- 2. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeVincenzo JP, Cehelsky J, Meyers R, Vaishnaw A, Nochur S, et al. (2007) Development of a human experimental infection model of Respiratory Syncytial Virus. Presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) Abstract # V-1257 Chicago, IL.
- 4. DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, et al. (2010) Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182:1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, et al. (2014) Oral GS-5806 Activity in a Respiratory Syncytial Virus Challenge Study. N Engl J Med 371:711–722. [DOI] [PubMed] [Google Scholar]
- 6. Ackermann MR (2014) Lamb model of respiratory syncytial virus-associated lung disease: insights to pathogenesis and novel treatments. ILAR J 55:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Derscheid RJ, van Geelen A, Gallup JM, Kienzle T, Shelly DA, et al. (2014) Human respiratory syncytial virus memphis 37 causes acute respiratory disease in perinatal lamb lung. Biores Open Access 3:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grosz DD, van Geelen A, Gallup JM, Hostetter SJ, Derscheid RJ, et al. (2014) Sucrose stabilization of Respiratory Syncytial Virus (RSV) during nebulization and experimental infection. BMC Res Notes 7:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derscheid RJ, van Geelen A, McGill JL, Gallup JM, Cihlar T, et al. (2013) Human Respiratory Syncytial Virus Memphis 37 Grown in HEp-2 Cells Causes more Severe Disease in Lambs than Virus Grown in Vero Cells. Viruses 5:2881–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eyles JE, Johnson JE, Megati S, Roopchand V, Cockle PJ, et al. (2013) Nonreplicating vaccines can protect african green monkeys from the memphis 37 strain of respiratory syncytial virus. J Infect Dis 208:319–329. [DOI] [PubMed] [Google Scholar]
- 11. DeVincenzo JP (2004) Natural infection of infants with respiratory syncytial virus subgroups A and B: a study of frequency, disease severity, and viral load. Pediatr Res 56:914–917. [DOI] [PubMed] [Google Scholar]
- 12. Perkins SM, Webb DL, Torrance SA, El Saleeby C, Harrison LM, et al. (2005) Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol 43:2356–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, DeVincenzo JP (2011) Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lo MS, Brazas RM, Holtzman MJ (2005) Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J Virol 79:9315–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stec DS, Hill MG III, Collins PL (1991) Sequence analysis of the polymerase L gene of human respiratory syncytial virus and predicted phylogeny of nonsegmented negative-strand viruses. Virology 183:273–287. [DOI] [PubMed] [Google Scholar]
- 16. Fuentes S, Tran KC, Luthra P, Teng MN, He B (2007) Function of the respiratory syncytial virus small hydrophobic protein. J Virol 81:8361–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fix J, Galloux M, Blondot ML, Eleouet JF (2011) The insertion of fluorescent proteins in a variable region of respiratory syncytial virus L polymerase results in fluorescent and functional enzymes but with reduced activities. Open Virol J 5:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reiche J, Schweiger B (2009) Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J Clin Microbiol 47:1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frabasile S, Delfraro A, Facal L, Videla C, Galiano M, et al. (2003) Antigenic and genetic variability of human respiratory syncytial viruses (group A) isolated in Uruguay and Argentina: 1993–2001. J Med Virol 71:305–312. [DOI] [PubMed] [Google Scholar]
- 20. Sato M, Saito R, Sakai T, Sano Y, Nishikawa M, et al. (2005) Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J Clin Microbiol 43:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine S, Klaiber-Franco R, Paradiso PR (1987) Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol 68 (Pt 9):2521–2524. [DOI] [PubMed] [Google Scholar]
- 22. Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, et al. (2001) CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738. [DOI] [PubMed] [Google Scholar]
- 23. Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, et al. (2006) Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 176:1600–1608. [DOI] [PubMed] [Google Scholar]
- 24. Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, et al. (2010) Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi Y, Mason CS, Jones LP, Crabtree J, Jorquera PA, et al. (2012) Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunol 25:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez I, Dopazo J, Melero JA (1997) Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J Gen Virol 78 (Pt 10):2419–2429. [DOI] [PubMed] [Google Scholar]
- 27. Zimmer G, Conzelmann KK, Herrler G (2002) Cleavage at the furin consensus sequence RAR/KR(109) and presence of the intervening peptide of the respiratory syncytial virus fusion protein are dispensable for virus replication in cell culture. J Virol 76:9218–9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The IMpact-RSV Study Group (1998) Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537. [PubMed] [Google Scholar]
- 29. Bates JT, Keefer CJ, Slaughter JC, Kulp DW, Slaughter JC, Schief WR, et al. (2014) Escape from neutralization by the respiratory syncytial virus-specific neutralizing monoclonal antibody palivizumab is driven by changes in on-rate of binding to the fusion protein. Virology 454–455:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Academy of Pediatrics, Infectious diseases and bronchiolitis guidelines committee (2014) Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 134:415–420. [DOI] [PubMed] [Google Scholar]
- 31. Grad YH, Newman R, Zody M, Yang X, Murphy R, et al. (2014) Within-Host Whole-Genome Deep Sequencing and Diversity Analysis of Human Respiratory Syncytial Virus Infection Reveals Dynamics of Genomic Diversity in the Absence and Presence of Immune Pressure. J Virol 88:7286–7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLellan JS, Chen M, Leung S, Graepel KW, Du X, et al. (2013) Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levely ME, Bannow CA, Smith CW, Nicholas JA (1991) Immunodominant T-cell epitope on the F protein of respiratory syncytial virus recognized by human lymphocytes. J Virol 65:3789–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Graaff PM, Heidema J, Poelen MC, van Dijk ME, Lukens MV, et al. (2004) HLA-DP4 presents an immunodominant peptide from the RSV G protein to CD4 T cells. Virology 326:220–230. [DOI] [PubMed] [Google Scholar]
- 35. Heidema J, de Bree GJ, de Graaff PM, van Maren WW, Hoogerhout P, et al. (2004) Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes. J Gen Virol 85:2365–2374. [DOI] [PubMed] [Google Scholar]
- 36. van Bleek GM, Poelen MC, van der Most R, Brugghe HF, Timmermans HA, et al. (2003) Identification of immunodominant epitopes derived from the respiratory syncytial virus fusion protein that are recognized by human CD4 T cells. J Virol 77:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brandenburg AH, de Waal L, Timmerman HH, Hoogerhout P, de Swart RL, et al. (2000) HLA class I-restricted cytotoxic T-cell epitopes of the respiratory syncytial virus fusion protein. J Virol 74:10240–10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goulder PJ, Lechner F, Klenerman P, McIntosh K, Walker BD (2000) Characterization of a novel respiratory syncytial virus-specific human cytotoxic T-lymphocyte epitope. J Virol 74:7694–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rock MT, Crowe JE Jr (2003) Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology 108:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venter M, Madhi SA, Tiemessen CT, Schoub BD (2001) Genetic diversity and molecular epidemiology of respiratory syncytial virus over four consecutive seasons in South Africa: identification of new subgroup A and B genotypes. J Gen Virol 82:2117–2124. [DOI] [PubMed] [Google Scholar]
- 41. Olson MR, Varga SM (2008) Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev Vaccines 7:1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumaria R, Iyer LR, Hibberd ML, Simoes EA, Sugrue RJ (2011) Whole genome characterization of non-tissue culture adapted HRSV strains in severely infected children. Virol J 8:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rebuffo-Scheer C, Bose M, He J, Khaja S, Ulatowski M, et al. (2011) Whole genome sequencing and evolutionary analysis of human respiratory syncytial virus A and B from Milwaukee, WI 1998–2010. PLoS One 6:e25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karron RA, Buonagurio DA, Georgiu AF, Whitehead SS, Adamus JE, et al. (1997) Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A 94:13961–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predicted amino acid sequence for RSV Memphis-37 NS1 protein and alignments. Alignments are as described for Figure 1, but for the NS1 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 NS2 protein and alignments. Alignments are as described for Figure 1, but for the NS2 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 N protein and alignments. Alignments are as described for Figure 1, but for the N protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 P protein and alignments. Alignments are as described for Figure 1, but for the P protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 M protein and alignments. Alignments are as described for Figure 1, but for the M protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 SH protein and alignments. Alignments are as described for Figure 1, but for the SH protein. Transmembrane domain: a.a. 23–41, Potential site for glycosylation: a.a. 52–54.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 M2-1 protein and alignments. Alignments are as described for Figure 1, but for the M2-1 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 M2-2 protein and alignments. Alignments are as described for Figure 1, but for the M2-2 protein.
(TIF)
Predicted amino acid sequence for RSV Memphis-37 L protein and alignments. Alignments are as described for Figure 1, but for the L protein.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files. Data have also been submitted to Genbank as accession number KM360090.