Abstract
We have studied the loading of the opioid hydromorphone into liposomes using ammonium sulfate gradients. Unlike other drugs loaded with this technique, hydromorphone is freely soluble as the sulfate salt, and, consequently, does not precipitate in the liposomes after loading. We have derived a mathematical relationship that can predict the extent of loading based on the ammonium ion content of the liposomes and the amount of drug added for loading. We have adapted and used the Berthelot indophenol assay to measure the amount of ammonium ions in the liposomes. Plots of the inverse of the fraction of hydromorphone loaded versus the amount of hydromorphone added are linear, and the slope should be the inverse of the amount of ammonium ions present in the liposomes. The inverse of the slopes obtained closely correspond to the amount of ammonium ions in the liposomes measured with the Berthelot indophenol assay. We also show that loading can be less than optimal under conditions where osmotically driven loss of ammonium ions or leakage of drug after loading may occur.
Keywords: liposomes, microencapsulation, encapsulation, controlled release, biophysical models
INTRODUCTION
The use of ammonium sulfate gradients for the efficient loading of weakly basic drugs into liposomes was first developed for the loading of doxorubicin into liposomes.1 Subsequently, this technique has been used for the loading of other weakly basic drugs into liposomes, including ciprofloxacin and bupivicaine.2–5 Loading is achieved when ammonium sulfate is first captured in the liposomes, and subsequently eliminated from the extraliposomal space by dialysis or desalting. Elimination of ammonium sulfate from the extraliposomal space causes rapid efflux of ammonia from the liposomes, which is produced by the dissociation of ammonium ions to give ammonia and protons, thereby reducing the intraliposomal pH. The pH gradient can be as much as 3–4 pH units between the intraliposomal and extraliposomal compartment. 1 Upon addition of a weakly basic drug to the liposomes, highly efficient loading occurs through the influx of the free base and subsequent accumulation of the protonated form in the acidic intraliposomal compartment.
Opioids are chemical substances with morphine-like action through binding to opioid receptors in the central and peripheral nervous system, and the gastrointestinal tract.6 They have long been used to treat acute and chronic pain. However, the rapid clearance of opioids by first pass metabolism in the liver, especially in species such as dogs, limits both the oral usage and the duration of the effect.7 Although a larger dose will increase the duration of the effect, dose is limited by the side-effects of opioids, especially at peak drug concentration, which include respiratory depression, sedation, coma, and death.6 Therefore, controlled release of opioids is a potential approach to overcoming the limited duration of effect, and we have recently documented the potential utility of liposome-encapsulated opioids for long-term management of pain in animals. The period of analgesia, which is about 2–4 h for an i.v. bolus injection, is increased when drug is administered subcutaneously in liposomes. The duration of serum concentration and analgesic effects can be extended to 24 h through the use of egg phosphatidylcholine/cholesterol liposomes. 8 The duration of serum concentration and analgesic effects is increased to 96 h through the use of dipalmitoylphosphatidylcholine/cholesterol liposomes.9 Initially, we used the dehydration–rehydration method of Kirby and Gregoriadis to prepare liposomes.10 More recently, we have used ammonium sulfate gradients to achieve more efficient loading of DPPC/cholesterol liposomes. Interestingly, the period of analgesia is greatly increased in macaque monkeys by ammonium sulfate loading of oxymorphone to as much as 3 weeks.11
Ammonium sulfate gradient loading efficiency of oxymorphone is typically 30–40%,11 while the efficiency of passive aqueous capture can be as low as 15% for hydromorphone.9 In contrast, doxorubicin loading efficiency can be as high as 99%.1 In order to learn more about the loading of opioids using ammonium sulfate gradients and how efficiency might be increased, we have studied the relationship between loading efficiency and the amount of drug added. We have developed a simple mathematical relationship capable of predicting the fraction of drug that will load into liposomes from the amount of ammonium ions trapped in the liposomes prior to loading, and from the amount of drug added to the liposomes during loading. As required for the relationship to be predictive, the saturating concentration of hydromorphone was found to exceed the concentration present in liposomes after loading.
MATERIALS AND METHODS
Dipalmitoylphosphatidylcholine (DPPC), egg phosphatidylcholine (egg PC), and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL). Hydromorphone hydrochloride was purchased from PCCA (Houston, TX). Sephadex G 50 was purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were reagent grade or better.
Liposome Preparation
A mixture of phospholipid/cholesterol 1.6:1 (mol/mol) dissolved in chloroform was evaporated in a rotary evaporator, and suspended in tert-butanol at 80 μmol/mL phospholipid, and freeze-dried to produce a microporous lipid mixture. Multilamellar vesicles (MLV) were prepared by suspending the lipid mixture at 80 μmol/mL phospholipid in aqueous medium with vigorous shaking for 5 min. The lipid dispersion was allowed to stand at 25°C under argon for 1 h prior to subsequent steps.
Small unilamellar vesicles (SUV) were prepared by ultrasonication of egg PC/cholesterol MLV for 10 min at 25°C using a bath type sonicator (Laboratory Supplies, Inc., Hicksville, NY). The suspension was sedimented at 160,000 × g for 4 h in an ultracentrifuge to eliminate any residual larger liposomes, leaving a highly uniform SUV population in the supernatant.12
Large unilamellar vesicles (LUV) were prepared by extrusion of MLV six times through a 0.2 μm polycarbonate filter, using a stainless steel extrusion cell (Mico Instruments, Middleton, WI) at a pressure of 120 psi at 25°C.
Size Distribution
Liposome size distribution was determined by dynamic light scattering (DLS), using a Nicomp Model 380 (Nicomp, Santa Barbara, CA).
Formation of Ammonium Sulfate Gradient
For MLV, the unencapsulated ammonium sulfate was removed by sedimentation of the liposomes at 300 × g in a benchtop centrifuge, the pellet was washed twice by resuspending it in 0.9% (w/v) NaCl and sedimenting as before. For SUV and LUV, the unencapsulated ammonium sulfate was removed by dialysis two times for 12 h against 1L 0.9% (w/v) NaCl at room temperature.
Ammonia Measurement
Ammonia was measured using an adaptation of the Berthelot indophenol method described by Jaenicke13 for measurement of nitrogenous materials. Liposomes were first extracted using the method of Bligh and Dyer.14 Briefly, 20 μL liposome sample was mixed with 140 μL water, 400 μL MeOH, and 200 μL CHCl3. Another 200 μL CHCl3 was added to give a cloudy mixture. Addition of 200 μL 0.9% NaCl and centrifugation at 300 rpm for 10 min gave two separate clear phases. The lower CHCl3 phase was aspirated out from under the upper phase, and the upper phase, which contained H2O, MeOH, and (NH4)2SO4, was transferred to large test tubes. After 0.4mL 10M sulfuric acid was added to each tube, the tubes were transferred to a 165°C hot block for 30 min to evaporate off the MeOH and water. The sulfuric acid was necessary to retain the ammonia. After cooling, 0.8mL 5M NaOH was added to each tube to neutralize the acid. After further cooling, 1mL 2.125% (w/v) phenol, 0.0125% (w/v) sodium nitroprusside was added to all tubes with agitation. Finally, 0.4mL 0.02M sodium hypochlorite in 2.5M NaOH was added with mixing. The solutions were allowed to stand at room temperature for exactly 20 min, and the absorbance at 578nm was measured using a Hitachi-3000 UV/Vis spectrophotometer (Hitachi Instruments, San Jose CA). In every batch of samples, standards were included that contained 0, 0.1, or 0.2 μmol NH3 (as ammonium sulfate). Standards were also subjected to extraction and evaporation.
Liposome Loading With Hydromorphone HCl
A solution of hydromorphone hydrochloride was added to 0.5mL of the liposome dispersion (20–80mM phospholipids) after the creation of ammonium sulfate gradient. The final volume was 1.0 mL, and the hydromorphone concentration was 0.25–2 mg/mL. The loading was performed by incubation at 25°C in a water bath for approximately 2 h. The loading was terminated by removal of unloaded free hydromorphone. All loading studies were performed in triplicate, and data were plotted as the mean ± the standard deviation.
Removal of Unloaded Hydromorphone HCl
For MLV, unloaded free hydromorphone was removed by sedimentation of the liposomes at 300 × g in a benchtop centrifuge, and the pellet was washed twice by resuspending it in 0.9% (w/v) NaCl and sedimenting as before. For LUV and SUV, unloaded free hydromorphone was removed by dialysis as described above or gel chromatography using a 1 × 5 cm2 Sephadex G 50 column eluted with 1M phosphate buffer, pH 7.4. One milliliter of liposomes was applied to the column. Fractions, 1 mL, were collected and analyzed for their hydromorphone content to ensure good separation of liposomes and free drug.
Hydromorphone Measurement
The hydromorphone concentration was determined using an Agilent 1100 HPLC system equipped with a reverse phase C18 column (Waters, Milford, MA). Prior to injection onto the column, 0.5mL of liposome dispersion was added into HPLC vials with 1.5mL MeOH and 0.5mL CHCl3 to make a clear homogeneous solution. The eluant was 75% MeOH/15% water/15% CHCl3 with 1% acetic acid, 1 mL/min flow rate. For a better separation, 0.5% SDS was used in some cases. The separation of hydromorphone was monitored by UV absorbance at 230 and 280nm and the separation of phospholipids and cholesterol was monitored with an ELS detector.
Lipid Concentration
Phospholipid concentrations were measured by phosphorus analysis following oxidative degradation using a modification of the method of Bartlett.15
Analysis of Data
In order to determine whether ammonium sulfate gradient loading of hydromorphone was optimal, we developed the following simple mathematical relationship for loading:
where and closely approximate the amount of drug added for loading, and the amount of ammonium ions encapsulated in the liposomes prior to loading, respectively, and F is the fraction of drug loaded. In order to apply this relationship to specific liposome preparations, liposome samples were loaded using different amounts of drug. Plots of 1/F versus the amount of drug added were prepared, and the slope and intercept values calculated by linear regression. Predicted plots were calculated using the amount of ammonium ions encapsulated in the liposomes prior to loading, measured with the Berthelot indophenol reaction. Derivation of this equation is shown in the appendix.
RESULTS
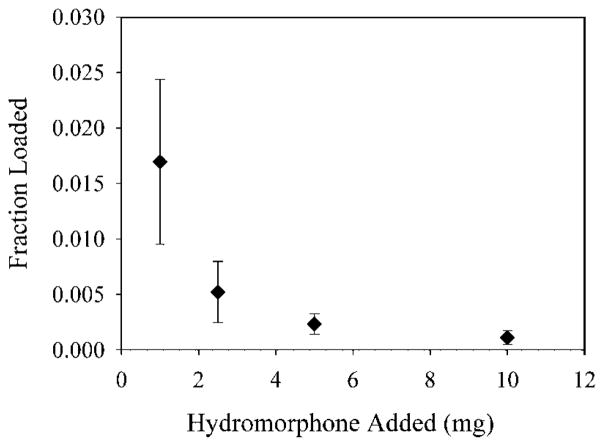
Hydromorphone (structure shown in Fig. 1) is a weak base, and, therefore, a potential candidate for active loading using ammonium sulfate. Ammonium sulfate gradient loading is shown schematically in Figure 2, and the drug to be loaded must be retained by liposomes if gradient loading is to be successful. We have previously shown that hydromorphone may readily be captured in liposomes by passive aqueous capture and that it is released slowly both in vitro and in vivo.8,9 In order to confirm the need for a gradient for loading of hydromorphone into liposomes, we determined whether hydromorphone would load passively without any gradient. Hydromorphone solution was incubated with DPPC/cholesterol MLV for 1 h above the Tm (55°C). The loading efficiency (Fig. 3) of hydromorphone into DPPC/cholesterol MLV with no gradient was extremely low at all hydromorphone concentrations studied. Only 0.02% of the drug loaded into the MLV when 1mg of drug was added to them. This observation demonstrated a minimal tendency to load, and confirms that the hydromorphone does not partition appreciably into the lipid bilayer despite the hydrophobicity of the neutral form.
Figure 1.
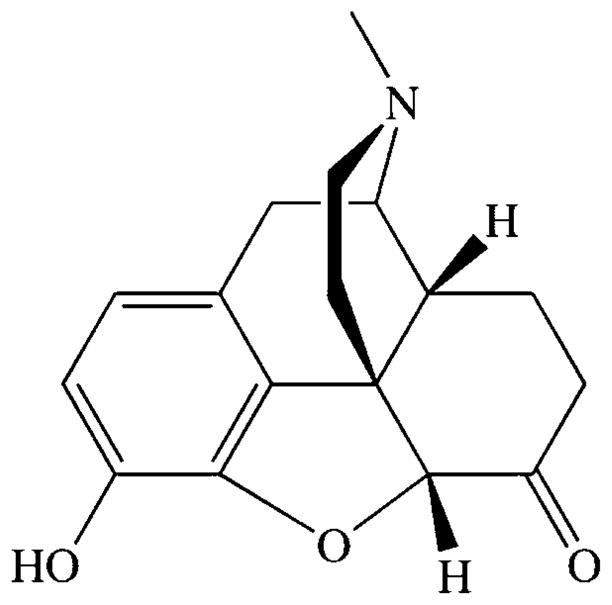
The structure of hydromorphone.
Figure 2.
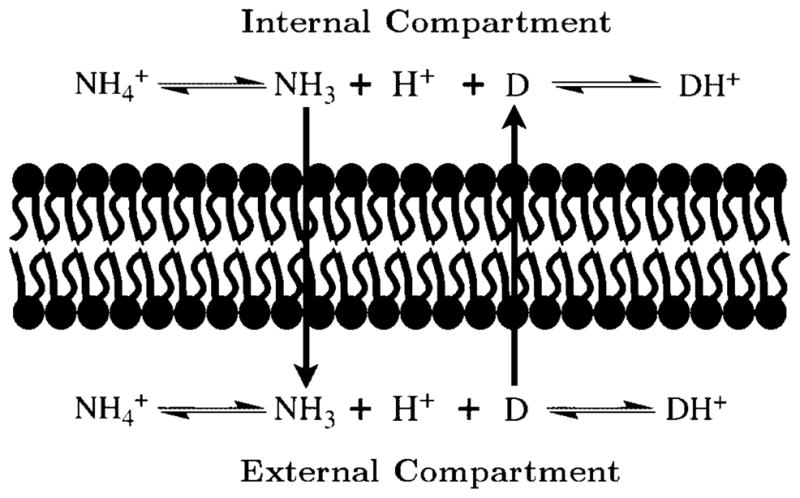
A schematic representation of ammonium sulfate gradient loading.
Figure 3.
The loading of hydromorphone into liposomes without a gradient. Each point is the mean of three values ± the standard deviation.
Hydromorphone Loading With Ammonium Sulfate Gradient in Liposomes
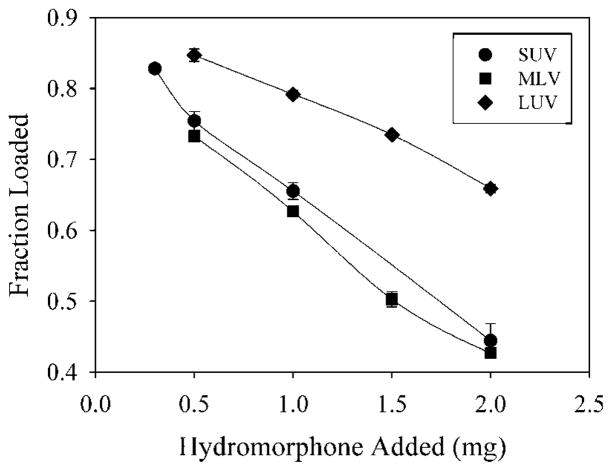
The loading of egg PC/cholesterol liposomes prepared in a 120mM ammonium sulfate solution is shown in Figure 4 as a simple plot of the fraction loaded versus the amount of hydromorphone added. As expected, the fraction of drug loaded decreases as the amount of hydromorphone added is increased. Loading of hydromorphone into LUV is an efficient process, giving 86–70% loading when between 0.5 and 2mg hydromorphone was added. Loading of SUV and MLV is less efficient, giving 75–50% loading when between 0.5 and 2mg hydromorphone was added. The difference is principally related to the amount of ammonium ions present in the liposomes, the amount being 14.08 μmol for LUV, 4.9 μmol for SUV, and 4.86 μmol for MLV.
Figure 4.
The fraction of drug loaded versus the amount of hydromorphone added for liposomes loaded with (NH4)2SO4 at 120mM. The liposomes were either LUV (diamonds), SUV (circles), or MLV (squares), and were prepared as described under the Materials and Methods Section. Each point is the mean of three values ± the standard deviation.
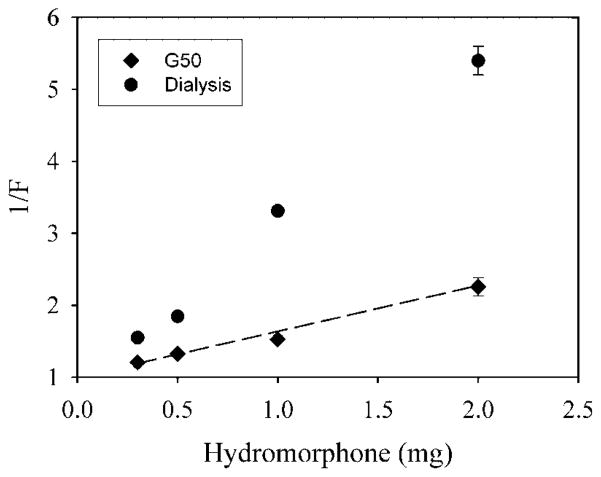
Figure 5 shows a plot of 1/F versus Odrug H+0 for the loading of egg PC/cholesterol SUV with hydromorphone. When excess hydromorphone is removed using Sephadex G50, the values are close to the curve predicted from . However, when excess hydromorphone is removed by dialysis at room temperature for 24 h, all data points are above the predicted curve, showing that the loading is much less than predicted. This suggests that prolonged dialysis may remove some of the drug that has been loaded into SUV.
Figure 5.
The inverse of fraction loaded (1/F) versus the amount of hydromorphone added for SUV. Excess hydromorphone was removed either using dialysis (circles), or Sephadex chromatography (diamonds). Each point is the mean of three values ± the standard deviation.
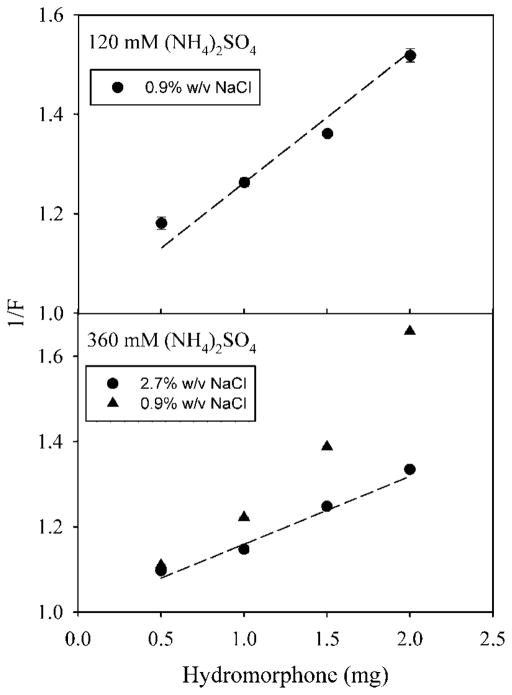
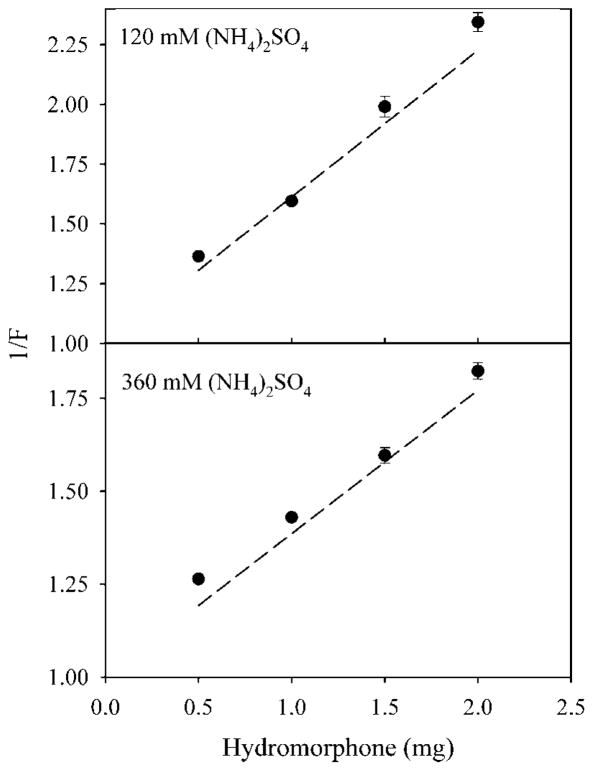
Figure 6 shows a plot of 1/F versus Odrug H+0 for the loading of hydromorphone into egg PC/cholesterol LUV. When LUV are made in 120mM ammonium sulfate, the data are close to the curve predicted from . When LUV are made in 360mM ammonium sulfate, loading in 0.9% (w/v) NaCl gave values that were above the predicted curve, and, therefore, loading was lower than predicted. In contrast, loading in 2.7% (w/v) NaCl gave values that were close to the predicted curve. Ammonium sulfate (360mM) is hypertonic to 0.9% NaCl, but isotonic to 2.7% NaCl. This suggests that a failure to match the tonicity for LUV may reduce loading efficiency through osmotic swelling and a resultant leakage of ammonium ions from the liposomes.
Figure 6.
The inverse of fraction loaded (1/F) versus the amount of hydromorphone added for LUV. LUV were loaded with (NH4)2SO4 at either 120mM (upper panel) or 360mM (lower panel). Liposomes were suspended in either 0.9% (w/v) NaCl (upper panel, circles; lower panel, triangles), or 2.7% (w/v) NaCl (lower panel, circles). Each point is the mean of three values ± the standard deviation.
Figure 7 shows a plot of 1/F versus for the loading of hydromorphone into egg PC/cholesterol MLV. The data are close to the curve predicted from for liposomes prepared in both 120 and 360mM ammonium sulfate. Both liposome preparations were loaded in 0.9% NaCl. Therefore, MLV must be less sensitive to hypotonic conditions than LUV.
Figure 7.
The inverse of fraction loaded (1/F) versus the amount of hydromorphone added for MLV. MLV were loaded with (NH4)2SO4 at either 120mM (upper panel) or 360mM (lower panel), and were suspended in 0.9% (w/v) NaCl. Each point is the mean of three values ± the standard deviation.
Table 1 summarizes the results of Figures 5–7 by showing the inverse of the slope, the intercept, and r2 for the regression curves for each set of data. The amount of ammonium ions in the liposomes prior to loading ( ), measured using the Berthelot indophenol reaction, is also shown. Based on the relationship plotted, the inverse of the slope should correspond to the value . For SUV freed from excess hydromorphone by dialysis, the inverse of the slope is only 27.6% of . Although r2 is 0.99, the intercept is only 0.87, which is appreciably less than 1, the expected value. For LUV prepared in 360mM ammonium sulfate, and loaded in 0.9% NaCl, the inverse of the slope is only 43% of . Although r2 is 0.98, the intercept is only 0.89, which is appreciably less than 1, the expected value. For all other cases, the inverse of the slopes are all within 5% of , suggesting a close agreement between the actual and theoretical value of . Furthermore, the value of r2 is greater than 0.98 in all cases, and the intercepts are all within 7% of unity. This suggests that loading closely adheres to the proposed mathematical relationship, provided loading conditions do not cause leakage of ammonium ions or loss of drug after loading.
Table 2.
The Concentration of Hydromorphone Inside Liposomes after Loading
| Liposomes | Loading Concentration (μmol/mL) | Phospholipid (μmol) | Captured (μmol) | Internal Volume (mL) | Hydromorphone Loaded (mg) | Internal Hydromorphone Concentration (mg/mL) |
|---|---|---|---|---|---|---|
| SUV | 240 | 20 | 9.8 | 0.041 | 0.92 | 22.39 |
| LUV | 240 | 66 | 28.16 | 0.117 | 1.33 | 11.38 |
| 720 | 68 | 39.2 | 0.054 | 1.5 | 27.76 | |
| MLV | 240 | 72 | 11.8 | 0.049 | 0.86 | 17.62 |
| 720 | 76 | 16.2 | 0.023 | 1.11 | 48.04 |
The internal volume of the liposomes was calculated from the amount of ammonium captured and the original ammonium concentration (NH4SO4 concentration times two), assuming passive aqueous capture. The amount of hydromorphone loaded is the highest observed fraction loaded for the greatest amount added (2 mg) times the amount added.
Figures 5–7 show plots of 1/F versus , which gives lines with a slope of , and intercepts equal to one. Alternatively, a plot of 1/F versus should give, for all cases where loading adheres to the relationship, a line with both slope and intercept equal to one. In Figure 8, 1/F was plotted against for every experiment shown above that closely adheres to the relationship. The regression line for all the data has a slope of 0.9788 and an intercept of 1.03, while r2 is 0.979. This shows once more that the results of all of these studies closely adhere to the relationship used.
Figure 8.
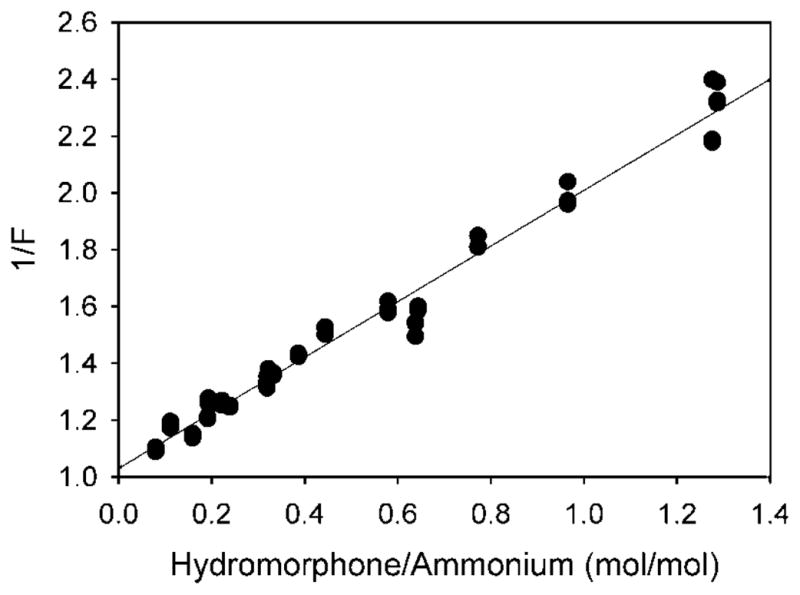
The inverse of fraction loaded (1/F) versus the (drug added)/(loaded ammonium ions) ratio. All experiments from Figures 3–5 are plotted, except for SUV separated by dialysis, and LUV loaded with 360mM (NH4)2SO4 and not tonicity matched during drug loading. For this plot the data points are individual values.
Hydromorphone is known to be highly soluble as the sulfate salt, and the relationship shown to closely predict hydromorphone loading assumes that precipitation did not occur. However, in order to establish that hydromorphone was in solution in the liposomes, we estimated its concentration in liposomes and have established a lower limit for its saturating concentration in the presence of ammonium sulfate at pH 5.5. Table 2 shows the concentration of hydromorphone in the liposomes after loading, calculated in the following way. For all liposome preparations, the internal volume of the liposomes was calculated from the amount of ammonium ions captured and the initial concentration of ammonium sulfate captured, assuming passive aqueous capture of ammonium sulfate. Based on the captured volume, we have further calculated the concentration of hydromorphone inside the liposomes from the highest fraction loaded after loading with 2mg hydromorphone. These calculations show that no concentration in the liposomes exceeded 48 mg/mL (Tab. 2). In solubility studies, it was established that the solubility of hydromorphone in the presence of ammonium sulfate at pH 5.5 was at least 60 mg/mL, and likely exceeded 200 mg/mL. In one experiment (Tab. 3), 30mg of hydromorphone hydrochloride was dissolved in 0.5mL of 120mM ammonium sulfate, pH 5.5, and sedimented at 300g for 10 min to remove any residual solid (none was visible). The solution was diluted 1000 times, and analyzed by HPLC. The concentration was 60.43mg/mL. In an additional study, where hydromorphone hydrochloride dissolution in 120mM ammonium sulfate was simply observed, 100mg appeared completely soluble in 0.5mL ammonium sulfate. Therefore, we may conclude that hydromorphone was in solution in liposomes after loading, and not precipitated, as is the case for drugs like doxorubicin.
Table 3.
Determination of the Concentration of Hydromorphone in Ammonium Sulfate at pH 5.5
| Sample | Peak Area
|
|||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Average | |
| 0.05 mg/mL | 6.37 | 6.3534 | ND | 6.3617 |
| 0.1 mg/mL | 12.43 | 11.91 | ND | 12.17 |
| 0.5 mg/mL | 57.6 | 57.9 | ND | 57.75 |
| Unknown | 7.733 | 7.734 | 7.334 | 7.600 |
Thirty milligrams of hydromorphone hydrochloride was suspended in 0.5mL 120mM ammonium sulfate, pH 5.5, sedimented, diluted 1000-fold, and measured using HPLC.
The concentration was found to be 60.43 mg/mL.
DISCUSSION
The experiments described above show that the loading of hydromorphone into liposomes using an ammonium sulfate gradient can be readily predicted using a simple mathematical relationship, which requires only that the amount of ammonium ions in the liposomes and the amount of drug added be known. The relationship described and validated here is potentially useful for establishing optimal loading conditions, and for demonstrating under what conditions loading is less than optimal. For example, less than predicted loading was observed above for LUV that were loaded in a solution hypotonic to the liposome contents. Such conditions probably led to the loss of ammonium sulfate from the liposomes during loading through osmotic rupture of the liposome membranes. The relationship can also be used to design conditions under which loading may be maximized. Principally, the fraction of drug loaded is determined by the ratio of encapsulated ammonium ions to drug added to the system for loading. Making this ratio as large as is feasible will maximize the amount of drug loaded. In previous studies, we have loaded 40mg oxymorphone and achieved a loading efficiency of 35%.11 In the present study loading efficiency for hydromorphone was as high as 90% when smaller amounts of drug were used.
The relationship we have developed is applicable only in those cases where the drug is completely in solution in the liposomes. We have established that hydromorphone solubility is at least 200 mg/mL at pH 4.5. Under the conditions used for loading, the internal hydromorphone concentration will not exceed 60 mg/mL, confirming that it will be completely in solution. Therefore, hydromorphone loading should be predicted by this relationship.
As shown theoretically by Ceh and Lasic,16 precipitation of the drug as a salt should, under many conditions, lead to loading that is higher than occurs if drug does not precipitate. Although this means that near-quantitative loading of hydromorphone cannot be achieved, as occurs with drugs that precipitate as sulfate salts, active loading of hydromorphone under optimal conditions is still more efficient than passive capture. In the experiments described in this article, the extraliposomal concentration was between 0.5 and 2 mg/mL. The concentration after loading in the liposomes was at least 20 times the concentration added to the extraliposomal medium. Therefore, even without precipitation, active loading of hydromorphone is still a concentrative process with all the advantages that this brings.
Despite the fact that our equation does not apply to doxorubicin and other precipitating compounds, it is a potentially useful way to demonstrate whether precipitation is occurring. For example, in Haran et al.,1 320 mmol/mol doxorubicin was loaded into a preparation of DPPC/cholesterol liposomes with close to 100% efficiency. The capture volume of these liposomes was 2.7 L/mol, and ammonium sulfate was captured in these liposomes at 110mM. Assuming that ammonium sulfate is captured by passive aqueous capture, the amount of ammonium ions captured prior to drug loading should have been 594 mmol/mol lipid. Using these values, the relationship described here predicts that loading would have been only 64% if doxorubicin did not precipitate. However, loading was close to quantitative, showing the effect of precipitation in increasing the loading.
The relationship we have developed is based upon the assumption that there will be no movement of cations across the liposome bilayer. This assumption appears justified, and is generally accepted for ammonium sulfate loading. There is a large difference in the permeability of ammonia (4.8 × 10−2 cm/s),17 and protons (1 × 10−12 cm/s).18 It is also well known that capture of ammonium sulfate with subsequent removal of ammonium sulfate from the extraliposomal compartment generates large pH gradients across the liposome membrane.1
In most studies of ammonium sulfate gradient loading of liposomes, the amount of ammonium ions captured in the liposomes has not been measured. In the present study, measurement of captured ammonium ions was critical for the validation of the relationship used. The method we have used here is a simple colorimetric assay, based on a method for measuring organic nitrogenous materials.13 The amount of ammonium ions captured is often not critical to efficient loading, particularly if the drug precipitates as the sulfate salt. However, we have shown in the present study that the amount of ammonium ions incorporated into liposomes is crucial to loading efficiency if the drug does not precipitate. Perhaps this method will find utility in other work also.
We have here demonstrated that this relationship is predictive for hydromorphone loading. However, it should be predictive for the loading of any weak base that does not precipitate as the sulfate salt. We hope to demonstrate its application to other opioids and to other weakly basic drugs.
Table 1.
Regression Analysis of Loading Studies
| Liposomes | (NH4)2SO4 (mM) | (μmol) | 1/Slope (μmol) | Intercept | r2 |
|
|
|---|---|---|---|---|---|---|---|
| SUV | 120a | 4.90 | 5.09 | 1.00 | 0.9847 | 104 | |
| 120b | 4.90 | 1.35 | 0.83 | 0.9957 | 28 | ||
| LUV | 120 | 14.08 | 14.09 | 1.05 | 0.9858 | 100 | |
| 360c | 19.65 | 19.23 | 1.00 | 0.9905 | 98 | ||
| 360 | 19.65 | 8.64 | 0.89 | 0.9810 | 44 | ||
| MLV | 120 | 4.86 | 4.69 | 0.99 | 0.9926 | 97 | |
| 360 | 8.10 | 8.47 | 1.07 | 0.9946 | 105 |
Excess drug was removed with Sephadex.
Excess drug was removed by dialysis.
Tonicity was matched with 2.7% (w/v) NaCl.
Acknowledgments
This publication was made possible by grant number RR 018802 from the National Institutes of Health, National Center for Research Resources.
APPENDIX: DERIVATION OF THE LOADING RELATIONSHIP
The following Eq. (1), predicts the coupled distribution of protons and weak bases in the protonated form between compartments separated by a membrane permeant to the free base, and impermeant to cations. The derivation of Eq (1) has been previously shown by other investigators.19,20
| (1) |
where I[H+], I[base H+] are the proton and protonated base concentrations in the liposome compartment, and O[H+], O[base H+] are the proton and protonated base concentrations in the external compartment.
This relationship is applicable both to ammonium ions and to the protonated weakly basic drug to be loaded. We can convert Eq (1) to total molar amounts, and for ammonium ions and the protonated weakly basic drug we may write:
| (2) |
| (3) |
where IH+, , Idrug H+ are the mol protons, ammonium ions, and protonated drug in the liposome compartment after loading, and OH+, , Odrug H+ are the mol protons, ammonium ions, and protonated drug in the external compartment after loading.
We can combine Eq. (2) and Eq. (3), because the first term in each is OH+/IH+, to give:
| (4) |
Hence, the coupled relationship between the loading of drug and the loss of ammonium ions from the liposomes is established. Because the membrane is permeant to ammonia and the free base of the drug and impermeant to all other species, there will be conservation of the amount of cationic species within each compartment during the loading process, which may be expressed as follows:
| (5) |
| (6) |
where are the mol protons and ammonium ions in the liposome compartment prior to loading, and are the mol protons and protonated drug in the external compartment prior to loading.
Assuming that the mol of protons in both compartments is negligible compared to the mol of ammonium ions or protonated drug, Eq. (5) and Eq. (6) may be simplified and rearranged to give:
| (7) |
| (8) |
It necessarily follows from the conservation of the amount of cationic species within each compartment that exchange of ammonium ions for protonated drug will be tightly coupled, and therefore:
| (9) |
Eq. (9) may be substituted into Eq. (8) to give:
| (10) |
Eq. (7), Eq. (9), and Eq. (10) may be substituted into the second and third terms of Eq. (4) to give:
| (11) |
Eq. (11) may be cross multiplied, simplified, and rearranged to give:
| (12) |
It may be recognized that:
| (13) |
where F is the fraction of protonated drug incorporated into liposomes by loading. Therefore, Eq. (12) may be restated as:
| (14) |
In all practical cases, and and, therefore, and closely approximate the amount of drug added for loading, and two times the amount of ammonium sulfate encapsulated in the liposomes prior to loading, respectively. Similarly, F closely approximates the fraction of drug incorporated into the liposomes by loading. A plot of 1/F versus the amount of drug added for loading should produce a straight line, whose slope is the inverse of the amount of ammonium ions encapsulated in the liposomes prior to loading and whose intercept is 1. Eq. (14) can also be rearranged to give a relationship that can be used for calculating F:
| (15) |
Strictly, this derivation should be expressed in terms of activities, which, for ionic species, deviate significantly from ideal. However, the activity coefficients of all ionic species may be taken as identical within each compartment. Inspection of Eq. (4), for example, will show that all activity coefficients may be dropped, since each term is a ratio of intraliposomal and extraliposomal species. Use of concentration, rather than activity, is, therefore, justified.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of ampbipathic weak bases. Biochim Biophys Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 2.Maurer N, Wong KF, Hope MJ, Cullis PR. Anomalous solubility behavior of the antibiotic ciprofloxacin encapsulated in liposomes: a 1H-NMR study. Biochim Biophys Acta. 1998;1374:9–20. doi: 10.1016/s0005-2736(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 3.Wong JP, Yang H, Blasetti KL, Schnell G, Conley J, Schofield LN. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J Control Release. 2003;92:265–273. doi: 10.1016/s0168-3659(03)00358-4. [DOI] [PubMed] [Google Scholar]
- 4.Oh YK, Nix DE, Straubinger RM. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection. Antimicrob Agents Chemother. 1995;39:2104–2111. doi: 10.1128/aac.39.9.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant GJ, Barenholz Y, Bolotin EM, Bansinath M, Turndorf H, Piskoun B, Davidson EM. A novel liposomal bupivacaine formulation to produce ultralong-acting analgesia. Anesthesiology. 2004;101:133–137. doi: 10.1097/00000542-200407000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe JH, Matin WR, editors. Opioid analgesics and antagonists, in Goodman and Gilman’s the pharmacological basis of therapeutics. New York: Macmillan Publishing Company; 1985. [Google Scholar]
- 7.Dohoo SE, Tasker RAR. Pharmacokinetics of oral morphine sulfate in dogs: a comparison of sustained release and conventional formulations. Can J Vet Res. 1997;61:251–255. [PMC free article] [PubMed] [Google Scholar]
- 8.Smith LJ, Krugner-Higby L, Clark M, Wendland A, Heath TD. A single dose of liposome-encapsulated oxymorphone or morphine provides long-term analgesia in an animal model of neuropathic pain. Comp Med. 2003;53:280–287. [PubMed] [Google Scholar]
- 9.Smith LJ, KuKanich B, Hogan BK, Brown C, Heath TD, Krugner-Higby LA. Pharmacokinetics of a controlled-release liposome-encapsulated hydromorphone administered to healthy dogs. J Vet Pharmacol Ther. 2008;31:415–422. doi: 10.1111/j.1365-2885.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby C, Gregoriadis G. Dehydration-Rehydration Vesicles: A Simple Method of High Yield Drug Entrapment in Liposomes. Nature Biotechnology. 1984;2:979–984. [Google Scholar]
- 11.Krugner-Higby L, KuKanich B, Schmidt B, Heath TD, Brown C, Smith LJ. Pharmacokinetics and behavioral effects of an extended-release, liposome-encapsulated preparation of oxymorphone in rhesus macaques. J Pharmacol Exp Ther. 2009;330:135–141. doi: 10.1124/jpet.108.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roseman M, Litman BJ, Thompson TE. Transbilayer exchange of phosphatidylethanolamine for phosphatidylcholine and N-acetimidoylphosphatidylethanolamine in single-walled bilayer vesicles. Biochemistry. 1975;14:4826–4830. doi: 10.1021/bi00693a008. [DOI] [PubMed] [Google Scholar]
- 13.Jaenicke L. A rapid micromethod for the determination of nitrogen and phosphate in biological material. Anal Biochem. 1974;61:623–627. doi: 10.1016/0003-2697(74)90429-1. [DOI] [PubMed] [Google Scholar]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 16.Ceh B, Lasic DD. A rigorous theory of remote loading of drugs into liposomes. Langmuir. 1995;11:3356–3368. doi: 10.1006/jcis.1996.4555. [DOI] [PubMed] [Google Scholar]
- 17.Antonenko YN, Pohl P, Denisov GA. Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys J. 1997;72:2187–2195. doi: 10.1016/S0006-3495(97)78862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutknecht J. Proton/hydroxide conductance through lipid bilayer membranes. J Membr Biol. 1984;82:105–112. doi: 10.1007/BF01870737. [DOI] [PubMed] [Google Scholar]
- 19.Nichols JW, Deamer DW. Catecholamine uptake and concentration by liposomes maintaining pH gradients. Biochim Biophys Acta. 1976;455:269–271. doi: 10.1016/0005-2736(76)90169-3. [DOI] [PubMed] [Google Scholar]
- 20.Bally MB, Mayer LD, Loughrey H, Redelmeier T, Madden TD, Wong K, Harrigan PR, Hope MJ, Cullis PR. Dopamine accumulation in large unilamellar vesicle systems induced by transmembrane ion gradients. Chem Phys Lipids. 1988;47:97–107. doi: 10.1016/0009-3084(88)90078-3. [DOI] [PubMed] [Google Scholar]