Abstract
TRAIL induces apoptosis in cancer cells whilst sparing normal tissues. Despite promising pre-clinical results, few patients responded to treatment with recombinant TRAIL (Apo2L/Dulanermin) or TRAIL-R2-specific antibodies, such as conatumumab (AMG655). It is unknown whether this was due to intrinsic TRAIL resistance within primary human cancers or insufficient agonistic activity of the TRAIL-R-targeting drugs. FcγR-mediated crosslinking increases the cancer-cell-killing activity of TRAIL-R2-specific antibodies in vivo. We tested this phenomenon using FcγR-expressing immune cells from patients with ovarian cancer. However, even in the presence of high numbers of FcγR-expressing immune cells, as found in ovarian cancer ascites, AMG655-induced apoptosis was not enabled to any significant degree, indicating that this concept may not translate into clinical use. On the basis of these results we next set out to determine whether AMG655 possibly interferes with apoptosis induction by endogenous TRAIL which could be expressed by immune cells. To do so, we tested how AMG655 affected apoptosis induction by recombinant TRAIL. This, however, resulted in the surprising discovery of a striking synergy between AMG655 and non-tagged TRAIL (Apo2L/TRAIL) in killing cancer cells. This combination was as effective in killing cancer cells as highly active recombinant isoleucine-zipper-tagged TRAIL (iz-TRAIL). The increased killing efficiency was due to enhanced formation of the TRAIL death-inducing signalling complex (DISC), enabled by concomitant binding of Apo2L/TRAIL and AMG655 to TRAIL-R2. The synergy of AMG655 with Apo2L/TRAIL extended to primary ovarian cancer cells and was further enhanced by combination with the proteasome inhibitor bortezomib or a SMAC mimetic. Importantly, primary human hepatocytes were not killed by the AMG655-Apo2L/TRAIL combination, also not when further combined with bortezomib or a SMAC mimetic. We therefore propose that clinical-grade non-tagged recombinant forms of TRAIL, such as dulanermin, could be combined with antibodies such as AMG655 to introduce a highly active TRAIL-R2-agonistic therapy into the cancer clinic.
Keywords: Apoptosis, ovarian, cancer, TRAIL, dulanermin, AMG655
Introduction
TNF-related apoptosis-inducing ligand (TRAIL) can kill tumour cells in vivo without harming normal tissues (1, 2), a finding that initiated the clinical development of TRAIL-receptor (TRAIL-R) agonists. Current TRAIL-R-agonistic drugs were developed during a period of concern about their potential hepatotoxicity (3). Hence, in comparison to highly active recombinant forms of TRAIL such as leucine-zipper- (1) or isoleucine-zipper-tagged TRAIL (LZ-TRAIL or iz-TRAIL) (4), the clinically used recombinant non-tagged TRAIL form, dulanermin (5), is a relatively weak agonist of TRAIL-R1 (also known as DR4) and TRAIL-R2 (also known as DR5, Apo2, KILLER, TRICK2), the two death-domain (DD)-containing TRAIL-Rs. In addition to its limited agonistic capacity, dulanermin has a short in-vivo half-life (5), and, like other recombinant forms of TRAIL, binds to the non-apoptosis-inducing TRAIL-Rs, TRAIL-R3 (DcR1), TRAIL-R4 (DcR2) and Osteoprotegerin (OPG), which may attenuate the pro-apoptotic activity exerted by its interaction with TRAIL-R1 and/or TRAIL-R2. Consequently, agonistic antibodies to TRAIL-R1 and TRAIL-R2 with a longer half-life and more restricted receptor specificity than recombinant TRAIL were also developed for clinical use (6). However, despite encouraging preclinical studies (2, 7) very few patients responded to either dulanermin (5, 8) or TRAIL-R1/2-targeting antibodies (9-11) in clinical trials conducted thus far. This suggests that the current clinical approaches of targeting TRAIL-R1 and TRAIL-R2 should be re-examined before further studies are undertaken in patients.
Interestingly, Fcγ receptors (FcγR) on immune cells were recently shown to be capable of crosslinking antibodies against DD-containing TRAIL-Rs which rendered these antibodies active in killing cancer cells in vivo (12, 13). We therefore tested whether we could identify conditions under which a clinically used TRAIL-R2-specific antibody, AMG655, which has so far not shown any significant clinical activity, could be rendered active by exploiting this phenomenon. Because the tumour microenvironment in ovarian cancer is rich in FcγR-expressing immune cells (14) and because TRAIL may serve as a treatment for ovarian cancer (6, 15, 16), we set out to investigate whether FcγR-expressing immune cells in the ovarian cancer microenvironment would enable AMG655-mediated killing of patient-derived ovarian cancer cells. Surprisingly, these experiments led us to discover a previously unrecognised synergy between AMG655 and TRAIL in killing primary ovarian cancer cells specifically which, importantly, is independent of the presence of immune cells.
Results and Discussion
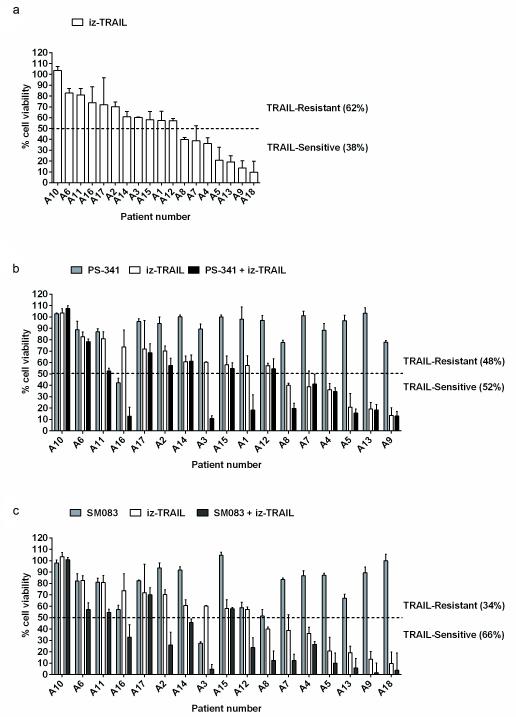
Treatment of primary ovarian cancer cells with bortezomib or SMAC mimetics enhances apoptosis induction by iz-TRAIL
We first used iz-TRAIL, a highly active recombinant form of TRAIL which we previously developed for preclinical studies (4), to determine whether primary ovarian cancer cells were TRAIL-sensitive or -resistant and whether proteasome inhibitors or SMAC mimetics enhanced their sensitivity to TRAIL. We obtained primary ovarian cancer cells from chemotherapy-resistant patients (Supplementary Table S1 and Supplementary Figure S1) and found that whilst treatment with iz-TRAIL was capable of killing these cells, this was only true for 38% of the cases (Figure 1a). Co-treatment with the proteasome inhibitor bortezomib/PS-341 (Figure 1b) or the SMAC mimetic compound SM083 (Figure 1c), however, rendered these cells sensitive to iz-TRAIL-induced apoptosis in 52% and 66% of the cases, respectively. These results confirm those obtained by others (15, 17), implying that a highly active clinical TRAIL-R agonist could be used to treat ovarian cancer patients, preferably in combination with a proteasome inhibitor or a SMAC mimetic compound.
Figure 1.
Treatment of primary ovarian cancer cells with bortezomib or SMAC mimetics leads to enhanced iz-TRAIL-induced cell death. Primary ovarian cancer cells were isolated from 18 patients with advanced ovarian cancer (Supplementary Table S1 and Supplementary Figure S1). (a) Primary ovarian cancer cells were cultured in 50% RPMI and 50% ascites which was 0.22 μm sterile filtered and supplemented with 1% penicillin/streptomycin/gentamycin/glutamine at 37°C in a humidified incubator with 5% CO2 and subsequently treated with iz-TRAIL [100 ng/ml] in the absence or presence of bortezomib/PS-341 [20 nM] (Selleck Chemicals) (b), or the SMAC mimetic SM083 [100 nM] (29) (c). Cell viability was measured after 48 hours using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Southampton, UK) according to the manufacturer’s instructions. Data represent the mean of 3 biological replicates (± S.D.) using cells from each donor.
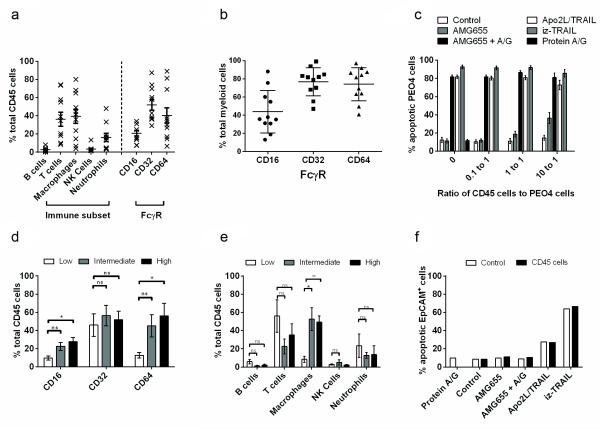
Primary ascites-derived human CD45-positive cells are inefficient enablers of FcγR-dependent TRAIL-R2-mediated apoptosis
Considering that FcγRs on immune cells were proposed to enable apoptosis induction by TRAIL-R-targeting antibodies by crosslinking them, in conjunction with the fact that the ovarian cancer microenvironment, especially in ascites, often contains high numbers of FcγR-expressing immune cells, we reasoned that TRAIL-R2 antibodies might be an effective treatment for ovarian cancer. We therefore tested the efficacy of the TRAIL-R2-specific antibody AMG655 at killing ovarian cancer cells in the presence of ascites-derived immune cells. We first confirmed the presence of different immune cell subsets and overall FcγR expression on CD45-positive (CD45) immune cells isolated from ovarian cancer ascites (Figure 2a). Myeloid cells (macrophages and neutrophils) were abundant in ovarian cancer ascites and expressed high levels of CD16 (FcγRIIIA), CD32 (FcγRIIA), and CD64 (FcγRIA) (Figure 2b). NK cells were found in lower numbers in ovarian cancer ascites and expressed CD16 (FcγRIIIA) (Supplemental Figure S2a).
Figure 2.
Primary ascites-derived human CD45-positive cells are inefficient enablers of FcγR-dependent TRAIL-R2-mediated apoptosis. (a) Flow-cytometric analysis (FACS) of FcγRIIIA (CD16), FcγRIIA (CD32) and FcγRIA (CD64) on the surface of CD45-positive immune cells obtained from ascites of 11 patients with ovarian cancer. Flow cytometry was performed on a BD FACSCalibur and analysed using FlowJo software from Tree star. FcγR expression was determined by staining cells for CD45, CD16, CD32, and CD64; B-cells were identified via CD45, CD3, CD19; macrophages via CD45, CD68, CD14; T-cells via CD45 and CD3; NK cells via CD45, CD16, CD56; neutrophils via CD45, CD66. All antibodies were purchased from BioLegend, (London, UK). (b) A forward and side scatter gate was used to identify macrophages (CD45, CD68, CD14) and neutrophils (CD45, CD66) within ovarian cancer ascites (Supplementary Figure S2). FcγR expression was determined by staining cells for CD45 and CD16, CD32, and CD64. (c) Ascites-derived primary human CD45 immune cells were co-cultured with PEO4 cells and treated with AMG655 [500 ng/ml], AMG655 and recombinant protein A/G, Apo2L/TRAIL [500 ng/ml], or iz-TRAIL [500 ng/ml]. Primary CD45 cells were co-cultured with PEO4 cells in RPMI supplemented with 10% FCS and 1% penicillin/streptomycin/gentamycin/glutamine. Tumour-cell-specific death was quantified from a mixed population of tumour and immune cells 24 hours after TRAIL treatment and determined by detection of cleaved CK18 (M30 CytoDeath, Peviva, Bromma, Sweden), a specific marker of epithelial cell death by FACS. Data shown are mean (± S.D.) of 11 independent experiments using CD45 cells from 11 patients. (d) The relative expression of CD16, CD32 and CD64 on immune cells between patients with low (<10%), intermediate (>10% and <25%), and high (>25%) induction of AMG655-induced apoptosis in PEO4 cells (within the 10:1 ratio treated group, Supplementary Figure S3). (e) Differences between the immune cell profiles of patients with low, intermediate, and high enablers of FcγR-dependent AMG655-induced apoptosis (within the 10:1 ratio-treated group). Data were analysed using GraphPad Prism 5 software (GraphPad Software). Statistical analysis was performed using a two-tailed Student’s t-test (n.s = p>0.05, *=p<0.05, **=p<0.01, ***=p<0.001). (f) Primary ovarian cancer cells were cultured with patient-derived immune cells that had been determined to be capable of enabling AMG655-induced apoptosis in PEO4 cells (Supplementary Figure S5), at a ratio of 10 immune cells to 1 ovarian cancer cell, in the absence or presence of AMG655 [500 ng/ml], AMG655 and protein A/G, Apo2L/TRAIL [500 ng/ml], or iz-TRAIL [500 ng/ml]. Specific death of cancer cells was determined flow-cytometrically after 24 hours by release of cleaved cytokeratin 18. Primary CD45 cells and EpCAM+ cells were cultured in 50% RPMI and 50% ascites which was 0.22 μm sterile filtered and supplemented with 1% penicillin/streptomycin/ gentamycin/glutamine at 37°C in humidified incubator with 5% CO2. Data are representative of 2 independent experiments performed using cells from the same patient and experiments performed using samples from other patients.
To determine the efficiency of FcγR-mediated TRAIL-R2-antibody crosslinking by ascites-derived immune cells and a potential increase in agonistic activity achieved thereby, we employed the highly TRAIL-sensitive PEO4 cell line. However, even at the highest ratio of ten immune cells to one PEO4 cell, the level of AMG655-induced apoptosis was modest (Figure 2c). The results from individual patients were divided into three groups, low (<10%), medium (>10% and <25%), and high (>25%), dependent on the capacity of their CD45+ FcγR-expressing cells to induce FcγR-dependent TRAIL-R2-mediated apoptosis of PEO4 cells (Supplementary Figure S3). This allowed the determination of the relative contribution of the different FcγRs and immune cell subsets towards AMG655-mediated crosslinking of TRAIL-R2. Higher expression of FcγRIIIA (CD16) and FcγRIA (CD64) expression and macrophages (within the 10 to 1 ratio treated group) were associated with enhanced AMG655-mediated apoptosis (Figure 2d, e). Although a polymorphism at FcγRIIA131H/H versus FcγRIIA131H/R was reported to enhance TRAIL-R2-mediated apoptosis (12), the polymorphism frequencies were similar between different patient groups (Supplementary Table S3). In summary, the efficiency of apoptosis induction by FcγR-crosslinked AMG655 never reached the levels achieved by iz-TRAIL or a non-tagged recombinant form of TRAIL that we synthesised according to known dulanermin specificities which is referred to as Apo2L/TRAIL herein (Figure 2c).
Thus, human FcγR-expressing immune cells were unable to enhance anti-TRAIL-R2-induced apoptosis in primary ovarian cancer cells to any significant level (Figure 2f). Yet, Apo2L/TRAIL and, even more potently, iz-TRAIL were capable of inducing apoptosis in primary ovarian cancer cells, suggesting that recombinant forms of TRAIL are more effective at multimerising TRAIL-R1 and/or TRAIL-R2 and, consequently, at inducing apoptosis than the TRAIL-R2-specific antibody AMG655 in these cancer cells. Although these are in-vitro studies, the ratio of immune to cancer cells that was sufficient to drive FcγR-dependent TRAIL-R2-mediated apoptosis was substantially higher than the most favourable ratios we found in solid deposits of ovarian cancer (Supplementary Figure S4). We therefore conclude that the concept of enabling TRAIL-R2-mediated apoptosis in cancer cells via FcγR-expressing human immune cells is most likely not viable for the majority of cancers.
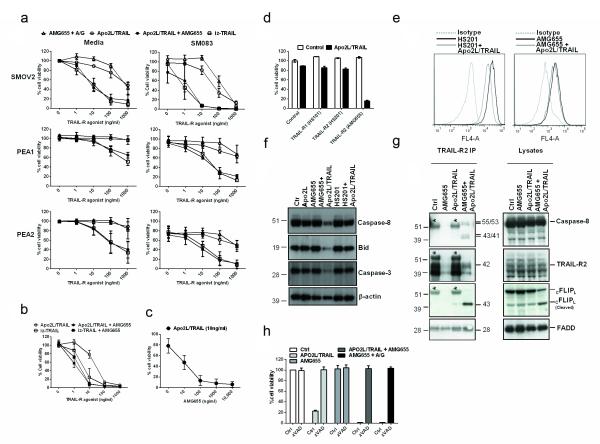
AMG655 and Apo2L/TRAIL synergise to potently kill ovarian cancer cells via TRAIL-R2
Based on these results, we hypothesized that the binding of AMG655 to TRAIL-R2 might interfere with the binding of endogenous TRAIL to TRAIL-R2. If true, AMG655 treatment might even be counterproductive by preventing the killing of cancer cells by endogenous TRAIL (18). Unexpectedly however, when testing this hypothesis, we found that AMG655 did not block, but instead profoundly enhanced the capacity of Apo2L/TRAIL to induce apoptosis in cancer cells. Together, AMG655 and Apo2L/TRAIL were as active in killing ovarian cancer cells as iz-TRAIL (Figure 3a). AMG655 enhanced Apo2L/TRAIL-induced apoptosis in TRAIL-sensitive and TRAIL-resistant ovarian cancer cell lines treated with or without SMAC mimetics (Figure 3a). The activity of iz-TRAIL was not inhibited but, in contrast to Apo2L/TRAIL, also not increased by AMG655 (Figure 3b). This suggests that there is most likely a maximum proapoptotic agonistic activity that is achievable by TRAIL-R-crosslinking which is reached by both iz-TRAIL alone and the combination of Apo2L/TRAIL with AMG655. The synergism between AMG655 and Apo2L/TRAIL was observed at concentrations of AMG655 as low as 100 ng/ml (Figure 3c), which is more than two orders of magnitude below the levels achieved in patients (5, 19). Importantly, the TRAIL-R2-specific antibody AMG655 we employed here has been used clinically and the non-tagged Apo2L/TRAIL form we employed mimics dulanermin which has also already been tested in clinical trials (2, 4). Hence, this combination represents a highly active TRAIL-R2-agonistic therapy that could be suitable for immediate use in patients.
Figure 3.
AMG655 and Apo2L/TRAIL synergise to potently kill ovarian cancer cells via TRAIL-R2. (a) All ovarian cancer cell lines were cultured in RPMI 1640 supplemented with 10% Foetal Calf Serum (FCS) and 1% penicillin/streptomycin/glutamine. SMOV2, PEA1, and PEA2 cells were treated with AMG655 and recombinant protein A/G, Apo2L/TRAIL, iz-TRAIL, or AMG655 [10 μg/ml] and Apo2L/TRAIL with (right panel) and without (left panel) SM083 at the indicated concentrations. Cell viability was measured after 24 hours using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega) according to the manufacturer’s instructions. (n=3 ± S.D.). The production and purification of iz-TRAIL was as described previously (4). Production and purification of Apo2L/TRAIL was performed as previously described by Ashkenazi et al. (2). (b) PEO4 cells were pre-incubated in the absence or presence of AMG655 [10 μg/ml] for 1 hour and then treated with Apo2L/TRAIL or iz-TRAIL at increasing concentrations. Cell viability was measured after 24 hours (n=5 ± S.D.). (c) PEO4 cells were treated with Apo2L/TRAIL [10 ng/ml] and increasing concentrations of AMG655. Cell viability was measured after 24 hours. (d) PEO4 cells were pre-treated with or without different TRAIL-R1- or TRAIL-R2-specific antibodies [10 μg/ml], and then treated with Apo2L/TRAIL [10 ng/ml]. The TRAIL-R1- and -R2-specific antibodies HS101 and HS201 are available from AdipoGen. (e) PEO4 cells were incubated with Apo2L/TRAIL [1 μg/ml] for 1 hour on ice, and then were washed 3 times, and incubated with HS201 [10 μg/ml] or AMG655 [10 μg/ml] or isotype control for 1 hour on ice. The cells were washed, and the binding of HS201 and AMG655 to TRAIL-R2 was determined by flow cytometry. (f) PEO4 cells were stimulated with control Apo2L/TRAIL [1 μg/ml], AMG-655 [10 μg/ml], AMG-655 [10 μg/ml] + Apo2L/TRAIL [1 μg/ml], HS201 [10 μg/ml], or HS201 [10 μg/ml] + Apo2L/TRAIL [1 μg/ml]. After 14 hours the cells were lysed, and equal amounts of proteins from lysates, as determined by the bicinchoninic acid (BCA) protein assay (Pierce), were separated by SDS-PAGE (NuPAGE) on 4-12% Bis-Tris-NuPAGE gels under reducing conditions (Invitrogen). Proteins were transferred onto nitrocellulose membranes (Amersham Pharmacia). Membranes were subjected to immunoprobing with antibodies against caspase-8 (clone C15, Enzo, Exeter, UK), Bid (R&D Systems, Abingdon, UK), caspase-3 (R&D Systems) and β-actin (Sigma, Gillingham, UK). Proteins were visualised using the Western Lightning® Plus-ECL, Enhanced Chemiluminescence Substrate ECL Western Blotting Detection Plus (GE Healthcare). (g) PEO4 cells were treated with either AMG655 [10 μg/ml], Apo2L/TRAIL [100 ng/ml], or Apo2L/TRAIL and AMG655 for 1 hour and then lysed. Either AMG655 [10 μg/ml] or Apo2L/TRAIL [100 ng/ml] was added to the lysates of cells stimulated in the absence of the respective substance before immunoprecipitation of TRAIL-R2 to ensure the observed changes in the TRAIL-R2 DISC were dependent on TRAIL-R2 stimulation (IP, immunoprecipitation. Protein concentrations of each lysate were determined as above. TRAIL-R2 was immunoprecipitated via AMG655, which was added to lysates if not used for stimulation, and uncoupled Protein G Sepharose (GE Healthcare). The beads were then washed and proteins were eluted from the beads with LDS buffer (NuPAGE, Invitrogen) containing DTT. Proteins were separated by SDS-PAGE as above and analysed by Western blotting as above. Membranes were subjected to immunoprobing with antibodies against TRAIL-R2 (Cell Signaling, Danvers, MA, USA), FADD (clone 1F7, Enzo), cFLIP (clone NF6, is available from Enzo) and Caspase-8 (clone C15, Enzo). *, unspecific bands representing the IgG1 heavy chain from the addition of exogenous AMG655 to the lysates. (h) PEO4 cells were treated for one hour with the pan-caspase inhibitor zVAD-fmk [20 μM] and subsequently with Apo2L/TRAIL [100 ng/ml], AMG655 [10 μg/ml] and Apo2L/TRAIL [100 ng/ml], AMG655 [10 μg/ml], or AMG655 and protein A/G. Cell viability was measured after 24 hours.
Intriguingly, the synergy of AMG655 with Apo2L/TRAIL in killing cancer cells was unique amongst three different TRAIL-R1/2-specific antibodies we tested (Figure 3d), indicating that not all TRAIL-R2-specific antibodies synergise with Apo2L/TRAIL to kill cancer cells. When examining the molecular basis for this specificity, we found that Apo2L/TRAIL significantly reduced the binding of HS201, but not of AMG655, to TRAIL-R2 (Figure 3e). Thus, Apo2L/TRAIL and HS201 appear to bind TRAIL-R2 at overlapping epitopes, thereby preventing these two proteins from acting in synergy. AMG655 instead binds TRAIL-R2 at an epitope that does not interfere with the receptor’s concomitant interaction with Apo2L/TRAIL so that both proteins can bind TRAIL-R2 simultaneously, resulting in enhanced TRAIL-R2 crosslinking and apoptosis-inducing capacity. In line with this, the combination of Apo2L/TRAIL with AMG655, but not with HS201, was able to trigger effective DISC-mediated caspase-8 activation with subsequent Bid cleavage (Figure 3f). We hypothesised that the effect of AMG655 on Apo2L/TRAIL-induced apoptosis could be mediated by concomitant binding to TRAIL-R2 which would lead to enhanced multimerisation of TRAIL-R2 and, consequently, increased formation of the TRAIL death-inducing signalling complex (DISC) (20). In line with this hypothesis, AMG655 significantly enhanced Apo2L/TRAIL-mediated recruitment of FADD, Caspase-8 and cFLIP and, hence, TRAIL DISC formation (Figure 3g). This indicates that the synergistic apoptosis-inducing activity of AMG655 and Apo2L/TRAIL is due to increased TRAIL DISC formation as a consequence of enhanced TRAIL-R2 crosslinking. Furthermore, synergistic cell death induction by AMG655-Apo2L/TRAIL was blocked by caspase inhibition (Figure 3h) but not by inhibition of JNK (21) (Supplementary Figure S6), indicating that the AMG655-Apo2L/TRAIL combination induces apoptotic cell death (2).
It was recently shown that some TRAIL-resistant ovarian cancer cell lines require activation of the mitochondrial pathway for TRAIL-induced apoptosis (21). We therefore next investigated whether the synergistic effect of AMG655 and Apo2L/TRAIL requires activation of the mitochondrial apoptotic pathway by using a genetically defined system of isogenic HCT-116 colon carcinoma cell lines which are either wild-type (HCT116-WT) or deficient for Bax and Bak (HCT116-Bax/Bak-dko) (Supplementary Figure S7a). HCT116-WT and HCT116-Bax/Bak-dko cells both expressed similar levels of TRAIL-R1 and TRAIL-R2 on their surface (Supplementary Figure S7b). Whereas HCT116-WT cells were sensitive to AMG655-Apo2L/TRAIL, HCT116-Bax/Bak-dko cells were resistant (Supplementary Figure S7c), indicating that the AMG655-Apo2L/TRAIL combination cannot overcome resistance to TRAIL-induced apoptosis that is due to a block in the apoptosis pathway at the mitochondrial level. However, addition of bortezomib re-sensitised HCT116-Bax/Bak-dko cells to AMG655-Apo2L/TRAIL (Supplementary Figure S7c), indicating that the mitochondrial block to the novel TRAIL-R-agonist combination can be overcome by bortezomib, as previously shown for other TRAIL-R agonists (22).
We next investigated whether the synergy between AMG655 and Apo2L/TRAIL was mediated solely by TRAIL-R2 or whether there was a contribution of TRAIL-R1. To do so, we performed transient knockdowns of TRAIL-R1 and TRAIL-R2 since these were both expressed on PEO4 cells (Supplementary Figure S8a). We found that suppression of TRAIL-R2 completely reversed the synergistic effect of AMG655 and Apo2L/TRAIL treatment on cell death (Supplementary Figure S8, b and c), whereas TRAIL-R1 knockdown only resulted in a minor reduction which was most likely attributable to co-suppression of TRAIL-R2 by TRAIL-R1 knockdown (Supplementary Figure S8b and c). These data show that the synergy of AMG655 with Apo2L/TRAIL in inducing apoptosis depends on TRAIL-R2.
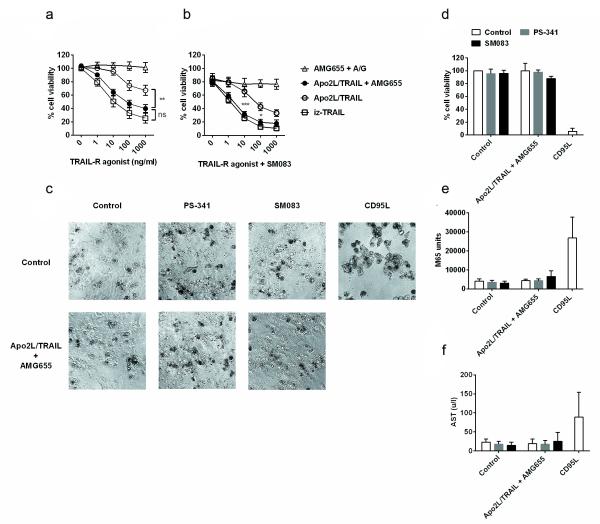
AMG655 and Apo2L/TRAIL synergise to kill primary ovarian cancer cells but not primary human hepatocytes
We next investigated whether AMG655 could also synergise with Apo2L/TRAIL in killing primary ovarian cancer cells. Indeed, the combination of AMG655 with Apo2L/TRAIL was as active as iz-TRAIL in killing primary ovarian cancer cells (Figure 4a). When used in combination with SM083, we observed that the AMG655-Apo2L/TRAIL combination was again as active as iz-TRAIL when combined with SM083 in killing primary ovarian cancer cells (Figure 4b). Thus, the combination of Apo2L/TRAIL with AMG655 can kill patient-derived primary ovarian cancer cells, and further combination with a SMAC mimetic drug, and/or possibly other TRAIL-sensitising agents, may hold great therapeutic promise for ovarian cancer.
Figure 4.
AMG655 and Apo2L/TRAIL synergise to kill primary ovarian cancer cells but not primary human hepatocytes (PHH). (a) Primary EpCAM+ ovarian cancer cells, isolated from 6 patients with advanced ovarian cancer, were treated with iz-TRAIL, AMG655 and recombinant protein A/G (A/G), Apo2L/TRAIL, or Apo2L/TRAIL and AMG655 [10 μg/ml], either alone (a) or in the presence of SM083 [100 nM] (b). Cell viability was measured after 48 hours (n = 6 ± S.D for (a) and n = 6 ± S.D. for (b). Data were analysed using GraphPad Prism 5 software (GraphPad Software) and two way ANOVA (non-significant (n.s) = p> 0.05, *=p<0.05, **=p<0.01, ***=p<0.001). Individual patient data are shown in Supplementary Figure S9. (c) PHH from 3 donors and PHH culture reagents were purchased from GIBCO® Life technologies. After thawing, the PHH were seeded as per the manufacturer’s recommendations and cultured in hepatocyte maintenance medium for 3 days. PHH were then treated with Apo2L/TRAIL [10 μg/ml] and AMG655 [10 μg/ml] with or without SM083 [100 nM] or bortezomib/PS-341 [20 nM]. Fc-CD95L [1 μg/ml] was used as a positive control. Fc-CD95L was produced and purified from HEK293T supernatant as previously described (4). (c) Phase contrast microscopy (10 × original magnification) of treated PHH from a representative donor (donor 3). (d) Viability of PHH was assessed 24 hours after the onset of treatment as in (a) (n=3 ± S.D.) using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega) according to the manufacturer’s instructions. (e) PHH death was determined 24 hours after onset of treatment as in (a) by measuring release of soluble keratin 18 (K18) using the M65® ELISA (Peviva) according to the manufacturer’s instructions (n=3 ± S.D.). (f) Presence of the liver enzyme AST in PHH culture supernatants was measured 24 hours after onset of treatment. Release of intracellular hepatocyte-specific AST liver enzymes was measured using the Reflovet plus instrument (Roche, Burgess Hill, UK) according to the manufacturer’s instructions. Individual donor data are shown in Supplementary Figure S10.
In mice, highly active forms of TRAIL and SMAC mimetic compounds can be combined without causing overt toxicity (23, 24). Given that AMG655 binds to human TRAIL-R2 but not to the only murine DD-containing TRAIL-R (mTRAIL-R/mDR5), the potential toxicities of therapeutic combinations of AMG655 cannot be evaluated in mouse models. Also, such xenograft models (2, 7) are not predictive of the potential clinical efficacy of AMG655 and dulanermin. Because of these considerations and previous concerns regarding the potential liver toxicity of TRAIL (3, 4, 25, 26), we next examined whether primary human hepatocytes were sensitive to apoptosis induction by the AMG655-Apo2L/TRAIL combination, either alone or in the presence of SM083 or bortezomib. As control we used CD95L, a potent killer of primary human hepatocytes (4). We found that treatment with the combination of Apo2L/TRAIL and AMG655 did not induce any significant changes in cellular morphology (Figure 4c), loss of cell viability (Figure 4d), or increase in the death of primary human hepatocytes (Figure 4e). None of the AMG655- and Apo2L/TRAIL-comprising treatments resulted in a significant increase in liver enzyme production (Figure 4f). These results are in line with our previous findings regarding the effects of highly active recombinant forms of TRAIL on primary human hepatocytes (4). Importantly, also further combination with SM083 or bortezomib did not induce a significant decrease in the viability of primary human hepatocytes (Figure 4d). Thus, combining AMG655 and Apo2L/TRAIL, with or without SM083 or bortezomib, does not kill primary human hepatocytes.
Taken together, on the basis of our findings we propose that it should be possible to overcome the limited efficacy of currently clinically employed TRAIL-R agonists, in a cancer-cell-specific manner, either by combining two of them, AMG655 and dulanermin, or by employing single-agent highly active forms of recombinant TRAIL (1, 4, 27), possibly in combination with SMAC mimetics, proteasome inhibitors or other potent TRAIL apoptosis sensitisers (28). We anticipate that our findings will contribute to the introduction of a highly active TRAIL-R-agonistic therapy into the cancer clinic.
Supplementary Material
Acknowledgements
We would like to thank the patients and the staff at Imperial College Healthcare NHS Trust for providing and collecting the ovarian cancer ascites samples for this research. AMG655 was kindly provided by AMGEN. We thank B. Vogelstein and R. Youle for providing cell lines. We would like to thank Dr C. Kantari for technical advice and helpful discussions.
Acknowledgments
This work has been funded by the Medical Research Council by providing a Clinical Research Training Fellowship for M.T. (MC_EX_G0802342), Cancer Research UK, and the Association for International Cancer Research. AMG655 was kindly provided by Amgen Inc. (Thousand Oaks, CA, USA). The SMAC mimetic compound SM083 (also known as SM 9a) was synthesised and kindly provided by P. Seneci and L. Manzoni.
Footnotes
Conflict of interest. H.W. is a named inventor on a patent underlying the development of AMG655. H.W. is a scientific advisor, co-founder, and shareholder of Apogenix GmbH. Otherwise the authors declare no competing interests.
References
- 1.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5(2):157–63. doi: 10.1038/5517. Epub 1999/02/04. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. The Journal of clinical investigation. 1999;104(2):155–62. doi: 10.1172/JCI6926. Epub 1999/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. United States. 2001:383–5. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 4.Ganten TM, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas TL, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(8):2640–6. doi: 10.1158/1078-0432.CCR-05-2635. Epub 2006/04/28. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(17):2839–46. doi: 10.1200/JCO.2009.25.1991. Epub 2010/05/12. [DOI] [PubMed] [Google Scholar]
- 6.Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis. 2009;14(4):607–23. doi: 10.1007/s10495-009-0321-2. Epub 2009/02/06. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer biology & therapy. 2010;9(8):618–31. doi: 10.4161/cbt.9.8.11264. Epub 2010/02/13. [DOI] [PubMed] [Google Scholar]
- 8.Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(33):4442–51. doi: 10.1200/JCO.2011.37.2623. Epub 2011/10/20. [DOI] [PubMed] [Google Scholar]
- 9.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Jr., Rocha-Lima CM, et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2012 doi: 10.1093/annonc/mds142. Epub 2012/06/16. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L, Balint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, et al. A Randomized Phase 2 Study of Paclitaxel and Carboplatin with or without Conatumumab for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013 doi: 10.1097/JTO.0b013e31827ce554. Epub 2013/02/02. [DOI] [PubMed] [Google Scholar]
- 11.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(20):6187–94. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 12.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer cell. 2011;19(1):101–13. doi: 10.1016/j.ccr.2010.11.012. Epub 2011/01/22. [DOI] [PubMed] [Google Scholar]
- 13.Haynes NM, Hawkins ED, Li M, McLaughlin NM, Hammerling GJ, Schwendener R, et al. CD11c+ dendritic cells and B cells contribute to the tumoricidal activity of anti-DR5 antibody therapy in established tumors. J Immunol. 2010;185(1):532–41. doi: 10.4049/jimmunol.0903624. [DOI] [PubMed] [Google Scholar]
- 14.Leinster DA, Kulbe H, Everitt G, Thompson R, Perretti M, Gavins FN, et al. The peritoneal tumour microenvironment of high-grade serous ovarian cancer. The Journal of pathology. 2012;227(2):136–45. doi: 10.1002/path.4002. Epub 2012/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saulle E, Petronelli A, Pasquini L, Petrucci E, Mariani G, Biffoni M, et al. Proteasome inhibitors sensitize ovarian cancer cells to TRAIL induced apoptosis. Apoptosis. 2007;12(4):635–55. doi: 10.1007/s10495-006-0025-9. Epub 2007/01/26. [DOI] [PubMed] [Google Scholar]
- 16.Petrucci E, Pasquini L, Bernabei M, Saulle E, Biffoni M, Accarpio F, et al. A small molecule SMAC mimic LBW242 potentiates TRAIL- and anticancer drug-mediated cell death of ovarian cancer cells. PloS one. United States. 2012:e35073. doi: 10.1371/journal.pone.0035073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrucci E, Pasquini L, Bernabei M, Saulle E, Biffoni M, Accarpio F, et al. A Small Molecule SMAC Mimic LBW242 Potentiates TRAIL- and Anticancer Drug-Mediated Cell Death of Ovarian Cancer Cells. PloS one. 2012;7(4):e35073. doi: 10.1371/journal.pone.0035073. Epub 2012/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12(2):214–9. doi: 10.1038/nm1356. Epub 2006/01/31. [DOI] [PubMed] [Google Scholar]
- 19.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(23):5883–91. doi: 10.1158/1078-0432.CCR-10-0631. Epub 2010/10/16. [DOI] [PubMed] [Google Scholar]
- 20.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. The international journal of biochemistry & cell biology. 2007;39(7-8):1462–75. doi: 10.1016/j.biocel.2007.02.007. Epub 2007/04/04. [DOI] [PubMed] [Google Scholar]
- 21.Goncharenko-Khaider N, Lane D, Matte I, Rancourt C, Piche A. The inhibition of Bid expression by Akt leads to resistance to TRAIL-induced apoptosis in ovarian cancer cells. Oncogene. England. 2010:5523–36. doi: 10.1038/onc.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillissen B, Richter A, Overkamp T, Essmann F, Hemmati PG, Preissner R, et al. Targeted therapy of the XIAP/proteasome pathway overcomes TRAIL-resistance in carcinoma by switching apoptosis signaling to a Bax/Bak-independent ‘type I’ mode. Cell death & disease. 2013;4:e643. doi: 10.1038/cddis.2013.67. Epub 2013/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8(8):808–15. doi: 10.1038/nm735. Epub 2002/07/16. [DOI] [PubMed] [Google Scholar]
- 24.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, et al. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer research. 2008;68(19):7956–65. doi: 10.1158/0008-5472.CAN-08-1296. Epub 2008/10/03. [DOI] [PubMed] [Google Scholar]
- 25.Ganten TM, Koschny R, Haas TL, Sykora J, Li-Weber M, Herzer K, et al. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology (Baltimore, Md) 2005;42(3):588–97. doi: 10.1002/hep.20807. Epub 2005/07/23. [DOI] [PubMed] [Google Scholar]
- 26.Koschny R, Ganten TM, Sykora J, Haas TL, Sprick MR, Kolb A, et al. TRAIL/bortezomib cotreatment is potentially hepatotoxic but induces cancer-specific apoptosis within a therapeutic window. Hepatology (Baltimore, Md) 2007;45(3):649–58. doi: 10.1002/hep.21555. Epub 2007/02/28. [DOI] [PubMed] [Google Scholar]
- 27.Gieffers C, Kluge M, Merz C, Sykora J, Thiemann M, Schaal R, et al. APG350 induces superior clustering of TRAIL receptors and shows therapeutic antitumor efficacy independent of cross-linking via Fcgamma receptors. Molecular cancer therapeutics. 2013;12(12):2735–47. doi: 10.1158/1535-7163.MCT-13-0323. Epub 2013/10/09. [DOI] [PubMed] [Google Scholar]
- 28.Lemke J, von Karstedt S, Abd El Hay M, Conti A, Arce F, Montinaro A, et al. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell death and differentiation. 2014;21(3):491–502. doi: 10.1038/cdd.2013.179. Epub 2013/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecis D, Mastrangelo E, Belvisi L, Bolognesi M, Civera M, Cossu F, et al. Dimeric Smac mimetics/IAP inhibitors as in vivo-active pro-apoptotic agents. Part II: Structural and biological characterization. Bioorganic & medicinal chemistry. 2012;20(22):6709–23. doi: 10.1016/j.bmc.2012.09.041. Epub 2012/10/16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.