Abstract
Ureaplasma spp. respiratory tract colonization is a significant risk factor for bronchopulmonary dysplasia (BPD), a chronic lung disorder in preterm infants. As an initial step preparatory to future clinical trials to evaluate the clinical efficacy of azithromycin to prevent BPD, we characterized the pharmacokinetics, safety, and biological effects of a single intravenous dose of azithromycin (10 mg/kg) in preterm neonates (n=12) 24–28 weeks gestation at risk for Ureaplasma infection and BPD. A two-compartment structural model with the clearance and volume of peripheral compartment (V2) allometrically scaled on body weight (WT) best described the pharmacokinetics of azithromycin in preterm neonates. The estimated parameters were: clearance [0.18 L/h × WT(Kg)0.75], intercompartmental clearance [1.0 L/h], volume of distribution of central compartment [0.93 L] and V2 [14.2 L × WT(Kg)]. There were no serious adverse events attributed to azithromycin. A single dose of azithromycin did not suppress inflammatory cytokines or myeloperoxidase activity in tracheal aspirates. Our results demonstrated the safety of azithromycin and developed a PK model that is useful for future simulation-based clinical trials for eradicating Ureaplasma and preventing BPD in preterm neonates.
Keywords: azithromycin, Ureaplasma, prematurity, pharmacokinetics, Bronchopulmonary Dysplasia
Introduction
Accumulating data suggest that respiratory colonization with the genital mycoplasma species, Ureaplasma parvum and Ureaplasma urealyticum is a risk factor for the development of the chronic lung disorder bronchopulmonary dysplasia (BPD) in preterm neonates1–7. Human and experimental studies confirmed that Ureaplasma spp. cause an augmented and dysregulated inflammatory response in the developing lung that contributes to arrested alveolarization and angiogenesis, chronic inflammation, and interstitial fibrosis that characterize the pathologic findings of BPD8–16. Although, early detection and eradication of Ureaplasma may prevent or ameliorate the severity of BPD in infected preterm neonates, there is currently no consensus among neonatologists concerning appropriate therapy. Development of effective therapies to eradicate Ureaplasma from the respiratory tract and/or prevent infection-mediated lung inflammation is critically important for the prevention of BPD.
Despite in vitro susceptibility of Ureaplasma species to erythromycin (a macrolide antibiotic)17, clinical trials involving erythromycin therapy in Ureaplasma colonized preterm infants have failed to demonstrate any efficacy to prevent BPD18, 19 or to eradicate respiratory tract colonization20. The 14-membered macrolides that are derivatives of erythromycin and the related 15-member azalides (e.g., azithromycin) are promising candidates for modulating the inflammatory effects associated with Ureaplasma infection. They affect neutrophil function (e.g. chemotaxis, cell adhesion, oxidative burst and phagocytosis), cytokine release (e.g., IL-1β, IL-8, IL-6)21 and nitric oxide production in vitro22. The macrolide antibiotics may exert these immunomodulatory effects in the setting of infection, and may occur independently of a direct bactericidal effect23. Azithromycin inhibits neutrophil influx and chemoattractant/cytokine release in murine lung noninfectious24, 25, as well as pneumonia26, 27 injury models. Pharmacokinetic studies in mice28 and humans have shown that azithromycin is preferentially concentrated in pulmonary epithelial lining fluid and alveolar macrophages29, 30. Since neutrophil recruitment and activation has been implicated in BPD pathogenesis31–33, the experimental effects observed with azithromycin in vitro and in vivo indicate that this drug may be beneficial in the treatment of Ureaplasma infection and the prevention of BPD in preterm infants.
Azithromycin exhibits bacteriostatic activity with higher potency than erythromycin against Ureaplasma isolates in vitro17, 34, 35 and suppresses cytokine release in cultured tracheal aspirate cells from preterm infants36. We hypothesize that intravenous azithromycin therapy will prevent BPD in Ureaplasma-colonized preterm infants by accelerating pathogen clearance and/or down-regulating the pulmonary inflammatory response. Although the safety and pharmacokinetics of intravenous azithromycin have been evaluated in children >6 months of age37, there are no safety or pharmacokinetic data in preterm neonates from which appropriate age-based dosing can be derived. In order to design appropriate randomized clinical trials to evaluate clinical efficacy of azithromycin to eradicate Ureaplasma and/or prevent BPD, we conducted a pilot study to evaluate the safety and pharmacokinetics of a single intravenous dose of azithromycin in mechanically ventilated, preterm neonates at high risk for Ureaplasma respiratory tract colonization and BPD development. In addition, we evaluated pulmonary inflammatory cytokines and neutrophil myeloperoxidase activity as potential azithromycin response biomarkers and the feasibility of a central reference laboratory for Ureaplasma cultures and PCR genotyping for planning for future randomized clinical trials.
Methods
Study design and infant enrollment
This study was a single-dose (10 mg/kg), pharmacokinetic study of i.v. azithromycin in mechanically ventilated preterm neonates born between 24–28 wk of gestation conducted from February to December, 2008. This dose was selected since it was previously reported to be safe in older children37. The Institutional Review Boards of the participating sites (University of Maryland, School of Medicine and the University of Virginia, School of Medicine) approved the study protocol. Neonatal intensive care unit admissions with gestational age 24wk 0d to 28wk 6d were screened for study eligibility. Inclusion criteria were: 1) appropriate weight for gestational age as determined by best obstetrical estimates38; 2) intubation and mechanical ventilation for any duration during the first 48 hours after birth; 3) presence of an indwelling intravenous line for drug administration; and 4) presence of an indwelling clinically indicated arterial line for blood sampling. Exclusion criteria were: 1) non-viability or planned withdrawal of life support; 2) major lethal congenital anomalies; 3) delivery for maternal indications (low risk of Ureaplasma colonization); 4) hypotension defined as mean arterial blood pressure < the gestational age in wks39 that persists for >6 h during the first 24 h of life; 5) electrocardiogram QT interval corrected for heart rate (Qtc) ≥450 ms; 6) significant renal impairment (urine output <0.5 mL/kg/hr); 7) significant hepatic impairment (liver function tests >2x upper limit normal range); 8) exposure to another macrolide antibiotic; 9) clinically suspected systemic Ureaplasma spp. infection or other confirmed systemic bacterial/viral infection; 10) maternal receipt of a macrolide antibiotic within 7 days prior to delivery and 11) participation in other clinical trials involving investigational or marketed products. Infants surviving to 36 wks post-menstrual age (PMA) were assessed for physiologic BPD. A timed oxygen reduction test was conducted for those infants on low fractional inspired oxygen40. Infants who could be weaned off of supplemental oxygen and remain on room air for 30 minutes were classified as non-BPD infants while those who were receiving positive pressure support at 36 wks PMA or failed the timed oxygen reduction test were classified as BPD infants.
Pharmacokinetic study design
After baseline laboratory tests and pre-dose cultures had been obtained, infants received an azithromycin dose of 10 mg/kg at a concentration of 2 mg/mL as a single intravenous infusion over 1 h using a functioning intravenous line. Blood draws were performed using an existing indwelling arterial catheter inserted for clinical indications. Sparse sampling technique was employed. Six total samples (0.5 mL each) were collected between 0–1, 1–4, 6–8, 24–48, 48–96, and 96–144 h post-dose with the exact time of each sample recorded. The sampling windows were selected to allow precise characterization of the distribution and the elimination phases of azithromycin and are based on the results of a previously published report in older infants and children37. The blood samples were collected into tubes containing anticoagulant EDTA, centrifuged, and the plasma aspirated and frozen at –80°C until analyzed for azithromycin concentrations using a validated LC/MS/MS detection method41.
Pharmacokinetic data analysis
Plasma concentration data were modeled using the non-linear mixed-effects modeling software NONMEM (version VI) (ICON Development Solutions, Ellicott City, MD). The first-order conditional estimation method with interaction was used throughout the modeling procedure. A two compartment structural model (ADVAN3 TRANS4 NONMEM subroutine) was used for the analysis. The model selection was based on evaluation of the objective function (OF) value, pharmacokinetic parameter estimates and their relative standard errors, physiologic plausibility of the parameter estimates, and inspection of goodness-of-fit plots. The Likelihood Ratio Test was used for comparing rival hierarchical models where a decrease in OF (−2 log likelihood) of 6.6 points was necessary to consider the improvement in model performance statistically significant at p =0.01 and 1 degree of freedom42. An exponential error model was used to describe the inter-individual variability in CL and V2 as follows:
Where ηi is the proportional difference between the hypothetical true parameter estimate of the ith subject (Pi) and the typical population parameter value (TVP) and is assumed to be normally distributed with a mean of 0 and a variance of ω2. The residual error (which includes model misspecification, intra-subject variability as well as errors in dosing, sampling times and sample analysis) was described using a proportional error model as follows:
Where Yobs is the observed plasma concentration, Ypred is the model predicted plasma concentration and ε is a normally distributed parameter with a mean of 0 and variance of σ2. The CL and V2 were allometrically scaled on body weight in the final model as follows
Were TVCLi and TVVpi are the typical values for clearance and volume of peripheral compartment for a subject i with a certain body weight (WTi). Such scaling resulted in statistically significant (p <0.01) drop in the OF (9 and 10 points drop in OF for scaling CL and V2, respectively, on body weight). The final pharmacokinetic model was then used to simulate different regimens of i.v. azithromycin dosing (combinations of different doses and treatment durations) using Pharsight® Trial Simulator software (version 2.2.1, Pharsight Corporation, Mountain View, CA). For each regimen, 1000 neonates were simulated and the median and 90 % prediction intervals (calculated from the 5th and 95th percentiles) for the simulated azithromycin concentrations were determined.
Respiratory specimen collection
Tracheal aspirates (TA) for Ureaplasma culture and cytokines were obtained at the time of clinically indicated endotracheal tube suctioning. Two tracheal aspirate samples were obtained 2–6 h apart pre-dose and, in infants who remain intubated additional aspirates were obtained at 2, 4–5 and 21 d post-dose. A single nasopharyngeal (NP) culture was obtained pre-dose and additional NP samples were obtained using the same schedule if the infant was extubated. For tracheal aspirate sampling, the first instillation of saline was collected for cytokine analysis and second instillation for culture. Lung lavage was performed by a standardized technique as previously described10. Culture specimens were immediately inoculated in 10B urea broth43 and kept on ice until processed.
Microbiological Methods
Ureaplasma culture and antimicrobial susceptibility testing
Screening cultures for Ureaplasma spp. on specimens of endotracheal or nasal secretions obtained as described above were performed on site at the University of Maryland, Baltimore (UMB). For each specimen, 4 ten-fold serial dilutions were performed in 10B broth and incubated under atmospheric conditions at 37°C. Cultures were observed for up to 72 h for broth color change from yellow to pink without turbidity, indicating pH change due to urease activity. Aliquots of all original TA and NP cultures from both sites and all positive cultures obtained at UMB were immediately frozen at −80°C and batch-shipped to the Diagnostic Mycoplasma Laboratory at the University of Alabama at Birmingham (UAB) for definitive culture and organism identification. Colonies of Ureaplasma spp. were identified presumptively by their characteristic granular brown appearance on A8 agar in the presence of the CaCl2 indicator43. Ureaplasma isolates from all culture-positive neonates were tested by microbroth dilution as described previously44 to determine their minimal inhibitory concentrations (MICs) for azithromycin. Treatment failure was defined as a confirmed positive culture at any time point post-dose.
Ureaplasma Genotyping by Real-time PCR Assay
In addition to culture, all clinical specimens were tested directly for the presence of U. urealyticum and U. parvum by a real-time PCR assay. Individual Ureaplasma isolates obtained by culture were also tested by PCR to determine their species designations. Genomic DNA from clinical specimens and the Ureaplasma isolates was extracted by the proteinase K method as described previously45. A multiplex real-time PCR assay was employed to detect and differentiate the two Ureaplasma species simultaneously using the Roche LightCycler 2.0 (Roche Diagnostics, Indianapolis, IN)46. The U. parvum primer/probe set anneals to the 477 bp UP063 gene (NP_077893), which encodes a conserved hypothetical protein that is identical in all 4 U. parvum serovars. The U. urealyticum primer/probe set anneals to a 15,072 bp open reading frame (ORF) that is almost perfectly (>99.97%) conserved in all 10 U. urealyticum serovars (serovar 10 Genbank ID ACI59931.1).
Cytokine and Myeloperoxidase analysis
Lung lavage fluid for cytokines was placed on ice, immediately centrifuged at 4000×g to pellet cells, the supernatant aliquoted and frozen at –80°C for later cytokine analysis by the UMB Cytokine Core Laboratory. IL-1β, IL-8, and IL-6 were measured by Luminex™ multianalyte immunoassay using reagents from Upstate Biotechnology. The cell pellet was resuspended in 100 µl lysis buffer (50 mM potassium phosphate buffer containing 0.5% hexadecyl trimethylammonium bromide (HTAB)), then frozen at −80°C for later myeloperoxidase assay (MPO). MPO activity was assayed in cell lysates as previously described47, 48.
Safety evaluation
Safety and tolerance evaluation in each infant included vital signs, frequency of apnea and bradycardia, cardiac rhythm, pulse oximetry, clinical laboratory testing and adverse events. Infants were assessed for morbidities associated with prematurity, concomitant medications, and adverse events on study days 3, 7 (± 3 d), 14 (± 3 d) and every 2 wks thereafter until discharge or transfer. Clinically relevant AE that were specifically evaluated included: local site reaction including phlebitis, gastrointestinal abnormalities (abdominal distension, gastric aspirates, emesis, heme-positive stools), intestinal obstruction including radiographic-confirmed pyloric stenosis, culture-proven sepsis with other bacterial or nonbacterial pathogens, necrotizing enterocolitis (NEC), and cardiac arrhythmias. A hearing screening test as part of clinical care was performed before discharge. Serial clinical laboratory tests (complete blood count with manual differential, electrolytes, liver function, and renal function) were performed pre- and post-dose.
Results
Clinical outcomes
Out of sixty-nine potential infants 24wk 0d to 28wk 6d gestation screened, 41 infants were eligible for the study. Parental consent was obtained for 14 infants, 19 refused consent, and 8 were not approached within the enrollment time window. Plasma samples were available from twelve subjects only since one subject was withdrawn prior to dosing secondary to clinical deterioration, and one subject was not included in the PK analysis secondary to receipt of an additional dose of azithromycin in violation of the protocol. Since pre-dose cultures were successfully obtained and processed for all 14 enrolled subjects and all subjects were followed for safety data and were assessed for BPD outcome, demographic data for all subjects are included in Table 1. The postnatal age at time of study drug dosing was 47 ± 28 h (mean±SD). As summarized in Table 2, three Ureaplasma-positive infants died (including the infant withdrawn prior to receiving study drug), while all Ureaplasma-negative infants survived. Sixty percent of surviving Ureaplasma-positive infants were classified as physiologic BPD compared to 17% of Ureaplasma-negative infants (Table 2). Overall, 75% of Ureaplasma-positive infants either died or developed BPD (Table 2).
Table 1.
Summary of infants’ demographics
| Infant Characteristics | mean ± SD or N (%) N=14 |
|---|---|
| Birth weight (g) | 855 ± 276 |
| Gestational age (wk) | 26 ± 1 |
| Postnatal age at dosing time (h) | 47 ± 28 |
| (wk) | 26.3 ± 0.95 |
| Length (cm) | 33.5 ± 3.4 |
| Body Surface Area (m2) | 0.09 ± 0.02 |
| Duration of IMV at study entry (h) | 29 ± 13 |
| Male gender | 7 (50) |
| Race: | |
| Black | 9 (64) |
| White | 2 (14) |
| Hispanic | 1 (7) |
| Asian | 1 (7) |
| Mixed race | 1 (7) |
| Ureaplasma positive | 8 (57) |
IMV: intermittent mandatory ventilation
Table 2.
Clinical outcomes
| Study number |
Ureaplasma culture status |
Status @ 36 wk PMA |
Timed O2 reduction test performed |
Results of Timed O2 reduction test |
Physiologic BPD @ 36 wk PMA |
|---|---|---|---|---|---|
| 1 | Pos | Died 56 d | |||
| 2 | Pos | Alive, SIMV |
No | Yes | |
| 3 | Pos | Alive, NC | Yes | Passed | No |
| 4 | Pos | Died 21 d | |||
| 5* | Pos | Alive, nCPAP |
No | Yes | |
| 6 | Pos | Alive, NC | Yes | Passed | No |
| 7 | Pos | Alive, SIMV |
No | Yes | |
| 8** | Pos | Died 3 d | |||
|
All positives |
3 deaths | 2/5 | 2 | 3/5 (60%) | |
| 9 | Neg | Alive, NC | Yes | Passed | No |
| 10 | Neg | Alive, NC | Yes | Passed | No |
| 11 | Neg | Alive, NC | Yes | Passed | No |
| 12 | Neg | Transferred, NC |
No | Yes | |
| 13 | Neg | Alive, room air |
No | No | |
| 14 | Neg | Alive, NC | Yes | Passed | No |
|
All negatives |
0 deaths | 4/6 | 4 | 1/6 (17%) | |
| Total | 3 deaths | 6 | 6 | 4/14 (29%) | |
received 2 additional doses azithromycin (protocol violation);
withdrawn prior to dosing due to clinical deterioration
PMA: post-menstrual age
NC: nasal cannula; nCPAP: nasal continuous positive pressure; SIMV: synchronized intermittent mandatory ventilation
Pharmacokinetics of azithromycin in preterm neonates
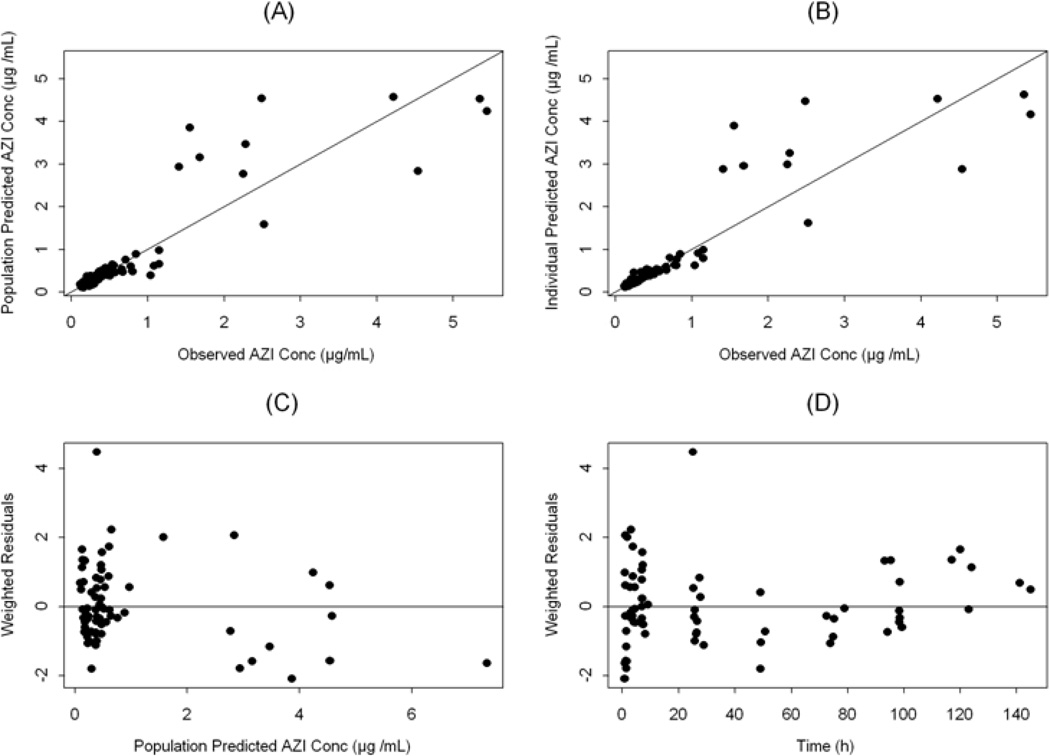
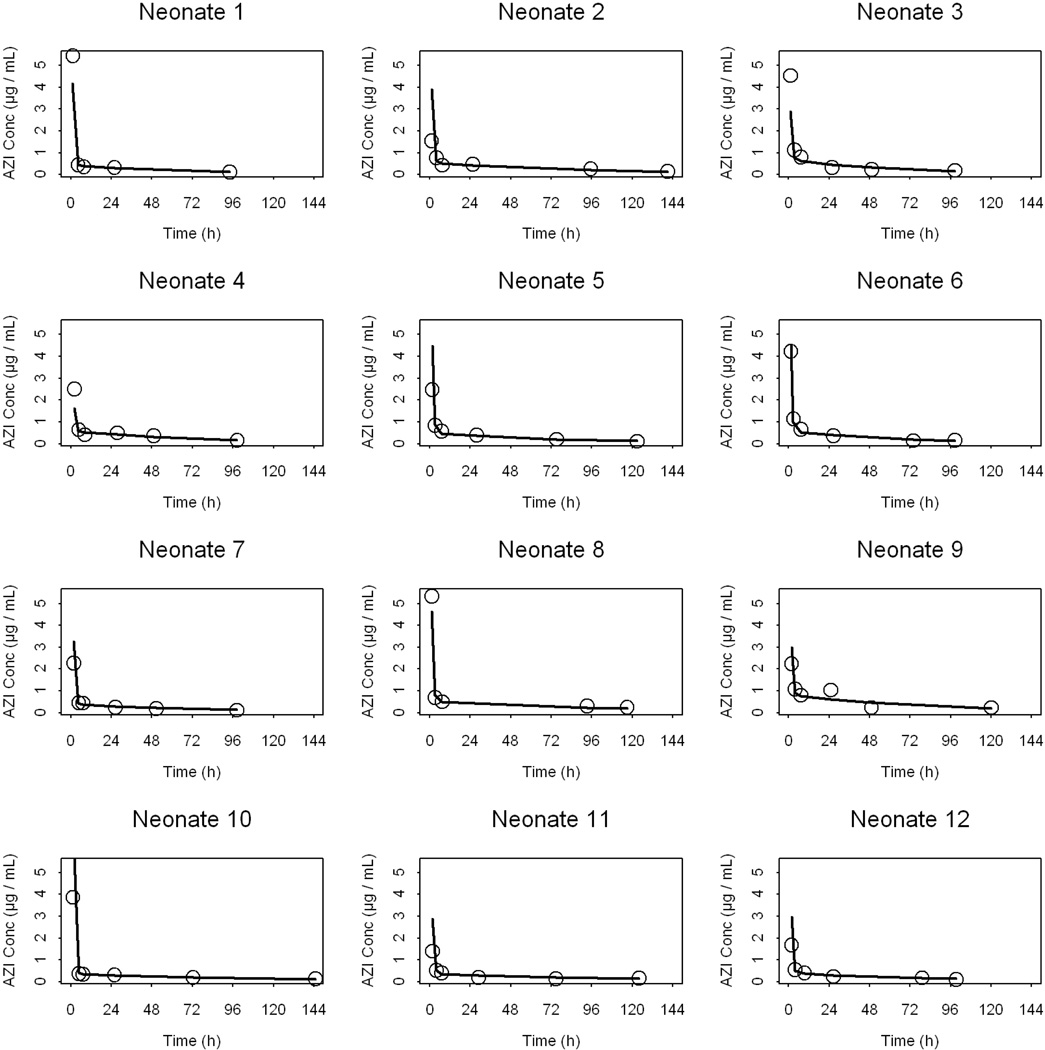
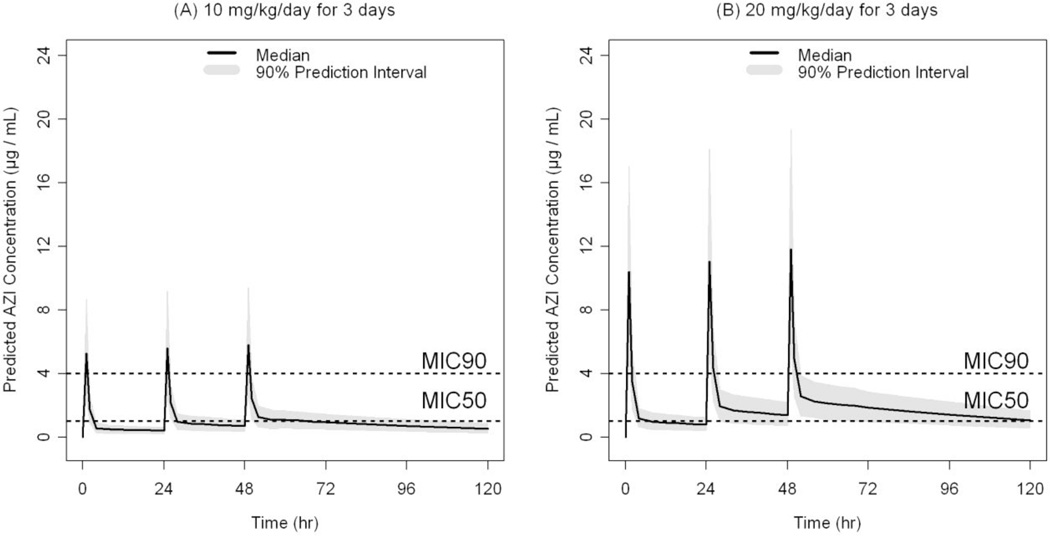
Data from 12 infants were included in the pharmacokinetic (PK) analysis. Each infant contributed 6 plasma samples collected in pre-specified intervals over 144 h post-dose. A population approach using NONMEM was used to analyze the relatively sparse PK data. Such approach is best suited for analyzing PK data derived from designs with sparse sampling42. The estimated population pharmacokinetic parameters of azithromycin in preterm infants are presented in Table 3. The structural model parameters were estimated with good precision. The clearance (CL) and the volume of the peripheral compartment (V2) were allometrically scaled on body weight (using an exponent of 0.75 for CL and 1 for V2) in the final model49. Using body weight as a covariate for CL and V2 accounted for ∼ 38 and 43%, respectively, of the initially estimated inter-subject variability in these parameters. The homogeneous nature of the dataset (narrow range of age) and the small number of neonates in the different categories of other covariates (e.g., sex and race) precluded robust examination of the influence of these covariates on the PK of azithromycin. Based on the final model parameters, the typical value for the elimination half-life (t1/2) is estimated to be 58 hours for a neonate weighing 1 kg. Goodness-of-fit plots for the final population PK model depicted in Figure 1 indicate that the model described the data well with no systematic bias. It is noteworthy that there was large variability in the plasma concentrations at the first sampling time. Sampling errors may have contributed to such variability when the plasma levels were declining rapidly with time. Figure 2 represents the observed and the model predicted plasma concentrations for each neonate (n=12) based on the empirical Bayesian estimates of the individual PK parameters. Non compartmental analysis of the data (details not shown) indicated that the harmonic mean t1/2 is 61 hours while the mean AUC24 and AUCinf were 17 and 56 µg.h/mL, respectively. Assuming 30% plasma-protein binding of azithromycin50, the AUC24 for unbound fraction of azithromycin (AUC24(u)) is ∼12 µg.h/mL. Neither single 10 mg/kg/day × 1 day (Figure 2, observed) nor multiple 10 mg/kg/day × 3 days (Figure 3A, simulated) azithromycin dosage regimens were adequate for maintaining azithromycin plasma concentrations above the MIC50 (1 µg/mL) derived from Ureaplasma isolates obtained from 25 neonates who received care at the University of Alabama at Birmingham. However, a dosage regimen of 20 mg/kg/day × 3 days i.v. azithromycin seems sufficient to maintain azithromycin plasma levels above the MIC50 (Figure 3B, simulated).
Table 3.
Population pharmacokinetic parameter estimates for azithromycin in preterm neonates
| Parameter | Estimate (% RSE) | % ISV (%RSE) |
|---|---|---|
| CL (L/h) | 0.18 x WT0.75 (8.2) | 23.5 (44.6) |
| V1 (L) | 0.93 (12.1) | NE |
| Q (L/h) | 1.0 (13.2) | NE |
| V2 (L) | 14.2 x WT (9.2) | 26.2 (50.6) |
| Residual Error (%) | 28.7 (15.0) | --- |
RSE: relative standard error
ISV: intersubject variability, calculated as the square root of the estimated variance of intersubject variability (i.e., ω) x100
WT: body weight in kilograms
NE: not estimated
Figure 1.
Diagnostic scatter plots for the population PK model. (A) population-predicted versus observed azithromycin plasma concentrations; (B) individual-predicted versus observed azithromycin (AZI) plasma concentrations; (C) weighted residuals versus population predicted plasma concentrations; and (D) weighted residuals versus time (The solid lines represent the lines of identity in A and B and zero weighted residuals in C and D).
Figure 2.
Observed (closed circles) and the post-hoc predicted (solid lines) azithromycin plasma concentrations versus time profiles for each neonate.
Figure 3.
Azithromycin i.v. simulated plasma concentration versus time profiles in preterm neonates after the following dosage regimens (A) 10 mg/kg/day × 3 days, n=1000, and (B) 20 mg/kg/day × 3 days, n=1000. Median plasma concentrations and 90% prediction interval (calculated from the 5th and 95th percentiles of the simulated concentrations) are depicted in the figure. The dotted lines represent the MIC50 (1 µg/mL) and MIC90 (4 µg/mL) of azithromycin against Ureaplasma.
Ureaplasma culture and polymerase chain reaction (PCR) results
Tracheal aspirate and nasopharyngeal samples were obtained pre-dose (day 0) and on study days 2, 4–5, and 21 of age for both Ureaplasma culture and PCR analysis. Prior to azithromycin dosing, eight of the fourteen enrolled infants (57%) were both Ureaplasma culture- and PCR-positive. Screening cultures performed at the University of Maryland, Baltimore (UMB) and confirmative cultures performed at the University of Alabama at Birmingham (UAB) agreed in all 8 positive infants. U. parvum alone was the most common species isolated from culture-positive infants (n=5, 63%). U. urealyticum alone was isolated from 1 culture-positive infant (13%) and both species were isolated from 2 infants (25%). Of the seven subjects who were culture-positive pre-dose and completed the study, 3 had at least one positive culture post-dose and were considered treatment failures (43%).
Azithromycin effects on indices of inflammation
Analyses of TAs pre- and post-dose for concentrations of pro-inflammatory cytokines (IL-1β), chemokines (IL-8), counter-regulatory cytokines (IL-6) and neutrophil myeloperoxidase (MPO) activity revealed a trend towards higher IL-6, IL-8, and IL-1β concentrations in TAs pre-dose in the azithromycin-treated Ureaplasma–positive infants compared to concentrations pre-dose in Ureaplasma-negative infants. However, a single 10 mg/kg dose of azithromycin did not significantly (p >0.05) suppress the TA cytokines or MPO of the treated neonates who remained intubated at later time points (Table 4).
Table 4.
Tracheal aspirate cytokine concentrations pre- and post-azithromycin therapy*
| Cytokine | Pre-dose (N=12) |
D2 post-dose (N=9) |
|---|---|---|
| IL-6 (pg/mL) | 204 (92–444) |
148 (67–255) |
| IL-6 Ureaplasma positive | 237 (128–400) |
147 (67–255) |
| IL-6 Ureaplasma negative | 107 (76–1070) |
148 (64–721) |
| IL-8 (pg/mL) | 4560 (1980–6595) |
2910 (2250–5850) |
| IL-8 Ureaplasma positive | 5850 (2140–-5850) |
3110 (2220–5850) |
| IL-8 Ureaplasma negative | 2700 (1820–14200) |
2750 (2250–8170) |
| IL-1β (pg/mL) | 20.14 (2–89) |
40.4 (25–91) |
| IL-1β Ureaplasma positive | 24 (2–95) |
63 (25–599) |
| IL-1β Ureaplasma negative | 4 (2–82) |
30 (5–91) |
| MPO (units/mg protein) | 0.6 (0.3–1.5) |
0.5 (0.3–0.8) |
| MPO Ureaplasma positive | 0.6 (0.3–2.0) |
1.2 (0.2–3.0) |
| MPO Ureaplasma negative | 0.5 (0.3–1.2) |
0.5 (0.1–0.8) |
Data are expressed as median with 25th to 75th percentiles in parentheses
Safety and serious adverse events
All infants were monitored until discharge for adverse events and serious adverse events (SAE). The SAEs observed were common morbidities of extreme prematurity and none was assessed as being related to azithromycin exposure. The SAEs were: a) one bilateral Grade 3 intraventricular hemorrhage (IVH) complicated by posthemorrhagic hydrocephalus treated with ventriculo-peritoneal shunt placement, b) one pneumomediastinum treated with increased ventilator support with high frequency oscillation, c) one worsening Respiratory Distress Syndrome treated with high frequency oscillator ventilation and nitric oxide administration, d) one event of direct hyperbilirubinemia one day post-dose that returned to baseline within 24 h, and e) two events of rapid progression of intestinal necrotizing enterocolitis (NEC). Both NEC events were followed by life support withdrawal and death. One infant failed the hearing screening test.
Discussion
To the best of our knowledge, this is the first study to evaluate azithromycin pharmacokinetics in preterm neonates. Azithromycin pharmacokinetics in this preterm sample followed bi-exponential disposition with a rapid initial decline in the plasma levels followed by a slower elimination phase (Figure 2). A population model for azithromycin pharmacokinetics in preterm neonates was developed using NONMEM. Incorporating body weight as a covariate resulted in statistically significant improvement in the model fit (p <0.01) and reduced the estimated random inter-subject variability in both CL and V2 (Table 3). The results from the present study indicate that preterm neonates have reduced azithromycin clearance and increased volume of distribution compared to older children37, 51, with considerable inter-subject variability (Table 3). The estimated clearance of azithromycin in the present study was 0.18 L/h for a typical preterm neonate weighing 1 kg. In a previous pharmacokinetic study involving 32 older children (0.5–16 yr) who received a single 10 mg/kg intravenous dose, azithromycin clearance was estimated to be 0.98 L/h/kg37. The reduced clearance of azithromycin in preterm neonates may be attributed to the immature or deficient biliary excretion pathways in preterm neonates relative to older children, which is the major route of azithromycin elimination52. Consistently, the systemic exposure of azithromycin was ∼ 4-fold greater in preterm neonates than previously reported in older children (AUCinf = 56 µg.h/mL in preterm neonates versus 15 µg.h/mL for 0.5 to 2 year old children37 at the same 10 mg/kg i.v. dose level). A wide range of azithromycin half-life values in older children (0.5–16 yr) was reported (approximately 26 to 83 h)37, 51. In our study, based on the final model, the estimated half-life of azithromycin in preterm neonates is approximately 58 hours for a typical 1 kg neonate.
For effective Ureaplasma eradication, the plasma concentration of free unbound azithromycin must be maintained above the minimum inhibitory concentration that is required to inhibit 50% (MIC50) of Ureaplasma. Although the MIC50 of azithromycin against Ureaplasma is determined in vitro, it provides target unbound drug concentrations to be reached/exceeded for desired clinical outcome. The MIC50 and MIC90 of azithromycin against neonatal Ureaplasma isolates at the University of Alabama at Birmingham are 1 and 4 µg/mL, respectively, and the MIC range from the isolates obtained from the 8 tested neonates in this study is 0.5 – 2 µg/mL. In the current study, a rapid immediate decline of azithromycin plasma concentrations below the MIC50 was observed (Figure 2) which may explain the 43% treatment failure rate of a single i.v. dose of azithromycin to eradicate Ureaplasma in culture-positive study subjects. The long elimination half-life of azithromycin (∼58 hr) enables short course azithromycin administration to be clinically effective53. Indeed, the recommended dosing duration of azithromycin in children and adults is 3–5 days51, 54–56. During this time interval, steady-state azithromycin plasma levels are not achieved since treatment for 5 half-lives (∼12 days) is required for steady state azithromycin plasma levels to be achieved. Therefore, we conducted our simulation analysis for not more than a 3-day period which indicated that even multiple dose administration of 10 mg/kg/day × 3 days azithromycin would be inadequate to maintain azithromycin plasma concentrations above the MIC50. Hence, we predict that multiple dose administration of 10 mg/kg will be insufficient for eradicating Ureaplasma. On the other hand, a dosage regimen of 20 mg/kg/day × 3 days would be sufficient to maintain azithromycin plasma concentration above the MIC50 as demonstrated by our simulation analysis (Figure 3B).
The pharmacodynamic parameter that is a good predictor for achieving azithromycin efficacy is the ratio of AUC24(u)/MIC90 50. The optimal AUC24(u)/MIC90 ratios for azithromycin to eradicate Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, and Moraxella catarrhalis were reported to be 0.5, 0.9, 14.8, 14.8, and 14.8 h, respectively50 however, the optimal AUC24(u)/MIC90 for azithromycin to effectively eradicate Ureaplasma is still unknown. Based on our calculated AUC24(u) (12 µg.h/mL) for azithromycin in the preterm sample and the MIC90 of 4 µg/mL, we predict that this ratio needs to be significantly higher than 3 h. This can be achieved either by accelerated azithromycin dosing or “front-loading” azithromycin (administering all intended therapeutic doses as one single bolus dose) to increase the 24 h systemic exposure of unbound azithromycin (AUC24(u)) as demonstrated in a previous preclinical study57.
Azithromycin has unique pharmacokinetic characteristics. Once administered, very little of the administered dose resides in the plasma and the vast majority of azithromycin accumulates intracellularly leading to a prolonged elimination t1/2 and extended mean residence time (MRT). These characteristics favor administering higher dosage regimens of azithromycin, a strategy that is commonly used for the management of acute otitis media in older children (10 mg/kg/day × 3 days or 30 mg/kg as single dose). In the current study with sparse information about the pharmacokinetics, safety and toxicity of azithromycin in preterm neonates, we would not have found it acceptable to evaluate the effects of a higher dosage regimen without first assuring the safety in this population. As such, an azithromycin dose previously evaluated in older children was used37. Infants in this study were closely monitored for signs of intolerance to azithromycin. No SAEs attributed to the drug were observed following single dose administration. Single (10 mg/kg/day) and multiple (10 mg/kg/day × 3 days) dosage regimens of azithromycin were deemed safe for older children and no azithromycin-related SAEs were reported37, 55.
In the current study, 57% of the mechanically ventilated preterm infants were both Ureaplasma culture and PCR positive pre-dose. Seventy-five percent of Ureaplasma-positive infants died or developed BPD while only seventeen percent of Ureaplasma-negative infants developed BPD and none died, highlighting the adverse outcomes in Ureaplasma-positive infants (Table 2). The dosage regimen used in this study (10 mg/kg/day) is likely insufficient against Ureaplasma, as three of seven Ureaplasma-positive infants remained culture positive post-dosing. Likewise, this dosage regimen was ineffective in suppressing the levels of inflammatory cytokines or the MPO activity in the lungs of these infants. In a single center RCT pilot study of 43 subjects, azithromycin (10 mg/kg/day × 7 days followed by 5 mg/kg/day up to 6 wks) did not reduce the incidence of BPD in infants <1000 g birthweight58. In a follow-up larger trial at the same institution, Ureaplasma clearance and BPD rates were similar in the placebo and azithromycin-treated groups59. These results suggest that 10 mg/kg azithromycin as a single or multiple dose is likely inadequate for Ureaplasma eradication and BPD prevention. Furthermore, they demonstrate the importance of performing appropriate PK/PD studies preparatory to phase II/III trials to assess microbiological and clinical efficacy of azithromycin.
In conclusion, the high rate of mortality and respiratory morbidity associated with Ureaplasma colonization of preterm infants underscores the need to develop effective treatment to eradicate the organisms and prevent lung injury in at-risk infants. The ultimate goal of this investigation was to determine PK parameters necessary to design and conduct future clinical trials of azithromycin in preterm neonates. The data presented were consistent with the safety of azithromycin and established a PK model for refining future simulation-based clinical trials to eradicate Ureaplasma and prevent BPD.
Acknowledgments
This work was supported by Federal funds under grants HD054876 and R01A1072577. The authors would like to thank Dr. Kang-Pil Kim, Dr. Pamela Voulalas, Rob Rogwsiki, Mary Spence, Elise Janofsky, Amy E. Blackman, Donna Crabb, and Amy Ratliff for their technical assistance.
Footnotes
Conflict of Interest/Disclosure
The authors declare no conflict of interest that may interfere with data interpretation. Dr. Ahmed Othman was an employee of University of Maryland at the time of the design and initiation of the study and is currently an employee of Abbott Laboratories.
References
- 1.Wang EL, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: Results of a metaanalysis. J Pediatr. 1995;127:640–644. doi: 10.1016/s0022-3476(95)70130-3. [DOI] [PubMed] [Google Scholar]
- 2.Castro-Alcaraz S, Greenberg EM, Bateman DA, Regan JA. Patterns of colonization with Ureaplasma urealyticum during neonatal intensive care unit hospitalizations of very low birth weight infants and the development of chronic lung disease. Pediatrics. 2002;110:E45–E45. doi: 10.1542/peds.110.4.e45. [DOI] [PubMed] [Google Scholar]
- 3.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz B, et al. Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J Clin Microbiol. 2005;43:4852–4854. doi: 10.1128/JCM.43.9.4852-4854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24:1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 6.Colaizy TT, Morris CD, Lapidus J, Sklar RS, Pillers DA. Detection of ureaplasma DNA in endotracheal samples is associated with bronchopulmonary dysplasia after adjustment for multiple risk factors. Pediatr Res. 2007;61:578–583. doi: 10.1203/pdr.0b013e318045be03. [DOI] [PubMed] [Google Scholar]
- 7.Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res. 2009;65:84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudd PT, Cassell GH, Waites KB, Davis JK, Duffy LB. Ureaplasma urealyticum pneumonia: experimental production and demonstration of age-related susceptibility. Infect Immun. 1989;57:918–925. doi: 10.1128/iai.57.3.918-925.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh WF, et al. A primate model of Ureaplasma urealyticum infection in the premature infant with hyaline membrane disease. Clin Infect Dis. 1993;17(Suppl 1):S158–S162. doi: 10.1093/clinids/17.supplement_1.s158. [DOI] [PubMed] [Google Scholar]
- 10.Patterson AM, et al. Ureaplasma urealyticum respiratory tract colonization is associated with an increase in IL-1β and TNF-α relative to IL-6 in tracheal aspirates of preterm infants. Pediatr Infect Dis J. 1998;17:321–328. doi: 10.1097/00006454-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Manimtim WM, et al. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69:3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viscardi RM, Manimtim WM, Sun CCJ, Duffy L, Cassell GH. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Devel Pathol. 2002;5:141–150. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 13.Viscardi RM, et al. Characterization of a murine model of Ureaplasma urealyticum pneumonia. Infect Immun. 2002;70:5721–5729. doi: 10.1128/IAI.70.10.5721-5729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoder BA, et al. Effects of antenatal colonization with Ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]
- 15.Viscardi R, et al. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol. 2006;9:143–151. doi: 10.2350/10-05-0112.1. [DOI] [PubMed] [Google Scholar]
- 16.Viscardi RM, et al. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res. 2006;60:141–146. doi: 10.1203/01.pdr.0000228322.73777.05. [DOI] [PubMed] [Google Scholar]
- 17.Renaudin H, Bebear C. Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum. Eur J Clin Microbiol Infect Dis. 1990;9:838–841. doi: 10.1007/BF01967388. [DOI] [PubMed] [Google Scholar]
- 18.Bowman ED, Dharmalingam A, Fan WQ, Brown F, Garland SM. Impact of erythromycin on respiratory colonization of Ureaplasma urealyticum and the development of chronic lung disease in extremely low birth weight infants. Pediatr Infect Dis J. 1998;17:615–620. doi: 10.1097/00006454-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson B, Rylander M, Faxelius G. Ureaplasma urealyticum, erythromycin and respiratory morbidity in high-risk preterm neonates. Acta Paediatr. 1998;87:1079–1084. doi: 10.1080/080352598750031428. [DOI] [PubMed] [Google Scholar]
- 20.Baier RJ, Loggins J, Kruger TE. Failure of erythromycin to eliminate airway colonization with ureaplasma urealyticum in very low birth weight infants. BMC Pediatr. 2003;3:10. doi: 10.1186/1471-2431-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin BK. Macrolides as biologic response modifiers. J Respir Dis. 2002;23:S31–S38. [Google Scholar]
- 22.Ianaro A, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292:156–163. [PubMed] [Google Scholar]
- 23.Tsai WC, Standiford TJ. Immunomodulatory effects of macrolides in the lung: lessons from in-vitro and in-vivo models. Curr Pharm Des. 2004;10:3081–3093. doi: 10.2174/1381612043383430. [DOI] [PubMed] [Google Scholar]
- 24.Kawashima M, et al. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung. 2002;180:73–89. doi: 10.1007/pl00021246. [DOI] [PubMed] [Google Scholar]
- 25.Beigelman A, et al. Azithromycin attenuates airway inflammation in a noninfectious mouse model of allergic asthma. Chest. 2009;136:498–506. doi: 10.1378/chest.08-3056. [DOI] [PubMed] [Google Scholar]
- 26.Tsai WC, et al. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am J Respir Crit Care Med. 2004;170:1331–1339. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- 27.Tsai WC, Hershenson MB, Zhou Y, Sajjan U. Azithromycin increases survival and reduces lung inflammation in cystic fibrosis mice. Inflamm Res. 2009;58:491–501. doi: 10.1007/s00011-009-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard AE, Cimochowski CR, Faiella JA. Correlation of increased azithromycin concentrations with phagocyte infiltration into sites of localized infection. J Antimicrob Chemother. 1996;37(Suppl C):9–19. doi: 10.1093/jac/37.suppl_c.9. [DOI] [PubMed] [Google Scholar]
- 29.Patel KB, et al. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob Agents Chemother. 1996;40:2375–2379. doi: 10.1128/aac.40.10.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capitano B, et al. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest. 2004;125:965–973. doi: 10.1378/chest.125.3.965. [DOI] [PubMed] [Google Scholar]
- 31.Arnon S, Grigg J, Silverman M. Pulmonary inflammatory cells in ventilated preterm infants: effect of surfactant treatment. Arch Dis Child. 1993;69:44–48. doi: 10.1136/adc.69.1_spec_no.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auten RL. & Ekekezie, II Blocking leukocyte influx and function to prevent chronic lung disease of prematurity. Pediatr Pulmonol. 2003;35:335–341. doi: 10.1002/ppul.10275. [DOI] [PubMed] [Google Scholar]
- 33.Liao L, et al. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res. 2006;60:299–303. doi: 10.1203/01.pdr.0000233058.08200.d6. [DOI] [PubMed] [Google Scholar]
- 34.Duffy LB, Crabb D, Searcey K, Kempf MC. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clindamycin against Mycoplasma spp. J Antimicrob Chemother. 2000;45(Suppl 1):29–33. doi: 10.1093/jac/45.suppl_3.29. [DOI] [PubMed] [Google Scholar]
- 35.Loza E, et al. Comparative in vitro activity of clarithromycin. Spanish Collaborative Group. Eur J Clin Microbiol Infect Dis. 1992;11:856–866. doi: 10.1007/BF01960892. [DOI] [PubMed] [Google Scholar]
- 36.Aghai ZH, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs RF, et al. Pharmacokinetics of intravenously administered azithromycin in pediatric patients. Pediatr Infect Dis J. 2005;24:34–39. doi: 10.1097/01.inf.0000148927.48680.fc. [DOI] [PubMed] [Google Scholar]
- 38.Donovan EF, et al. Inaccuracy of Ballard scores before 28 weeks' gestation. National Institute of Child Health and Human development Neonatal Research Network. J Pediatr. 1999;135:147–152. doi: 10.1016/s0022-3476(99)70015-6. [DOI] [PubMed] [Google Scholar]
- 39.Pellicer A, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: A randomized, blinded, clinical trial. Pediatrics. 2005;115:1501–1512. doi: 10.1542/peds.2004-1396. [DOI] [PubMed] [Google Scholar]
- 40.Walsh MC, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 41.Barrett B, et al. Validated HPLC-MS-MS method for determination of azithromycin in human plasma. Anal Bioanal Chem. 2005;383:210–217. doi: 10.1007/s00216-005-0018-5. [DOI] [PubMed] [Google Scholar]
- 42.Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8:195–222. [PubMed] [Google Scholar]
- 43.Waites KB, Duffy LB, Schwartz S, Talkington DF. Mycoplasma and Ureaplasma. In: Isenberg H, editor. Clinical Microbiology Procedure Handbook. Edn. 2nd. Washington, DC: ASM Press; 2004. [Google Scholar]
- 44.Novy MJ, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 45.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amnioitic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–S153. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 46.Xiao L, et al. Detection and Characterization of Human Ureaplasma Species and Serovars by Real-time PCR. J Clin Microbiol. doi: 10.1128/JCM.01877-09. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobson JR, et al. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 48.Roviezzo F, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 49.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 50.Zeitlinger M, Wagner CC, Heinisch B. Ketolides--the modern relatives of macrolides : the pharmacokinetic perspective. Clin Pharmacokinet. 2009;48:23–38. doi: 10.2165/0003088-200948010-00002. [DOI] [PubMed] [Google Scholar]
- 51.Nahata MC, Koranyi KI, Luke DR, Foulds G. Pharmacokinetics of azithromycin in pediatric patients with acute otitis media. Antimicrob Agents Chemother. 1995;39:1875–1877. doi: 10.1128/aac.39.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garey KW, Amsden GW. Intravenous azithromycin. Ann Pharmacother. 1999;33:218–228. doi: 10.1345/aph.18046. [DOI] [PubMed] [Google Scholar]
- 53.Gordon EM, Blumer JL. Rationale for single and high dose treatment regimens with azithromycin. Pediatr Infect Dis J. 2004;23:S102–107. doi: 10.1097/01.inf.0000112523.95762.f5. [DOI] [PubMed] [Google Scholar]
- 54.Dunne MW, et al. Randomized, double-blind study of the clinical efficacy of 3 days of azithromycin compared with co-amoxiclav for the treatment of acute otitis media. J Antimicrob Chemother. 2003;52:469–472. doi: 10.1093/jac/dkg358. [DOI] [PubMed] [Google Scholar]
- 55.Ruuskanen O. Safety and tolerability of azithromycin in pediatric infectious diseases: 2003 update. Pediatr Infect Dis J. 2004;23:S135–139. doi: 10.1097/01.inf.0000112528.75956.41. [DOI] [PubMed] [Google Scholar]
- 56.Cohen R, et al. Comparison of two dosages of azithromycin for three days versus penicillin V for ten days in acute group A streptococcal tonsillopharyngitis. Pediatr Infect Dis J. 2002;21:297–303. doi: 10.1097/00006454-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Girard D, Finegan SM, Dunne MW, Lame ME. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J Antimicrob Chemother. 2005;56:365–371. doi: 10.1093/jac/dki241. [DOI] [PubMed] [Google Scholar]
- 58.Ballard HO, Anstead MI, Shook LA. Azithromycin in the extremely low birth weight infant for the prevention of bronchopulmonary dysplasia: a pilot study. Respir Res. 2007;8:41. doi: 10.1186/1465-9921-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballard HO, et al. Use of azithromycin for early treatment of Ureaplasma spp. in preterm infants: a randomized, double-blind, placebo-controlled trial. EPAS2009. 2009;4515:4513. [Google Scholar]