Abstract
We developed a coating method to produce functionalized small quantum dots (sQDs), ~ 9 nm in diameter, that were stable for over a month. We made sQDs in four emission wavelengths, from 527 nm to 655 nm and with different functional groups. AMPA receptors on live neurons were labeled with sQDs and post-synaptic density proteins were visualized with super-resolution microscopy. Their diffusion behavior indicates that sQDs access the synaptic clefts significantly more often than commercial QDs.
Keywords: AMPA receptors, fluorescent probes, quantum dots, single-molecule imaging
Quantum dots (QDs), made of semiconductor nanocrystals, are highly advantageous in fluorescence applications due to their brightness and high photo-stability[1]. In particular, they are widely used in single molecule experiments such as single particle tracking in living cells[2–5]. However, their large diameters, typically around 15–35 nm for commercially available QDs[6], significantly reduce their access to spatially confined cellular environments such as cell junctions[2]. There have been attempts to make smaller QDs, generally with diameters on the order of 10 nm[7–9]. However, small quantum dots usually suffer either from chemical instability or poor labeling specificity[2].
Achieving specific labeling with small QDs is particularly challenging on neurons, especially when labeling proteins in synaptic clefts, the ~30 nm thin spaces separating two neurons at synapses (Fig. 1a). The commercially available QDs (we refer to them as big QDs, or bQDs), have been successfully used to label neurons[10,11], although the majority of bQDs (typically ~70%–90%) label extra-synaptic sites, rather than sites within synapses, due to their large sizes[7,11].
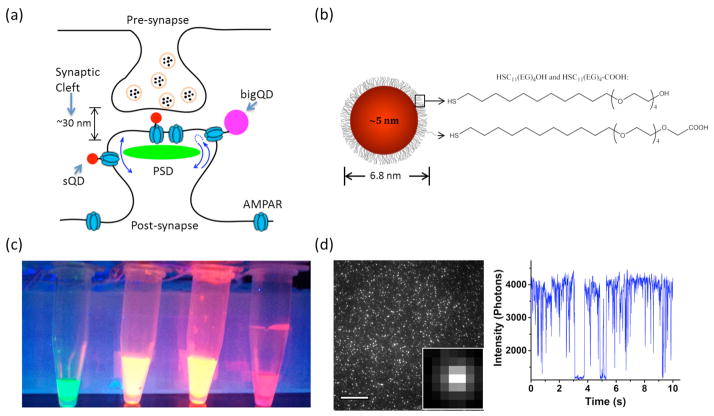
Figure 1. Characterizations of small quantum dots (sQDs).
(a) Schematic diagram of the synaptic region with AMPA receptors (AMPAR) labeled with sQDs and commercial QDs. (b) Schematic diagram of the sQD. sQDs are coated with PEGylated alkanethiol ligands by self-assemble-monolayer. (c) Fluorescence of sQDs with emission 527 nm, 615 nm, 620 nm and 655 nm in PBS (pH = 7.3) under UV illumination. All sQDs are soluble and mono-dispersed in PBS. (d) Single molecule fluorescent image of sQD-620-streptavidin bound to biotins fixed on a coverglass. Fluorescent intensity of sQD-620 over time is shown at right. Blinking behavior is observed. Scale bar: 15 μm.
The large size of bQDs primarily results from their passivating surface coating and attached biomolecules. The quantum dot crystal, often made of a cadmium selenide (CdSe) core with a zinc sulfide (ZnS) shell, is typically 2–7 nm in diameter, and is coated with selected groups of chemicals for dispersion and stabilization in water[12]. Commercial QDs are usually coated with thick polymer layers that attach to many bioaffinity groups (e.g. streptavidins), together resulting in a large size. Other coating methods have been developed to reduce the size of QDs[5,7,8,13–17]. In particular, Howarth et al. reported the use of DHLA-PEG derivatives for coating CdSe/ZnCdS QDs which reduced the size of QDs to 11 nm with stability at 37°C for more than 4 hours[7]. The reduced-sized QDs were reported to have better accessibility to the synaptic cleft compared to bQDs. However, problems with chemical stability of the coating and variability of the QD cores ultimately led to an abandonment of this procedure.
Here we report that coating with a thin, but stable, ligand-layer over the usual CdSe/ZnS–QD cores, allows for compact probes with substantially improved access to neuronal synapses. We use a self-assembled monolayer[18] of hydrophilic ligands with the structure HS-(CH2)11-(EG)4-OH/COOH (EG: Ethylene glycol) (Fig. 1b; and SI Fig. 1). Both the ligand structure and coating method are critical. The undecanethiol termination provides a hydrophobic attachment to the nanoparticle surface that resists desorption, and unlike bulkier DHLA-based ligands, packs densely on the QD surface. The short EG4 chain provides hydrophilicity for stable aqueous dispersion. The key parameter in the coating procedure is the use of elevated temperature without oxygen (60°C, 4 hr, N2) to drive replacement of the native hydrophobic ligands with a dense layer of the new ligands. The ligand monolayer makes them stable in water (> 1 month) and brightly fluorescent (28% quantum yield). They are also resistant to non-specific binding to biomolecules, across a wide panel of QD crystal sizes (3.2–8 nm), sources (NN-labs, Invitrogen, and laboratory-made), and batches, yielding a broad spectrum of fluorescence wavelengths (Fig. 1c). For the sake of simplicity, we call them small QDs (sQDs).
Streptavidin (SA) was conjugated to the COOH-functionalized sQDs via a straightforward EDC coupling method. Individual sQDs were bound to biotin molecules on a PEGylated coverslip, and fluorescent blinking was observed (Fig. 1d). The average intensity was approximately 1/3 that of the bQDs (SI methods). By only using a small number of COOH groups (10%) in the ligand layer, the rest being OH groups, nonspecific labeling is minimized and only a small number of bioaffinity molecules can attach to maintain a small particle size. Dynamic light scattering (DLS) measurements showed that the hydrodynamic diameter of the SA-functionalized sQD-620 was 8.9 ± 0.2 nm (SI Fig. 2a). These functionalized sQDs are stable for over one month at 4°C (SI Fig. 2b–2e).
A major limitation in labeling live neurons with sQDs is non-specific interactions, between sQDs and neurons, or between sQDs and substrate, particularly positively charged poly-lysine that is often used to attach neurons onto the coverslip. To test the binding specificity of our sQDs, we co-transfected neurons with the postsynaptic density (PSD) protein Homer1[12] and the AMPA receptor (AMPAR) subunit GluA2[20]. Homer1 was fused with a fluorescent protein (FP), dsRed, for the visualization of the PSDs. GluA2 was modified by genetically attaching a 15-mer amino acid acceptor peptide (AP) tag at the extracellular domain[21], which was biotinylated by co-transfected biotin ligase (BirA) in the endoplasmic reticulum[21,22]. Biotinylation of GluA2-AP allowed labeling by SA-sQDs when on the surface of transfected neurons. As shown in Fig. 2, overlap between Homer1 and AMPARs was observed; minimum binding of the SA-sQDs was found on untransfected neurons. The high-labeling specificity on neurons was also achieved with other sQDs at different emission wavelengths (SI Fig. 3). In summary, sQDs, like bQDs, specifically labeled biotinylated AMPARs, allowing for the tracking of single AMPARs.
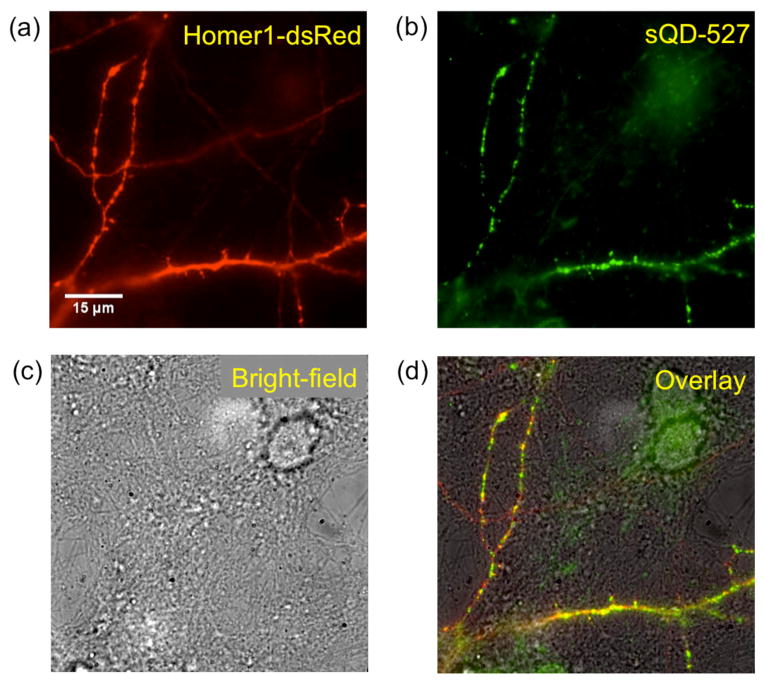
Figure 2. Specific labeling of sQDs on biotinylated AMPA receptors expressed in live neurons.
Neurons were co-transfected with post-synaptic Homer1-dsRed and biotinylated GluA2-AP (see text). (a) Fluorescent image of a neuron expressing Homer1-dsRed (red). (b) Fluorescent image of 527 nm emitting sQD-streptavidin bound to biotinylated GluA2-AP subunit of the AMPAR (green). (c) Bright-field image of the same region as in (a) and (b) show that the field of view is covered with neurons. (d) Overlay of Homer1-dsRed, sQD-labeled AMPARs, and brightfield image (a, b, c) shows that the labeling was highly specific to the transfected neuron. Very few sQDs were found on untransfected neurons or on the coverglass.
We next tracked AMPARs labeled with either sQDs or bQDs and compared their accessibility to synaptic clefts and compared their diffusion patterns. To improve the resolution of PSDs, we fused Homer1 with the photoactivatable FP (paFP) mGeos-M[23], which emits in the GFP channel when activated by near-UV (405 nm) light. Via Photoactivated Localization Microscopy (PALM)[24,25], the mGeos-M molecules can be localized with precision of 14 nm in the x, y-plane[23]. The sQDs for AMPAR labeling were then chosen with an emission wavelength different than the paFP — in this case, at 620 nm — and therefore could be detected separately via 3-D single-particle-tracking by a modified form of Fluorescence Imaging with One Nanometer Accuracy (FIONA)[26,27]. By combining single particle tracking and PALM we were able to observe 3-D diffusion of QD-labeled AMPARs at synapses with high spatial resolution (Fig. 3).
Figure 3. Different diffusion behavior of sQD- AMPARs and commercial QD- AMPARs.
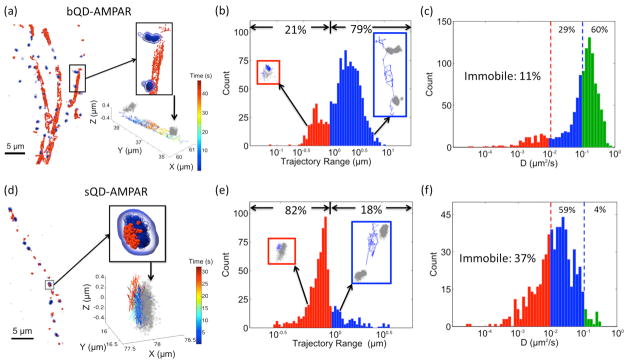
(a) Images of AMPARs labeled with commercial QDs (bQDs) diffusing between synapses. The blue clusters depict localizations of post-synaptic marker Homer1-mGeos-M, and the red dots are localizations of bQD-labeled receptors at different time points on trajectories. One region is enlarged to show the AMPAR diffusing along the dendrite between two synapses. The same region is shown at the bottom with trajectories colored by time. Grey clusters indicate the synapses. (b) Histogram of “trajectory range” of AMPARs labeled with commercial QDs (bQDs). The trajectory range is the maximum distance of the trajectory in either the x, y, or z dimension. It quantifies the spatial confinement of AMPAR diffusion. The fraction of trajectories with range < 1 μm are 21%, shown as red bar. The fraction of trajectories with range > 1μm are shown as blue bar. Insets are example trajectories (blue) around the synapse (gray). (c) Histograms of diffusion coefficients of AMPARs labeled with bQD. For AMPARs labeled with bQDs, only 11% of the receptors are immobile (D < 0.01 μm2/s, red bars), and 60% are diffusing rapidly (D > 0.1 μm2/s, green bars), with 29% having intermediate diffusion coefficients (blue bars). (d) Same scheme as (a) except AMPARs are labeled with sQDs showing that sQD-labeled AMPARs are primarily localized around synapses. (e) Same scheme as (b) except AMPARs are labeled with sQDs. (f) Same scheme as (c) except AMPARs are labeled with sQDs. For the sQDs-labeled AMPARs, 37% of receptors are immobile, and only 4% are diffusing fast, with 59% having intermediate diffusion coefficients. A comparison of (b)–(c) and (e)–(f) clearly shows the labeling difference between the sQDs and the bQDs. All trajectories in each histogram were collected from 4 dendritic regions. Figure (a) and (d) were partially rendered using the QuickSurf representation in VMD 1.9.2[36].
We observed different diffusion behavior for the sQD-labeled and bQD-labeled AMPARs. AMPARs labeled with bQDs diffuse rapidly along dendrites, often moving between synapses (Fig. 3a, and SI movie 1). The majority (79%) of the bQD-AMPARs travelled distances larger than 1 μm in either x-, y-, or z- directions (Fig. 3b). We further calculated the diffusion coefficients of the bQD-AMPARs and found that only 11% of the receptors were immobile (diffusion coefficient D < 0.01 μm2/s), 29% of the receptors were slowly diffusing (0.01 μm2/s < D < 0.1 μm2/s), and the majority of receptors (60%) were rapidly diffusing (D > 0.1 μm2/s) (Fig. 3c). In contrast, the sQD-AMPARs tended to diffuse within a confined range. As shown in Fig. 3d and SI movie 2, a population of the sQD-AMPARs were located within PSDs, with another population labeled specifically at other regions along the dendrites lacking fluorescent Homer1 molecules. Whether these other regions are synapses, or other subcellular domains, was not determined. Specifically, only a small fraction (18%) of the sQD-AMPARs travelled large distances along the dendrites, with trajectory-range > 1 μm (Fig. 3e). In particular, 37% of sQD-AMPARs were immobile, 59% showed slow diffusion and only 4% were fast diffusing (Fig. 3f). In summary, 82% of the sQD-AMPARs are confined to diffuse over a limited (<1 μm) space, while only 21% of the bQD-AMPARs are limited in their diffusion.
The two possibilities that can explain the results of slower diffusion of the sQDs compared to the bQDs, are that the surface chemistry is somehow different between the two, or that the sQDs are hindered (or, in the extreme case, immobilized), by the interaction with the PDSs more than the bQDs.
The difference in the diffusion behavior between sQDs and bQDs labeled AMPARs on neurons is not due to the different surface chemistry of QDs. We perform the same experiments and analysis above on surface of HEK 293 cells, where no spatially confined domains exist, such as synapses. On HEK cells, we observed similar diffusion behavior between bQDs and sQDs (SI Fig. 4). The majority of AMPARs (87% with bQDs and 73% with sQDs) were fast diffusing (D > 0.1 μm2/s). Therefore, the reduced ranges and diffusion coefficients of sQD-AMPARs on neurons result from the reduced QD size.
Synaptic receptors such as AMPARs are known to diffuse slower in the postsynaptic sites than in extrasynaptic site due to higher membrane viscosity in the postsynapse[28], as well as receptor interaction with scaffold proteins in the PSD[10,29]. From our data, it appears that the constrained diffusion of the sQDs is because they have greater access than the bQDs to the AMPARs in the PSDs. This further suggests that sQD-labeled AMPARs in the synapse move in a confined space, resulting in a higher population of slow diffusion than the bQD-labeled AMPARs. To test this possibility, we examined the trajectories of AMPARs within 2 μm radius of the locations of Homer1 and found that 37% of sQD trajectories were synaptic, compared to 10% of bQD trajectories (SI Fig. 5). Our results are consistent with previous reports of an increased number of sQD (~11 nm) labeled AMPARs at synapses, compared to AMPARs labeled with bQDs[7,11]. Despite differences in detailed protocol and analysis algorithm used, Howarth et al. estimated that 24% of bQDs and 46% of sQDs co-localized at synapses[7]; Groc et al. using a Cy3-antibody estimated 9.4% at synapses, 20.3% using Cy3-bungarotoxin and 5.4% using a bQD-antibody to a GFP[11].
We note that Farlow et al.[30] developed a monovalent quantum dot (~12 nm in diameter) by steric exclusion. They used a functionalized oligonucleotide to wrap the QDs, making it monovalent and therefore reduce the possibility of cross-linking target proteins. However, it carries large amount of negative charges and would cause significant non-specific binding to the poly-lysine coated coverslip when labeling neurons.
It is possible that, in our experiments, a streptavidin (with 4 biotin binding sites), or multiple streptavidin per QD can induce cross-linking between AMPARs, and therefore reduce the AMPAR mobility. However, our data indicates this in unlikely. The reason is that the diffusion constant of AMPARs labeled with bQD-SA are similar to that labeled with organic fluorescent dye conjugated antibodies. (Large-scale cross-linking is unlikely --though still possible-- to be induced by dye-labeled antibodies). In particular, we find that mobile AMPAR (GluA2 subunit) labeled with bQD-SA has a median diffusion constant of 0.15 μm2/s, in rough agreement with AMPAR diffusion measured with Cy3-antibodies by Groc et al. (median 0.08 μm2/s for mobile GluA2)[31], and with ATTO647N-anti-GluA2 by Giannone et al. (median 0.10 μm2/s)[32]. Furthermore, the number of SA per sQD is even less than with bQD, and therefore, it is unlikely that sQD induces cross-linking. Finally, we also tested AMPAR diffusion on the cytoplasmic side of HEK cells (SI Fig. 4). Here the diffusion of AMPARs measured by bQD and sQD are similar, which also support the fact that sQDs do not induce AMPAR-cross-linking.
Nevertheless, to further reduce the chances of cross-linking, one could separate out sQD with single copy of SA by gel electrophoreses [7,16,33], or one could reduce the percentage of –COOH in the coating (e.g. from 10% to approximately 2.5%), resulting in fewer SAs per sQD. In addition, it is straightforward to functionalize sQD with other biomolecules, such as biotin or GBP[34] (a 13kD nanobody against GFP) instead of SA (SI Fig. 6), which could be used to produce monovalent sQD. It is also possible to functionalize sQD with a PEGylated O6-alkylguanine derivative to label fusion proteins with a SNAP tag (19.4 kD)[35]. By making the sQD monofunctional and using smaller molecules as functional groups, the effective size of sQD can be further reduced, making it a better fluorescent probe to function in crowded cellular environment.
In conclusion, we have developed a simple method to coat quantum dot cores, producing a smaller streptavidin-functionalized sQDs (8.9 ± 0.2 nm in diameter) than commercially available QDs (15–35 nm). Our sQDs are also chemically more stable than the previous generation of sQDs[7]. These sQDs enable specific labeling of AMPARs and allowed much greater access to the synaptic region than bQDs. Combined with three-dimensional super-resolution imaging, which can localize pre- and post- synaptic region, the biomolecules labeled with sQDs can be investigated by single particle tracking. Their application is not limited to the synapse but extends to any subcellular structures, particularly confined spaces or crowed spaces in cells where small fluorescent probes with tremendous photostability are advantageous.
Supplementary Material
Footnotes
This work was supported by NSF grants MCB1216342, DBI 1243568 (to P.R.S.) and PHY0822613 (to P.R.S and K.S.) and NIH GM086214, R21 NS087413 (to P.R.S). We would also like to acknowledge NIH grant 9P41GM104601 to K.S.. A.M.S. acknowledges the NIH grant R00 CA153914. W.N.G. acknowledges the NIH grants NS043782 and DA035430, and W.N.G. and P.R.S. acknowledge summer fellowships from Marine Biological Laboratory in 2010 and 2011. We thank P. Dionne and P. De Koninck for sharing their constructs and labeling protocol with us. We thank K. W. Teng for technical assistance and D. G. Fernig for initial consultation and advice.
Contributor Information
Dr. En Cai, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801.
Dr. Pinghua Ge, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801.
Dr. Sang Hak Lee, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801
Dr. Okunola Jeyifous, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801. Department of Neurobiology, University of Chicago, Chicago, IL, USA
Dr. Yong Wang, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801
Dr. Yanxin Liu, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801. Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Dr. Katie M. Wilson, Department of Structural and Chemical Biology and Institute of Integrative Biology, University of Liverpool, Liverpool, UK
Dr. Sung Jun Lim, Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Michelle A. Baird, The National High Magnetic Field Laboratory and Department of Biological Science, The Florida State University, Tallahassee, FL, USA
John E. Stone, Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Dr. Kwan Young Lee, Department of Molecular and Integrative Physiology, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Michael W. Davidson, The National High Magnetic Field Laboratory and Department of Biological Science, The Florida State University, Tallahassee, FL, USA
Prof. Dr. Hee Jung Chung, Department of Molecular and Integrative Physiology, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Prof. Dr. Klaus Schulten, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801. Beckman Institute, University of Illinois at Urbana-Champaign, Urbana, IL, USA. Center for Biophysics and Computational Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Prof. Dr. Andrew M. Smith, Department of Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Prof. Dr. William N. Green, Department of Neurobiology, University of Chicago, Chicago, IL, USA. Marine Biological Laboratory, Woods Hole, MA, USA
Prof. Dr. Paul R. Selvin, Email: selvin@illinois.edu, Department of Physics and Center for the Physics of Living Cells, University of Illinois at Urbana-Champaign, Urbana, IL, USA., 1110 W Green St., Urbana, IL 61801. Center for Biophysics and Computational Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA. Marine Biological Laboratory, Woods Hole, MA, USA
References
- 1.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinaud F, Clarke S, Sittner A, Dahan M. Nat Methods. 2010;7:275–285. doi: 10.1038/nmeth.1444. [DOI] [PubMed] [Google Scholar]
- 3.Echarte MM, Bruno L, Arndt-Jovin DJ, Jovin TM, Pietrasanta LI. FEBS Lett. 2007;581:2905–2913. doi: 10.1016/j.febslet.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You C, Wilmes S, Beutel O, Löchte S, Podoplelowa Y, Roder F, Richter C, Seine T, Schaible D, Uzé G, et al. Angew Chem Int Ed. 2010;49:4108–4112. doi: 10.1002/anie.200907032. [DOI] [PubMed] [Google Scholar]
- 6.Sperling RA, Liedl T, Duhr S, Kudera S, Zanella M, Lin CAJ, Chang WH, Braun D, Parak WJ. J Phys Chem C. 2007;111:11552–11559. [Google Scholar]
- 7.Howarth M, Liu W, Puthenveetil S, Zheng Y, Marshall LF, Schmidt MM, Wittrup KD, Bawendi MG, Ting AY. Nat Methods. 2008;5:397–399. doi: 10.1038/nmeth.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J Am Chem Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan N, Palui G, Safi M, Ji X, Mattoussi H. J Am Chem Soc. 2013;135:13786–13795. doi: 10.1021/ja405010v. [DOI] [PubMed] [Google Scholar]
- 10.Dahan M, Lévi S, Luccardini C, Rostaing P, Riveau B, Triller A. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 11.Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L. J Neurosci. 2007;27:12433–12437. doi: 10.1523/JNEUROSCI.3349-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill R, Zayats M, Willner I. Angew Chem Int Ed. 2008;47:7602–7625. doi: 10.1002/anie.200800169. [DOI] [PubMed] [Google Scholar]
- 13.Fernig DG, Duchesne L. Nanoparticle Conjugates. 20110165647 A1 US. 2011
- 14.Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie S. Annu Rev Anal Chem. 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Greytak AB, Lee J, Wong CR, Park J, Marshall LF, Jiang W, Curtin PN, Ting AY, Nocera DG, et al. J Am Chem Soc. 2010;132:472–483. doi: 10.1021/ja908137d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke S, Pinaud F, Beutel O, You C, Piehler J, Dahan M. Nano Lett. 2010;10:2147–2154. doi: 10.1021/nl100825n. [DOI] [PubMed] [Google Scholar]
- 17.Pinaud F, King D, Moore HP, Weiss S. J Am Chem Soc. 2004;126:6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchesne L, Gentili D, Comes-Franchini M, Fernig DG. Langmuir. 2008;24:13572–13580. doi: 10.1021/la802876u. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama Y, Kawabata I, Sobue K, Okabe S. Nat Methods. 2005;2:677–684. doi: 10.1038/nmeth783. [DOI] [PubMed] [Google Scholar]
- 20.Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 21.Howarth M, Takao K, Hayashi Y, Ting AY. Proc Natl Acad Sci U S A. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howarth M, Ting AY. Nat Protoc. 2008;3:534–545. doi: 10.1038/nprot.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang H, Zhang M, Ji W, Chen J, Zhang Y, Liu B, Lu J, Zhang J, Xu P, Xu T. Proc Natl Acad Sci. 2012;109:4455–4460. doi: 10.1073/pnas.1113770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Wang W, Bates M, Zhuang X. Science. 2008;319:810–813. doi: 10.1126/science.1153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR. Science. 2003;300:2061–2065. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 27.Henriques R, Lelek M, Fornasiero EF, Valtorta F, Zimmer C, Mhlanga MM. Nat Methods. 2010;7:339–340. doi: 10.1038/nmeth0510-339. [DOI] [PubMed] [Google Scholar]
- 28.Renner M, Choquet D, Triller A. J Neurosci. 2009;29:2926–2937. doi: 10.1523/JNEUROSCI.4445-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czöndör K, Mondin M, Garcia M, Heine M, Frischknecht R, Choquet D, Sibarita J-B, Thoumine OR. Proc Natl Acad Sci. 2012:201109818. doi: 10.1073/pnas.1109818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farlow J, Seo D, Broaders KE, Taylor MJ, Gartner ZJ, Jun Y. Nat Methods. 2013;10:1203–1205. doi: 10.1038/nmeth.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- 32.Giannone G, Hosy E, Levet F, Constals A, Schulze K, Sobolevsky AI, Rosconi MP, Gouaux E, Tampe R, Choquet D, et al. Biophys J. 2010;99:1303–1310. doi: 10.1016/j.bpj.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itano MS, Neumann AK, Liu P, Zhang F, Gratton E, Parak WJ, Thompson NL, Jacobson K. Biophys J. 2011;100:2662–2670. doi: 10.1016/j.bpj.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothbauer U, Zolghadr K, Muyldermans S, Schepers A, Cardoso MC, Leonhardt H. Mol Cell Proteomics. 2008;7:282–289. doi: 10.1074/mcp.M700342-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Colombo M, Mazzucchelli S, Montenegro JM, Galbiati E, Corsi F, Parak WJ, Prosperi D. Small. 2012;8:1492–1497. doi: 10.1002/smll.201102284. [DOI] [PubMed] [Google Scholar]
- 36.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.