Abstract
Purpose.
We investigated bilateral tear cytokine levels in patients with unilateral bacterial keratitis (BK) as associated with in vivo confocal microscopic (IVCM) alterations in corneal nerves and dendritiform immune cells (DCs).
Methods.
A total of 54 (13 BK, 13 contralateral, 28 healthy controls) tear samples was collected prospectively and analyzed by multiplex microbeads assay. The IVCM of the central cornea was performed on the same day, and assessed for corneal nerve and DC alterations.
Results.
Interleukin-1β, IL-6, and IL-8 were significantly elevated only in affected eyes (66.6 ± 26.8, 7174 ± 2430, and 810 ± 315 ρg/mL, respectively; P = 0.04, P < 0.001, and P < 0.001, respectively), compared to healthy controls (13.0 ± 4.0, 171.8 ± 32.1, and 56.5 ± 33.8 ρg/mL). Levels of chemokine ligand 2 (CCL-2), IL-10, and IL-17a were elevated only in contralateral eyes (813 ± 478, 86.7 ± 38.3, and 3350 ± 881 ρg/mL, respectively; P = 0.02, P = 0.01, and P = 0.04, respectively), compared to controls (73.7 ± 25.3, 17.5 ± 4.9, and 1350 ± 337 ρg/mL). Triggering receptor expressed on myeloid cells (TREM)-1 was significantly elevated in affected (551 ± 231 ρg/mL, P = 0.02) and contralateral unaffected (545 ± 298 ρg/mL, P = 0.03) eyes compared to controls (31.3 ± 12.4 ρg/mL). The density of DCs was significantly increased in affected (226.9 ± 37.3 cells/mm2, P < 0.001) and unaffected (122.3 ± 23.7 cells/mm2, P < 0.001) eyes compared to controls (22.7 ± 5.9 cells/mm2). Sub-basal nerve density significantly decreased in affected (3337 ± 1615 μm/mm2, P < 0.001) and contralateral (13,230 ± 1635 μm/mm2, P < 0.001) eyes compared to controls (21,200 ± 545 μm/mm2). Levels of IL-1β, IL-6, and IL-8 were significantly correlated with DC density (R = 0.40, R = 0.55, and R = 0.31, all P < 0.02) and nerve density (R = −0.30, R = −0.53, and R = −0.39, all P < 0.01).
Conclusions.
Proinflammatory tear cytokines are elevated bilaterally in patients with unilateral BK, and are correlated strongly with alterations in DCs and nerve density as detected by IVCM.
Keywords: infectious keratitis, dendritic cells, corneal infection, tears, cytokines
Proinflammatory tear cytokines are elevated bilaterally in patients with unilateral bacterial keratitis, and are correlated strongly with alterations in dendritiform immune cells and nerve density as detected by in vivo confocal microscopy.
Introduction
Bacterial keratitis (BK) is a potentially blinding ocular condition of the cornea, which can result in severe loss of vision due to corneal scarring, corneal perforation, or endothalmitis.1,2 Risk factors for BK include contact lens wear, ocular surface disease, trauma, chronic use of topical steroids, corneal surgery, diabetes, and neurotrophic keratopathy.3–5 During the past decade, in vivo confocal microscopy (IVCM) has been used increasingly to evaluate the structural and cellular changes in various corneal diseases.6–12 It is being used in the diagnosis and management of corneal diseases, as well as in the assessment of disease severity.8,13 This technique is further being used to understand the pathophysiology of corneal diseases in patients, and has led to new insights into ocular and systemic diseases, such as keratoconus, herpetic keratitis, and diabetic neuropathy.6,14–17 Recently, we demonstrated bilateral nerve alterations in patients with unilateral herpes simplex keratitis (HSK) and herpes zoster ophthalmicus (HZO) by IVCM.15,16 Moreover, we showed an inverse correlation between the nerve density and density of dendritiform immune cells (DCs) in patients with infectious keratitis, including bacterial, fungal, and Acanthamoeba keratitis.18 However, the mechanism and consequences of bilateral nerve alterations in unilateral infectious keratitis remain poorly understood.
Recent studies have suggested that proinflammatory cytokines in tears may have a key role in the pathogenesis of several corneal diseases, including dry eye disease,19 keratoconus,20,21 graft-versus-host disease (GVHD),22 conjunctivitis,23 as well as in the development of corneal neovascularization.24 However, currently, to our knowledge, there have been no reports on the alterations of cytokine levels in patients with infectious keratitis. Furthermore, previous studies have assessed tear cytokine changes in either affected eyes21,24 or in bilateral diseases,22–25 but to our knowledge contralateral changes in tear cytokines in unilateral diseases have not been assessed before. Finally, although previous reports suggested that tear cytokine levels correlated with clinical findings, such as keratorefractive values in keratoconus20 or with clinical severity score in dry eye patients,25,26 it is unclear if intracorneal cellular changes in DCs and corneal nerves as shown by IVCM correspond with changes in tear cytokines.
We hypothesized that alterations in corneal DCs and corneal nerves by IVCM would correlate with changes in proinflammatory tear cytokines, and that unilateral BK would alter bilateral tear cytokine levels. Thus, in the current study, we aimed to quantify bilateral tear cytokines levels, and correlate them with alterations in corneal nerves and DCs as detected by IVCM in unilateral BK.
Patients and Methods
Patients
A prospective, single-center study was conducted in a masked fashion. Subjects with acute BK at presentation were recruited from the Cornea & Refractive Surgery Service at the Massachusetts Eye and Ear Infirmary (Boston, MA, USA), between 2011 and 2013. A total of 10 patients with a diagnosis of acute unilateral BK was included in this study and compared to 14 normal age- and sex-matched healthy control subjects. We collected 54 tear samples from the affected and contralateral clinically unaffected eyes of BK patients as well as from both eyes of healthy control subjects. In three BK patients, tear collection and IVCM were performed twice (Patients 8–10) to analyze changes over a 1- to 2-week time period after the initial visit. In all 3 patients, the infiltration and corneal epithelial defect improved after the treatment was started. All patients and healthy control subjects underwent slit-lamp biomicroscopy. Bacterial keratitis was diagnosed according to the patient's history, clinical examinations, and/or cultures. Patients with other causative organism, such as viral, fungal, and Acanthamaoeba keratitis, were excluded. None of the healthy controls had trauma, surgery, contact lens use, or other ocular surface diseases, such as dry eye disease or conjunctivitis. None of the BK patients or healthy control subjects had any systemic immunological diseases or diabetes. The study protocol was approved by the Institutional review Board/Ethics Committee from the authors' institution, complied with the Health Insurance Portability and Accountability Act, and was conducted in accordance with the provision of the Declaration of Helsinki. All patients provided written informed consent after a detailed explanation of the nature of the study.
Tear Collection
Tear collection was conducted before the IVCM examination and instillation of any eye drops as described previously.27 Briefly, 60 μL of sterile saline solution were instilled into the inferior fornix by using a micropipette. Next, subjects were asked to look left, right, up, and down, four times without blinking, to mix the tear fluid content. Then, the diluted tears with sterile saline were collected from the inferior fornix using the micropipette and transferred to an Eppendorf tube. This process was repeated for the contralateral eye and for both eyes of control subjects, always using a new micropipette sterile tip for each eye. All tear samples were stored in individual Eppendorf tubes at −80°C.
In Vivo Confocal Microscopy
Laser IVCM (Heidelberg Retina Tomograph 3/Rostock Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany) was performed on the central cornea of BK patients and control subjects bilaterally after tear collection. This microscope uses a 670-nm wavelength diode laser source and is equipped with a ×63 objective immersion lens with a numerical aperture of 0.9 (Olympus, Tokyo, Japan). The microscope provides a 400 × 400–μm section of the cornea with axial resolution of 1 to 4 μm. The technique used was the same as reported previously.18 Briefly, a disposable sterile polymethylmethacrylate (PMMA) cap (TOMO-cap; Heidelberg Engineering GmbH, Heidelberg, Germany) filled with hydroxypropyl methylcellulose 2.5% (GenTeal gel; Novartis Ophthalmics, East Hanover, NJ, USA) was mounted in front of the cornea module objective lens for each examination. After the instillation of topical anesthesia of 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX, USA) in both eyes, a drop of hydroxypropyl methylcellulose 2.5% (GenTeal gel) was applied to the inferior fornix. Further, hydroxypropyl gel was added to the outside tip of the TOMO-cap to improve optical coupling and the lens was advanced until the cap made contact with the surface of the cornea. Images were obtained from the epithelial layer to the endothelial layer using multiple scans in the sequence mode. A total of four to six sequence scans was recorded for each eye, at least 3 of which were sequence scans with particular focus on the subepithelial area, the sub-basal nerve plexus, and epithelial DCs, typically at a depth of 50 to 80 μm.
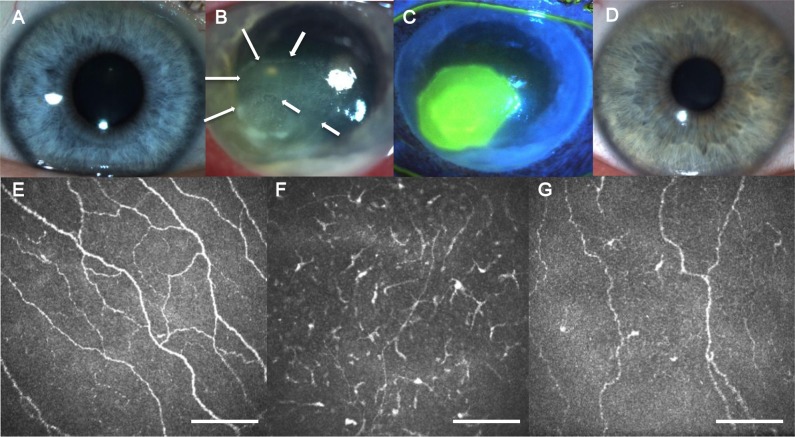
When a corneal ulcer was present with an epithelial defect or severe ulceration in eyes with BK, the ulcer and periphery of the ulcer were scanned. If the images of the ulcer were not suitable due to severe edema or opacity, the periphery of the ulcer (Fig. 1B, arrows) was chosen for the analysis to minimize the bias from the opacity or edematous ulceration. A minimum of 3 representative images of the sub-basal nerve plexus and epithelial DCs were selected for analysis for each eye. The images were selected from the layer immediately at or posterior to the basal epithelial layer and anterior to the Bowman's layer. The criteria to select the images were the best-focused and complete images, with the whole image in the same layer, without motion, without folds, and good contrast. Sub-basal nerve fibers and DC density and morphology were analyzed as described previously.18 Particularly, image analysis was performed using the ImageJ (developed by Wayne Rasband, National Institutes of Health [NIH], Bethesda, MD, USA; available in the public domain at http://rsb.info.nih.gov/ij/; accessed March 2014) and NeuronJ (available in the public domain at http://www.imagescience.org/meijering/software/neuronj/; accessed March 2014), a semiautomated tracing plugin for ImageJ, a free image analysis software distributed by the NIH. Three masked observers (TY, BMC, and YQ) evaluated the confocal images for the parameters as described below. Nerve density was assessed by measuring the total length of the nerve fibers in micrometers per frame of 0.16 mm2 area and expressed as μm/mm2. Number of nerves was defined as the number of total nerve fibers per frame of 0.16 mm2 area. The DC parameters included DC density, DC area, numbers of dendrites per cell, and DC field. The DCs were defined morphologically as bright dendritiform, well-demarcated structures, predominantly found in the sub-basal area. The DC density was calculated by dividing the total number of DCs per each frame of 0.16 mm2, expressed as cells/mm2. The DC area was determined as the mean actual cell area of 5 representative DCs/frame from 3 representative frames (total 15 DCs) and was expressed as μm2/cell. The number of dendrites is defined as the mean number of dendrites per cells of 5 representative DCs/frame from 3 representative frames. The DC field was defined as the span of each cell, which included the area of its mean cell body and dendrites of 5 representative DCs/frame from 3 representative frames and was expressed as μm2/cell.
Figure 1.
Slit-lamp photographs and in vivo confocal microscopy images. The representative slit-lamp photographs of a normal control eye (A), a clinically affected eye with unilateral BK (B, C), and its contralateral clinically unaffected eye (D). The IVCM images of a healthy control eye (E), a clinically affected eye with unilateral BK (F), and its contralateral clinically unaffected eye (G). Scale bars: 100 μm.
Tear Cytokine Protein Levels
The cytokine levels (IL-1Ra, IL-1β, IL-2, IL-5, IL-6, IL-7, IL-8, IL-10, IL-17a, fibroblast growth factor [FGF]-2, granulocyte/macrophage colony stimulating factor [GM-CSF], chemokine ligand [CCL]-2, and triggering receptor expressed on myeloid cells [TREM]-1) of tear samples were measured by Luminex (Luminex Corporation, San Antonio, TX, USA) beads-based multiplex immunoassay according to previous reports.23 Briefly, 20 μL of each tear sample were incubated with antibody-coated capture beads in an incubation buffer (PBS + 0.1% Tween-20 + 0.05% sodium azide) at room temperature. After 1 hour of incubation in the dark, the beads were washed with washing buffer (PBS + 0.1% Tween-20) by centrifugation, and biotinylated secondary antibodies were added for 30 minutes of incubation in the dark at room temperature. Plates were washed with washing buffer by centrifugation, and Phycoerythrin-labeled streptavidin was added for 15 minutes of incubation in the dark at room temperature. After being washed twice in washing buffer by centrifugation, plates were resuspended in 50 μL washing buffer, and assays were performed using a Luminex 200 instrument (Luminex Corporation). We used the STarStation v2.3 and STarCollate software program (Applied Cytometry Systems, Dinnington, UK) to determine sample cytokine levels by standard curve analysis using known concentrations of cytokines as standards.
Statistical Analysis
Data were analyzed using Prism for Windows version 6.04 (GraphPad Software, Inc., San Diego, CA, USA). To compare the sub-basal nerve density; DC density and morphology; and cytokine levels between control eyes, affected BK eyes, and contralateral unaffected eyes; 1-way ANOVA with Tukey's multiple comparisons post-test was performed. Spearman's correlation coefficient analysis was used to evaluate the correlation among sub-basal nerve density, DC parameters, and cytokine levels. For each test, differences were considered statistically significant at a P value of less than 0.05 and data were presented as mean ± SE.
Results
We collected 26 tear samples from 10 patients with BK and 28 tear samples from 14 healthy control subjects. Figure 1 shows the slit-lamp photographs and IVCM images of the representative cases of each group. Demographic data of BK and the healthy control group are summarized in Table 1. Clinical data of BK patients are presented in Table 2. The causative organisms were identified using culture examination in 5 of 10 patients. Five other patients were diagnosed with BK by their history and clinical findings, as well as their rapid response to the antibacterial therapy.
Table 1.
Demographics of Patients and Control Subjects
|
Healthy Controls |
BK |
Contralateral |
|
| Subjects, n | 14 | 10 | 10 |
| Tear samples, n | 28 | 13 | 13 |
| Mean age ± SD, y | 38.7 ± 12.8* | 40.1 ± 18.9 | 40.1 ± 18.9 |
| Sex, M/F | 8/6† | 5/5 | 5/5 |
P = 0.21, comparing age between healthy controls and BK group (t-test).
P = 0.53, comparing sex between healthy controls and BK group (Fisher's exact test).
Table 2.
Summary of Cases and Characteristics of Patients With BK
|
Case |
Age |
Sex |
Cause of BK |
Causative Organism |
Initial CDVA |
Topical Treatment |
Tear Samples,
n |
| 1 | 78 | M | Trauma | CNS | CF | VCM, TOB, BAC | 1 |
| 2 | 40 | F | CL-related | NA | CF | GFLX, TOB, Pred | 1 |
| 3 | 24 | F | CL-related | NA | HM | VCM, TOB | 1 |
| 4 | 58 | F | CL-related | NA | 20/40 | VCM, TOB | 1 |
| 5 | 26 | M | CL-related | NA | HM | VCM, MFX | 1 |
| 6 | 66 | F | CL-related | P. aeruginosa | 20/400 | VCM, TOB, CPFX | 1 |
| 7 | 22 | M | CL-related | P. aeruginosa | 20/70 | TOB, Pred | 1 |
| 8 | 18 | F | CL-related | P. aeruginosa | 20/200 | VCM, TOB | 2 |
| 9 | 56 | M | Long-term topical steroid use | Streptococcus pneumoniae | HM | VCM, TOB | 2 |
| 10 | 45 | F | Trauma | NA | 20/25 | MFX | 2 |
M, male; F, female; NA, not available; CNS, coagulase negative streptococcus; P, Pseudomonas; TOB, tobramycin; VCM, vancomycin; BAC, bacitracin; MFX, moxifloxacin; GFLX, gatifloxacin; Pred, prednisolone; CPXF, ciprofloxacin.
Corneal Sub-Basal Nerve Plexus and DC Parameters by IVCM
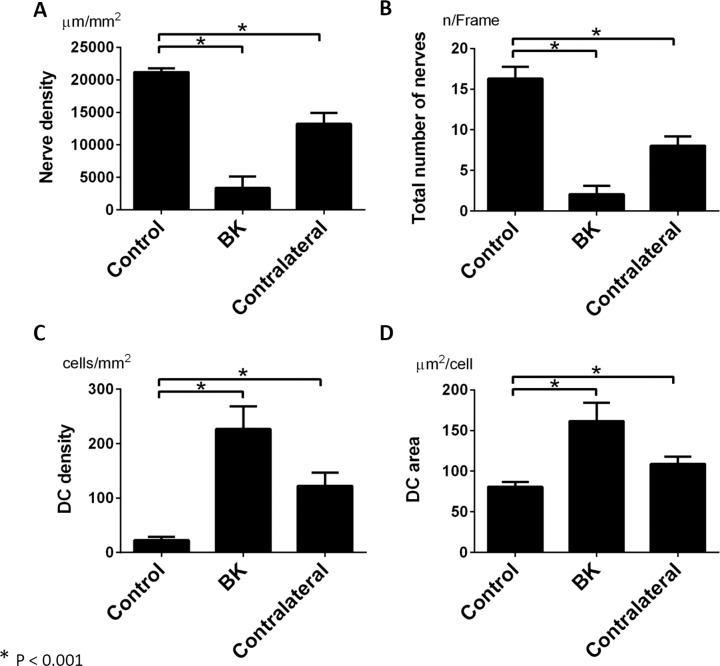
Quantitative analysis of affected and unaffected eyes of BK patients and controls are shown in Figure 2. Patients with unilateral BK showed significant reduction in the sub-basal nerve plexus density and total number of nerve fibers in both eyes, when compared to controls. In particular, the mean nerve density was reduced to 3337 ± 1615 μm/mm2 in affected BK eyes, and to 13,230 ± 1635 μm/mm2 in contralateral eyes, compared to 21,200 ± 545 μm/mm2 in controls (ANOVA, P < 0.001; Fig. 2A). The total number of nerve fibers was reduced to 2.0 ± 0.9/frame in BK eyes and 7.6 ± 1.1/frame in contralateral eyes, compared to 16.4 ± 1.4/frame in controls (ANOVA, P < 0.001; Fig. 2B). The DC density was increased to 226.9 ± 37.3 cells/mm2 in BK eyes and 122.3 ± 23.7 cells/mm2 in contralateral eyes, compared to 22.7 ± 5.9 cells/mm2 in healthy controls (ANOVA, P < 0.001; Fig. 2C). The DC area was increased to 161.7 ± 20.2 μm2 in BK eyes and 109.0 ± 8.5 μm2 in contralateral eyes, compared to 80.7 ± 5.8 μm2 in healthy controls (ANOVA, P < 0.001; Fig. 2D). There were no significant differences in number of dendrites and DC field among the groups (ANOVA, P = 0.247 and P = 0.263, respectively).
Figure 2.
Corneal sub-basal nerves and dendritiform cells. The nerve density (A) and number of nerves (B) in eyes with unilateral BK and contralateral eyes were significantly decreased compared to normal eyes (P < 0.001, 1-way ANOVA). The DC density (C) and mean DC area (D) were significantly higher in eyes with unilateral BK and in contralateral unaffected eyes than in eyes of healthy controls (P < 0.001, 1-way ANOVA). A P value of less than 0.05 was considered statistically significant. Graph bars: represent average SE.
Tear Cytokine Concentrations
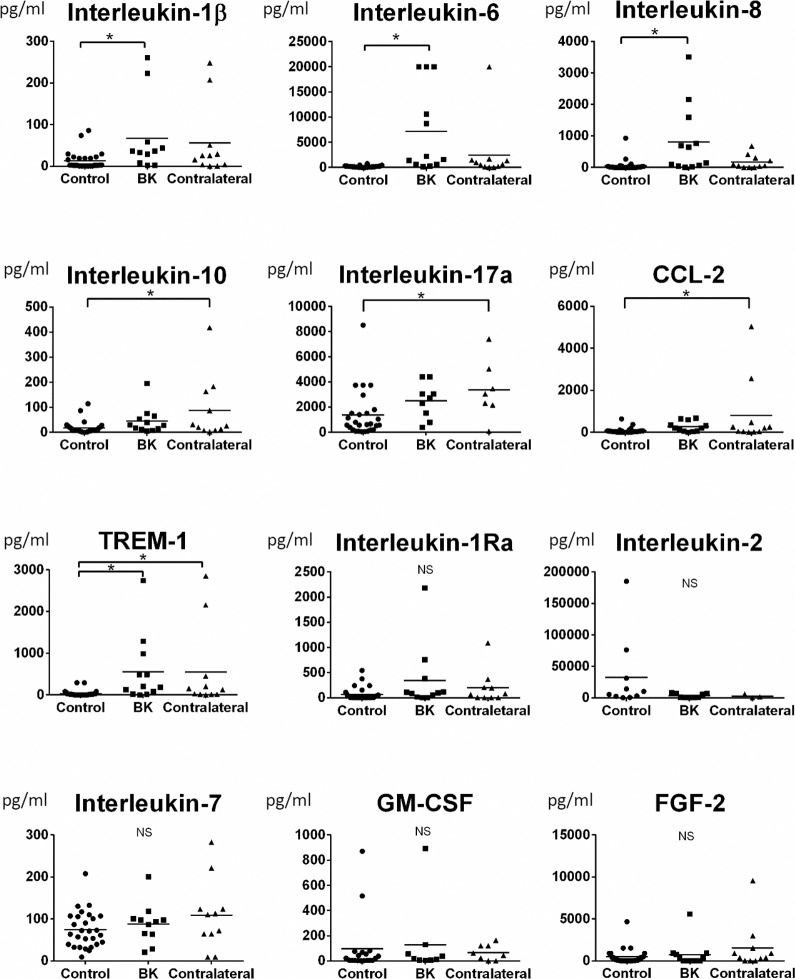
The detailed tear cytokine concentrations measured by the multiplex assay are shown in Figure 3. The concentrations of IL-1β, IL-6, and IL-8 were significantly increased in affected BK eyes (P = 0.04, P < 0.001, and P < 0.001), but not contralateral eyes (P > 0.05), compared to healthy controls. In contrast, the tear concentrations of IL-10, IL-17a, and CCL-2 were only significantly increased in contralateral unaffected eyes (P = 0.01, P = 0.04, and P = 0.02), but not affected eyes (P > 0.05), compared to healthy controls. The tear concentration of TREM-1 was significantly increased in affected eyes with BK (P = 0.02), as well as in contralateral unaffected eyes (P = 0.03), compared to controls. There were no significant differences in levels of IL-1Ra, IL-2, IL-7, FGF-2, and GM-CSF among the groups (P > 0.05).
Figure 3.
Tear cytokine levels. The IL-1β, IL-6, and IL-8 levels in affected BK eyes (P = 0.04, P < 0.001, and P < 0.001), but not contralateral eyes (P > 0.05), were significantly increased compared to controls. In contrast, IL-10, IL-17a, and CCL-2 were only significantly increased in contralateral unaffected eyes (P = 0.01, P = 0.04, and P = 0.02), but not in affected eyes (P > 0.05), compared to controls. The level of TREM-1 was significantly increased in affected eyes with BK (P = 0.02), as well as in contralateral unaffected eyes (P = 0.03), compared to normal eyes. There were no significant differences in levels of IL-1Ra, IL-2, IL-7, GM-CSF, and FGF-2 among the groups (P > 0.05). Error bars: represent the mean value for each group. A P value of less than 0.05 was considered statistically significant.
Correlation of Tear Cytokines With DC Parameters by IVCM
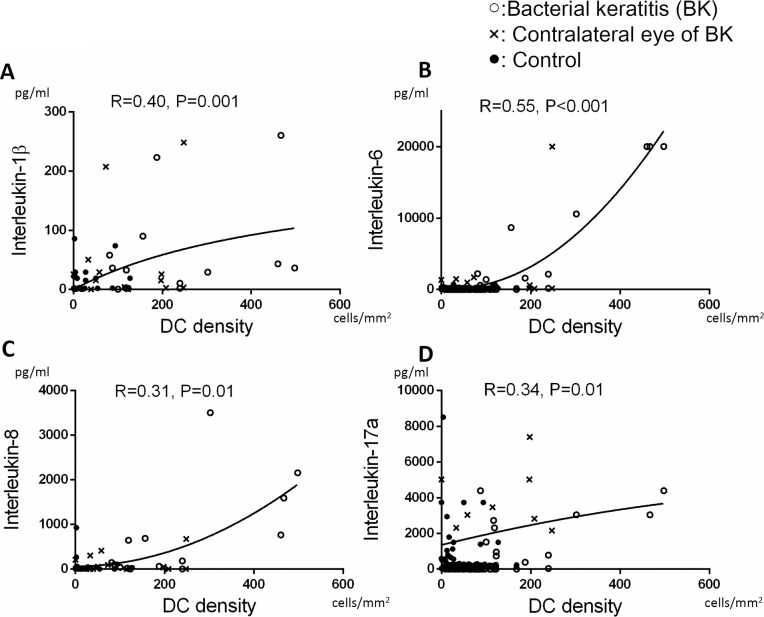
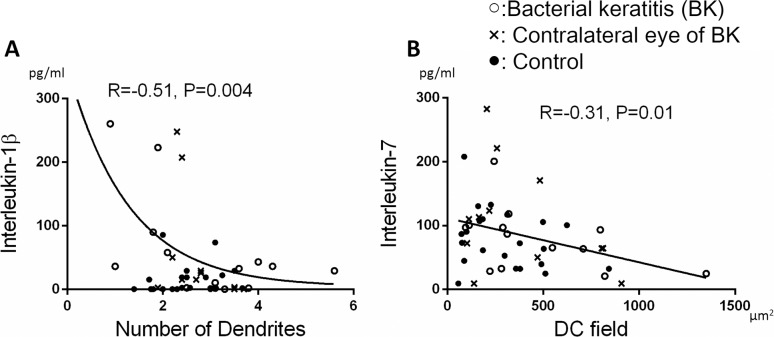
Tear concentrations of IL-1β, IL-6, IL-8, and IL-17a were positively correlated with DC density (R = 0.40, P = 0.001; R = 0.55, P < 0.001; R = 0.31, P = 0.01; and R = 0.34, P = 0.01, respectively; Figs. 4A–D). Interleukin-1β was correlated inversely with the number of dendrites (R = −0.51, P = 0.004; Fig. 5A), and IL-7 was inversely correlated with DC field (R = −0.31, P = 0.01; Fig. 5B). However, other cytokines did not correlate with morphological parameters for DCs (P > 0.05).
Figure 4.
Correlation between tear cytokine levels and DC density. Levels of IL-1β (R = 0.40, P = 0.001 [A]), IL-6 (R = 0.55, P < 0.001 [B]), IL-8 (R = 0.31, P = 0.01 [C]), and IL-17a (R = 0.34, P = 0.01 [D] significantly correlated with DC density. A P value of less than 0.05 was considered statistically significant.
Figure 5.
Correlation between cytokine levels and DC morphology. The number of dendrites was significantly correlated with IL-1β (R = −0.51, P = 0.004 [A]). The DC field was significantly correlated with IL-7 (R = −0.31, P = 0.01 [B]). A P value of less than 0.05 was considered statistically significant.
Correlation of Tear Cytokines and Sub-Basal Nerve Density
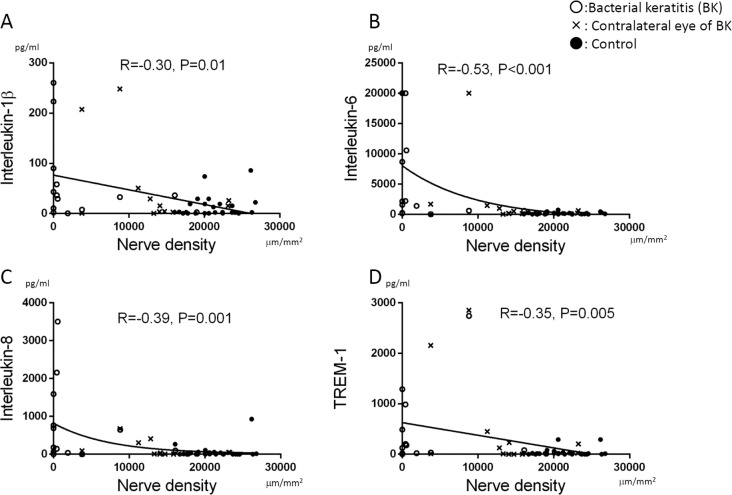
The tear concentrations of IL-1β, IL-6, and IL-8 were inversely correlated with sub-basal nerve density (R = −0.30, P = 0.01; R = −0.53, P < 0.001; and R = −0.39, P = 0.001, respectively; Figs. 6A–C). In addition, concentrations of TREM-1 were inversely correlated with sub-basal nerve density (R = −0.35, P = 0.005; Fig. 6D). There were no significant correlations between all other cytokines and corneal nerve density (P > 0.05).
Figure 6.
Correlation between nerve density and cytokine levels. Nerve density was significantly inversely correlated with IL-1β (R = −0.30, P = 0.01 [A]), IL-6 (R = −0.53, P < 0.001 [B]), IL-8 (R = −0.39, P = 0.001 [C]), and TREM-1 (R = −0.35, P = 0.005 [D]). A P value of less than 0.05 was considered statistically significant.
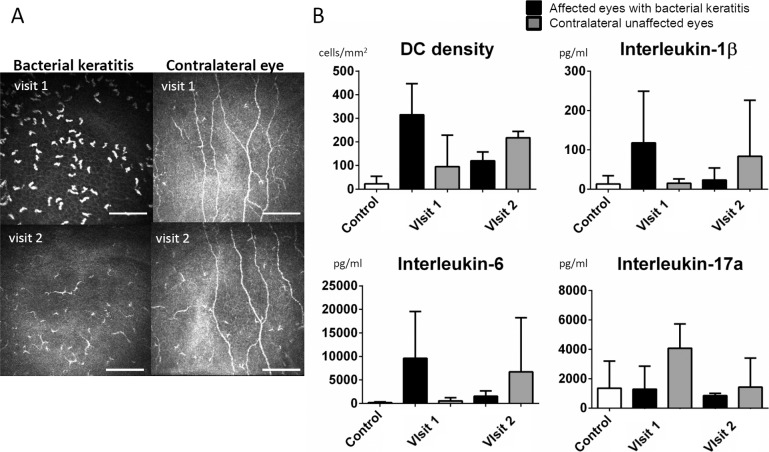
Alterations of Cytokine Concentrations in Patients Over Time
In 3 cases (cases 8–10), the tear samples and IVCM images were obtained at 2 time points (Fig. 7A). In all these 3 patients, the BK improved clinically after the antibiotic treatment started. The average of DC density, IL-1β, IL-6, and IL-17a decreased in the affected eyes as the BK was improving (Fig. 7B). However, surprisingly, the DC density, IL-1β, and IL-6 increased in the contralateral eyes, although they remained clinically unaffected. The IL-17a level in the contralateral eyes were elevated at visit 1 and decreased as the BK improved.
Figure 7.
Alterations of DC density and tear cytokines over time. In 3 cases (case 8–10 in Table 2), tear samples and IVCM were obtained on the same day at visits 1 and 2. In all of these 3 patients, the BK improved clinically after the antibiotic treatment started. The representative IVCM images at visits 1 and 2 (A). Size bars: represent 100 μm. The DC density, IL-1β, IL-6, and IL-17a in the affected eyes decreased from visit 1 to visit 2. The DC density, IL-1β, and IL-6 in the contralateral eyes gradually increased from visit 1 to visit 2, whereas IL-17a in the contralateral eyes increased at visit 1 and decreased at visit 2 (B).
Discussion
The current study evaluates the tear cytokine concentrations and alterations in corneal DCs and nerves by IVCM in healthy control subjects and patients with unilateral BK. We demonstrated correlation of proinflammatory tear cytokine levels with DC density and DC morphology as shown by IVCM. Recent advances in the technique of cytokine protein measurements from small volumes of fluid have enabled us to evaluate the tear cytokine concentrations in ocular diseases, such as dry eye disease, keratoconus, GVHD, and corneal neovascularization.19–25 Recently, Villani et al.26 evaluated tear cytokine concentration and IVCM data in patients with rheumatoid arthritis and reported a decrease in tear IL-1 and IL-6 levels, as well as in DC density after systemic therapy. The current study assesses and correlates bilateral tear cytokine concentrations and microscopic cellular findings of corneal sub-basal nerves and DCs in affected and clinically unaffected contralateral eyes of patients with unilateral BK and compares them to normal control eyes. The bilateral changes in tear cytokines in unilateral BK are in line with our previous studies, demonstrating bilateral changes in corneal nerves in patients with unilateral HSK and HZO.15,16 To date, to our knowledge, the underlying mechanism of the bilateral alterations in IVCM finding in unilateral clinical corneal diseases have not been elucidated.
Interestingly, our study demonstrates significant correlations of proinflammatory cytokines to increased DC density and alteration in their morphology as shown by IVCM, as well as correlations of upregulated tear cytokine levels in BK with reductions in corneal sub-basal nerve density. Antigen-presenting cells, most prominently dendritic cells, have a crucial role in the defense against foreign organisms through the secretion of cytokines, presentation of foreign antigens, pathogen clearance, and wound healing. During acute infections, various cytokines have been shown to increase in response to the tissue damage and pathologic antigens in the corneal epithelium.28,29 However, to our knowledge the tear cytokines in eyes with BK have not been investigated to date.
In the current study, bilateral increase in the concentration of TREM-1 is shown in patients with unilateral BK. The TREM-1, a molecule increased bilaterally in BK patients, is expressed at high levels on monocytes and macrophages, and functions as an amplifier of inflammation in tissues infected by bacteria or fungi. The TREM-1 can stimulate the production of proinflammatory cytokines, and stimulates rapid neutrophil degranulation and oxidative burst.30 In contrast, while TREM-1 is not upregulated in noninfectious diseases, TREM-2 regulates DCs, microglia and osteoclast during inflammation in the central nervous system and rheumatoid arthritis.30 During corneal infections, TREM-1 has been shown to be upregulated by Pseudomonas aeruginosa or lipopolysaccharides (LPS) in an animal model.31 Although there were no correlations between TREM-1 levels and DC parameters in our study, TREM-1 concentration was inversely correlated with corneal nerve density. The bilateral increase in TREM-1 concentration in tear samples of patients with unilateral BK, to our knowledge, is the first report on TREM-1 elevation in patients with ocular diseases.
The tear concentrations of IL-1β, IL-6, and IL-8 were elevated only in the affected eyes with BK, but not in contralateral clinically unaffected eyes. Interleukin-1β, which is elevated in our study, has been shown to induce additional proinflammatory mediators, such as IL-6, IL-8, FGF-2, prostaglandin E2, and cyclooxgenase-2.32–34 Moreover, IL-1β enhances host defense against infections, by augmenting antimicrobial function of macrophages and initiating T helper (Th) 1 and Th17 adaptive immune responses.35 Interleukin-6, which also was increased in tears of patients with unilateral BK, is a major proinflammatory molecule in corneal wound healing,36–39 infection,28,29 inflammation,28,40 and corneal transplantation models.41,42 During inflammation, IL-6 stimulates the maturation and trafficking of DCs, promotes the differentiation of B cells, and IL-2 production by T cells, and, thus, mediates adaptive immune responses against pathogens.43,44 Both IL-1β and IL-6 have been established as important mediators of fever induced by LPS from gram-negative bacteria45 and are produced by epithelial cells46 and plasmacytoid dendritic cells (pDCs).47 Interleukin-8 is an important inflammatory mediator during viral and bacterial infections,48 and increased IL-8 has been reported with LPS stimulation in corneal epithelial cells.49 As such, elevated IL-8 concentration in tear samples of the affected eyes would be expected as shown in the current study.
While the changes in tear cytokines and corneal DC density can be rationalized by infection-induced inflammation in affected eyes, alteration of cytokine concentrations in the contralateral clinically unaffected eyes is intriguing. Interestingly, IL-17a was only significantly elevated in the contralateral eyes, but not the affected BK eyes compared to controls. Interleukin-17a regulates prophylactic host defense against infection via Th17 cell responses, which improves the mucocutaneous barrier function. Interleukin-17 also stimulates the release of antimicrobial peptides and chemokines for neutrophil recruitment.35 Recently, in response to IL-6 stimulation, a population of neutrophils have been shown to produce IL-17a in an autocrine manner to activate fibroblasts and epithelial cells to produce chemokines and proinflammatory cytokines, which leads to enhancing reactive oxygen species and antifungal activity.50 Interleukin-17 expression was reported to be elevated by 2000-fold in human corneal tissues with filamentous fungal keratitis51; however, to our knowledge, there have been no reports on contralateral alterations of IL-17a concentrations in human samples or animal experiments. Clinically, the incidence of bilateral corneal infection is 1% to 3% and is relatively rare.52,53 It is tempting to speculate that the elevation of IL-17a in contralateral eyes in this study may potentially be due to prophylactic defense mechanisms in these eyes to prevent infection in the fellow eye.
Specific mechanisms have to be substantiated by comprehensive studies in animals and human, to determine the sequence of events that take place after BK with regards to tear cytokine increase and alterations in DC density and morphology. While some of the increased cytokines may be pathogen-specific, others may be upregulated nonspecifically as a response to inflammation. In this study, IL-7 was negatively correlated with DC field. Interleukin-7 has been proposed to be the primary driver of homeostatic T cell proliferation and also regulates the expression of major histocompatibility complex (MHC)-II or human leukocyte antigen (HLA)-DR on DCs via IL-7 receptor α signaling.54 While resident central corneal DCs demonstrate negative to low expression of MHC-II expression during steady state, DCs are capable of upregulating MHC-II during inflammation and migrate to draining lymph nodes.55 Correlation of tear IL-7 concentration with morphological changes of DCs, suggestive of DC activation in patients with BK, may result in the use of IL-7 as a primer for DC activation in other inflammatory ocular surface diseases.
Our study has several limitations. These include the lack of serum cytokine level measurements, the limited number of cases and the methodology of tear collection after instillation of saline. In systemic diseases, serum as well as local tissue cytokine/chemokine concentrations are found to be elevated, such as in dry eye disease with systemic disease56,57 and allergic conjunctivitis.23 However, in local eye disease, such as in keratoconus,20 serum cytokine levels remain within normal limits, while tear cytokine levels are elevated. In the case of infectious keratitis, the inflammation generally is limited to the eye.58 However, given that we did not obtain serum levels, we cannot comment on systemic cytokine alterations after BK. Further, our tear samples were collected after instillation of 60 μL of saline.27 Thus, the cytokine levels in the current study reflected the total amount of cytokines on the ocular surface.
In conclusion, our study demonstrated that increased proinflammatory tear cytokines correlated with increased corneal DC density and size. Moreover, we demonstrated that unilateral BK can result in bilateral alterations in proinflammatory tear cytokines, which potentially could result in the development of chronic bilateral ocular surface disease in patients with unilateral BK.
Acknowledgments
Supported by NIH Grants K08-EY020575 (PH) and L30-EY021919 (PH), Research to Prevent Blindness Career Development Award (PH), and an Uehara Memorial Foundation Fellowship (TY). The authors alone are responsible for the content and writing of the paper.
Disclosure: T. Yamaguchi, None; B.M. Calvacanti, None; A. Cruzat, None; Y. Qazi, None; S. Ishikawa, None; A. Osuka, None; J. Lederer, None; P. Hamrah, None
References
- 1. Daniell M: Overview. Initial antimicrobial therapy for microbial keratitis. Br J Ophthalmol. 2003; 87: 1172–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butler TK, Spencer NA, Chan CC, Singh Gilhotra J, McClellan K. Infective keratitis in older patients: a 4 year review, 1998-2002. Br J Ophthalmol. 2005; 89: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prokosch V, Gatzioufas Z, Thanos S, Stupp T. Microbiological findings and predisposing risk factors in corneal ulcers. Graefes Arch Clin Exp Ophthalmol. 2008; 250: 369–374. [DOI] [PubMed] [Google Scholar]
- 4. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008; 27: 22–27. [DOI] [PubMed] [Google Scholar]
- 5. Webb RM, Duke MA. Bacterial infection of a neurotrophic cornea in an immunocompromised subject. Cornea. 1985; 4: 14–18. [PubMed] [Google Scholar]
- 6. Niederer RL, McGhee CN. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res. 2010; 29: 30–58. [DOI] [PubMed] [Google Scholar]
- 7. Erie JC, Patel SV, McLaren JW, Nau CB, Hodge DO, Bourne WM. Keratocyte density in keratoconus. A confocal microscopy study(a). Am J Ophthalmol. 2002; 134: 689–695. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto Y, Dogru M, Sato EA, et al. The application of in vivo confocal scanning laser microscopy in the management of Acanthamoeba keratitis. Mol Vis. 2007; 13: 1319–1326. [PubMed] [Google Scholar]
- 9. Kobayashi A, Sakurai M, Shirao Y, Sugiyama K, Ohta T, Amaya-Ohkura Y. In vivo confocal microscopy and genotyping of a family with Thiel-Behnke (honeycomb) corneal dystrophy. Arch Ophthalmol. 2003; 121: 1498–1499. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi A, Fujiki K, Murakami A, Sugiyama K. In vivo laser confocal microscopy findings and mutational analysis for Schnyder's crystalline corneal dystrophy. Ophthalmology. 2009; 116: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 11. Kaufman SC, Musch DC, Belin MW, et al. Confocal microscopy: a report by the American Academy of Ophthalmology. Ophthalmology. 2004; 111: 396–406. [DOI] [PubMed] [Google Scholar]
- 12. Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Exp Ophthalmol. 2009; 37: 100–117. [DOI] [PubMed] [Google Scholar]
- 13. Sivaskandarajah GA, Halpern EM, Lovblom LE, et al. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013; 36: 2748–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petropoulos IN, Alam U, Fadavi H, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014; 55: 2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010; 117: 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013; 120: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamrah P, Sahin A, Dastjerdi MH, et al. Cellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2012; 119: 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011; 52: 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. VanDerMeid KR, Su SP, Krenzer KL, Ward KW, Zhang JZ. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011; 17: 1056–1063. [PMC free article] [PubMed] [Google Scholar]
- 20. Jun AS, Cope L, Speck C, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011; 6: e16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lema I, Duran JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005; 112: 654–659. [DOI] [PubMed] [Google Scholar]
- 22. Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012; 18: 797–802. [PMC free article] [PubMed] [Google Scholar]
- 23. Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006; 36: 777–784. [DOI] [PubMed] [Google Scholar]
- 24. Zakaria N, Van Grasdorff S, Wouters K, et al. Human tears reveal insights into corneal neovascularization. PLoS One. 2012; 7: e36451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999; 19: 201–211. [DOI] [PubMed] [Google Scholar]
- 26. Villani E, Galimberti D, Del Papa N, Nucci P, Ratiglia R. Inflammation in dry eye associated with rheumatoid arthritis: cytokine and in vivo confocal microscopy study. Innate Immun. 2013; 19: 420–427. [DOI] [PubMed] [Google Scholar]
- 27. Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002; 43: 1004–1011. [PubMed] [Google Scholar]
- 28. Arranz-Valsero I, Schulze U, Contreras-Ruiz L, et al. Involvement of corneal epithelial cells in the Th17 response in an in vitro bacterial inflammation model. Mol Vis. 2013; 19: 85–99. [PMC free article] [PubMed] [Google Scholar]
- 29. Duan F, Liao J, Huang Q, Nie Y, Wu K. HSV-1 miR-H6 inhibits HSV-1 replication and IL-6 expression in human corneal epithelial cells in vitro. Clin Dev Immunol. 2012; 2012: 192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006; 7: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 31. Wu M, Peng A, Sun M, et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating Toll-like receptor signaling and Th1/Th2-type immune responses. Infect Immun. 2011; 79: 2709–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JG, Kay EP. Common and distinct pathways for cellular activities in FGF-2 signaling induced by IL-1beta in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2009; 50: 2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee YA, Choi HM, Lee SH, et al. Synergy between adiponectin and interleukin-1beta on the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in fibroblast-like synoviocytes. Exp Mol Med. 2012; 44: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee HT, Lee JG, Na M, Kay EP. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004; 279: 32325–32332. [DOI] [PubMed] [Google Scholar]
- 35. van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011; 32: 110–116. [DOI] [PubMed] [Google Scholar]
- 36. Gallucci RM, Simeonova PP, Matheson JM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000; 14: 2525–2531. [DOI] [PubMed] [Google Scholar]
- 37. Gallucci RM, Sugawara T, Yucesoy B, et al. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001; 21: 603–609. [DOI] [PubMed] [Google Scholar]
- 38. McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010; 184: 7219–7228. [DOI] [PubMed] [Google Scholar]
- 39. Ebihara N, Matsuda A, Nakamura S, Matsuda H, Murakami A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Invest Ophthalmol Vis Sci. 2011; 52: 8549–8557. [DOI] [PubMed] [Google Scholar]
- 40. Sakimoto T, Sugaya S, Ishimori A, Sawa M. Anti-inflammatory effect of IL-6 receptor blockade in corneal alkali burn. Exp Eye Res. 2012; 97: 98–104. [DOI] [PubMed] [Google Scholar]
- 41. Funding M, Vorum H, Nexo E, Moestrup SK, Ehlers N, Moller HJ. Soluble CD163 and interleukin-6 are increased in aqueous humour from patients with endothelial rejection of corneal grafts. Acta Ophthalmol Scand. 2005; 83: 234–239. [DOI] [PubMed] [Google Scholar]
- 42. Nosov M, Wilk M, Morcos M, et al. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am J Transplant. 2012; 12: 1313–1322. [DOI] [PubMed] [Google Scholar]
- 43. Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003; 299: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 44. Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J. 2004; 18: 1439–1441. [DOI] [PubMed] [Google Scholar]
- 45. Romanovsky AA, Almeida MC, Aronoff DM, et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005; 10: 2193–2216. [DOI] [PubMed] [Google Scholar]
- 46. Sugaya S, Sakimoto T, Shoji J, Sawa M. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011; 55: 277–282. [DOI] [PubMed] [Google Scholar]
- 47. Michea P, Vargas P, Donnadieu MH, et al. Epithelial control of the human pDC response to extracellular bacteria. Eur J Immunol. 2013; 43: 1264–1273. [DOI] [PubMed] [Google Scholar]
- 48. Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000; 72: 391–398. [PubMed] [Google Scholar]
- 49. Erdinest N, Shmueli O, Grossman Y, Ovadia H, Solomon A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012; 53: 4396–4406. [DOI] [PubMed] [Google Scholar]
- 50. Taylor PR, Roy S, Leal SM Jr, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nature Immunol. 2014; 15: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karthikeyan RS, Leal SM Jr, Prajna NV, et al. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis. 2011; 204: 942–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003; 87: 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bharathi MJ, Ramakrishnan R, Shivakumar C, Meenakshi R, Lionalraj D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in south India. Indian J Ophthalmol. 2010; 58: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nature Immunol. 2009; 10: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002; 195: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsubota K, Fujihara T, Takeuchi T. Soluble interleukin-2 receptors and serum autoantibodies in dry eye patients: correlation with lacrimal gland function. Cornea. 1997; 16: 339–344. [PubMed] [Google Scholar]
- 57. Oh JY, Kim MK, Choi HJ, et al. Investigating the relationship between serum interleukin-17 levels and systemic immune-mediated disease in patients with dry eye syndrome. Korean J Ophthalmol. 2011; 25: 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tervo T, van Setten GB, Hovi M, Pakarinen M, Tarkkanen A, Valtonen V. C-reactive protein serum levels in patients with ocular disease. Acta Ophthalmol (Copenh). 1994; 72: 110–113. [DOI] [PubMed] [Google Scholar]