Abstract
Lyophilized formulations of keratinocyte growth factor-2 (KGF-2) were prepared with a range of disaccharide (sucrose or trehalose) and hydroxyethyl starch (HES) mass ratios. Protein degradation was assessed as a function of time of storage of the dried formulations at 40, 50 and 60 °C. Lyophilized and stored samples were rehydrated, and protein degradation was quantified by measuring loss of monomeric protein with size exclusion chromatography and by determining chemical degradation in the soluble fraction with reverse-phase chromatography. The secondary structure of the protein in the lyophilized formulations was studied with infrared spectroscopy. The magnitudes of degradation were compared the key physical properties of the formulations including retention of protein native secondary structure, glass transition temperature (Tg), inverse mean square displacements <u2>−1 for hydrogen atoms (fast β relaxation), and the relaxation time τβ, which correlates with relaxation due to fast Johari-Goldstein motions in the glass[1]. In addition, specific surface areas of the lyophilized formulations were determined by Brunauer-Emmet-Teller analysis of krypton adsorption isotherms and used to estimate the fraction of the KGF-2 molecules residing at the solid-air interface. KGF-2 degradation rates were highest in formulations wherein the protein’s structure was most perturbed, and wherein β relaxations were fastest, but the dominant factor governing KGF-2 degradation in freeze-dried formulations was the fraction of the protein found at the glass solid-air interface.
Keywords: KGF-2, protein stability, lyophilization, glass transition temperature, glass dynamics, global mobility, structural relaxation time, local mobility, protein interfacial adsorption, protein structure
Introduction
A lyophilized formulation is often the choice of dosage form when an aqueous solution formulation of a therapeutic biologic molecule is not sufficiently stable to achieve its desired shelf life. However, even in lyophilized formulations, proteins are prone to instabilities such as aggregation or chemical degradation. To minimize protein degradation, various excipients are added to the lyophilized formulation[2]. Key to the development of robust formulations that offer proteins protection against degradation is the formation of a glassy matrix by the added excipients. Commonly used glass-forming excipients include the disaccharides trehalose and sucrose, as well as polymers such as hydroxyethyl starch (HES) [2].
During storage proteins in lyophilized formulations that contain stabilizing excipients can still degrade, albeit slowly. The factors that control the rates of this degradation are not well understood, and there is still substantial debate about the physical properties of lyophilized formulations that govern rates of degradation. The extent of native structural retention during freeze-drying[3–5] and the dynamic properties of the glassy matrix[6, 7] are most often considered critical to the long-term storage stability of proteins in lyophilized formulations. Other phenomena, such as phase separation[8–12] and interfacial adsorption[13, 14] of protein molecules have been shown to affect protein stability during lyophilization, but their effects on stability during storage have not been investigated. In the current study, we examine the possibility that in addition to the degree of native protein structural retention and glass dynamics properties, the accumulation of proteins on the surface of lyophilized solids may be of importance in determining degradation rates.
In the current study as a model protein, we used keratinocyte growth factor-2 (KGF-2), a protein with a beta trefoil structure (with 12 beta strands folded into three units with each unit consisting of beta-beta-beta-loop-beta) that is found in numerous growth factors and some human interleukins[15]. The storage stability of KGF-2 was examined in various glassy, lyophilized formulations that contained mixtures of HES and the disaccharides sucrose or trehalose. The rates of aggregation and chemical degradation of KGF-2 were measured, and correlated with measures of native secondary structure retention and glassy matrix dynamics. In addition, we measured the specific surface areas of various lyophilized disaccharide-HES formulations, and used these data to estimate the amount of KGF-2 present at the glass-air interface. We propose a simple model that accounts for the possibility that the protein molecules adsorbed at interfaces may degrade more rapidly than protein molecules found in the bulk glass.
Materials and Methods
Materials
KGF-2 was a generous donation from Human Genome Sciences Inc, Rockville, Maryland. Hydroxyethyl starch / viastarch (HES) was obtained from Fresenius Kabi, Austria, GmbH. Sucrose was purchased from Pfanstiehl laboratories, Waukegan, IL, and trehalose and sodium phosphate was obtained from J.T Baker, PA.
Preparation of KGF-2 freeze-dried formulations
KGF-2 was simultaneously dialyzed and concentrated into 2mM sodium phosphate buffer, pH 6.2 to 10 mg/mL in centrifugal concentrators (Vivaspin®, Millipore) at 10°C. Formulation excipients and their percent weight masses used for preparing formulations of KGF-2 are listed in Table. 1. Each of the initial aqueous solution formulations of KGF-2 was prepared by weighing excipients into a 50 ml container followed by addition of 5 ml of the dialyzed and concentrated 10mg/mL concentrated KGF-2 solution. 2 mM sodium phosphate buffer was added to obtain a final volume of 50 ml and a final protein concentration of 1mg/mL. Aliquots of 1ml solution were pipetted into 5 cc glass vials (13 mm FNT BB LYO). Vial filling took place in a cold room at approximately 2–8°C. The vials were then partially stoppered using double vent Fluorotec rubber stoppers (Daikyo Fluorotec stoppers, West Pharmaceutical, Lititz, PA).
Table 1.
Formulation excipients and their percent weight masses used for preparing formulations of KGF-2
| Formulation# | Formulation additives, % by weight |
Buffer used |
|---|---|---|
| 1 | Trehalose 5% | 2mM sodium phosphate, pH 6.2 |
| 2 | Trehalose 4%, HES1% | |
| 3 | Trehalose 2.5%, HES2.5% | |
| 4 | Trehalose 1%, HES 4% | |
| 5 | Sucrose 5% | |
| 6 | Sucrose 4%, HES 1% | |
| 7 | Sucrose 2.5%, HES 2.5% | |
| 8 | Sucrose 1%, HES 4% | |
| 9 | HES 5% |
Freeze drying conditions for KGF-2 formulations
The glass vials containing the liquid KGF-2 formulations were loaded onto the shelves of a FTS Durastop® microprocessor-controlled freeze-dryer (SP Industries, Warminster PA) equipped with a Dura-dry MP® condenser unit. The initial shelf temperature of the freeze dryer was 10°C. Vials were allowed to equilibrate at this temperature for 60 min. The shelf temperature then was reduce to −5°C at 1°C/min and held for 20 minutes, followed by a second ramp to −45°C at 1.3°C / min. After 30 minutes at a shelf temperature of −45°C the chamber was evacuated to a pressure of 70 mTorr, and shelf temperature was increased from −45°C to −20°C over • 10 minutes (2.5°C / min) and held at this temperature for the duration of primary drying (1400min). At the end of primary drying, the shelf temperature was increased from −20°C to +33°C over 3 hours (a ramp rate of • 0.3 °C/min) and held at this temperature for 2 hours for secondary drying. After secondary drying was completed, the chamber was vented with dry nitrogen and the vials were stoppered within the freeze-drying chamber. The vial stoppers were later crimped onto the vials with aluminum seals.
Water content determination in KGF-2 lyophilize formulations
The residual water content in the freeze-dried formulations was determined using a Mettler DL37 coulometric moisture analyzer (Hightstown, NJ) with pyridine free vessel solutions (Photovolt Instruments, Inc., St. Louis Park, MN). Water standards were used to verify the accuracy of the instrument. The vials with freeze-dried samples were placed in a glove box (purged with dry nitrogen gas), and the dried formulations were dissolved in anhydrous dimethylformamide (DMF) (<50 ppm water). Water content in the samples was determined after subtraction of water level in DMF.
Surface Area Measurement
Surface areas of lyophilized formulations were calculated from krypton adsorption isotherms measured using a Quantachrome Autosorb-1 (Boynton Beach, FL). For each formulation, the contents of five vials of lyophilized placebo formulation without protein were inserted into the sample cell, and then the cell with samples was placed under vacuum to remove the moisture.
Krypton adsorption at liquid nitrogen temperature was measured at five points, with helium serving as an inert carrier gas. Brunauer–Emmett–Teller (BET) adsorption theory[16] was used -- over a pressure range of 0.10–0.30 of the saturation pressure of krypton -- to calculate the specific surface areas.
Storage Stability of KGF-2
The lyophilized KGF-2 formulations were incubated in temperature-controlled incubators at 40, 50 or 60°C. At various time points (0, 1, 4 9 and 16 weeks), samples were removed for the analyses described below.
Infrared Spectroscopic (IR) Analysis of KGF-2 Secondary Structure
IR spectra of KGF-2 in aqueous solution and in freeze-dried formulations were acquired using a BOMEM instrument (ABB Bomem Inc., Quebec, Canada) equipped with GRAMS® software (Galactic Industries Corp., Salem, NH). The IR spectrum of native KGF-2 in aqueous solution was obtained first. An aliquot of 10 mg/mL KGF-2 in 10 mM sodium citrate buffer, pH 6.2, was placed in a variable path length cell with CaF2 windows (Biotools Inc., Jupiter, FL). Using a path length of 6 µm, a total of 128 interferograms in single-beam transmission mode (resolution of 4 cm−1) were collected from 4000 to 1000 cm−1 and averaged. After subtraction of water vapor spectra as described by Dong et al,[17] absorbance data in the amide I region of 1720-1580 were converted to second derivative using a seven point Savitzy-Golay smoothing function. The second derivative spectra were baseline-corrected and their areas normalized to unity. This second derivative spectra was used as an ‘aqueous solution control’ for evaluation of the lyophilization-induced secondary structure perturbations of KGF-2 in the freeze-dried formulations.
To measure IR spectra for KGF-2 in the lyophilized formulations, the freeze dried KGF-2 sample (approx 200 µg protein) was gently ground with 500 mg of KBr (Thermo scientific, USA) using a mortar and pestle. This mixture was transferred into a stainless steel die (13mm internal diameter) and pressed with a hydraulic press (Carver Model ‘‘C’’, Wabash, IN) to form a pellet. IR spectra were acquired as described above converted to second derivative spectra. Water vapor spectra were subtracted, and the resulting protein second derivative spectra were baseline corrected and area normalized to unity. The secondary structural changes of KGF-2 in a freeze-dried formulation was assessed using area of the overlap of between a second derivative amide I spectrum for the protein in a lyophilized formulation and that of liquid native protein.[4] In addition, spectra were compared by determining the peak width at half height (W1/2) for the major second derivative amide I band for KGF-2 at 1647 cm−1. For evaluating the potential changes in secondary structure using the W1/2 method, W1/2 of the spectrum for the native protein in ‘liquid reference control’, was subtracted from the W1/2 of freeze-dried KGF-2 to obtain the relative difference in peak width (ΔW1/2). The values are presented as mean and standard error of duplicate samples of each lyophilized formulation.
Quantitation of KGF-2 Aggregatio
Aggregation of KGF-2 in the incubated and rehydrated freeze-dried formulations was quantified using size exclusion-high performance liquid chromatography (SE-HPLC). Triplicate, freeze-dried samples for each formulation, temperature and time point were reconstituted with distilled water, centrifuged at 13500 RPM to pellet potential insoluble aggregates, and the supernatant was collected for analysis. An Agilent 1100 HPLC system equipped with Chemstation™ software was used, together with a TSK Gel G2000SWXL column (30cm×7.8mm i.d., 5µm particle size). The supernatant (40 µL volume) from reconstituted and centrifuged KGF-2 samples was injected into the HPLC system, and the protein was eluted at 0.5ml/min using a mobile phase containing 100mM sodium citrate, 1M sodium chloride, pH 6.2. Eluting protein was monitored by optical absorbance at 280nm. No soluble aggregates were detected by SE-HPLC in this study. Therefore, aggregation was determined directly from the loss of monomeric KGF-2 relative to an un-lyophilized liquid control sample.
Quantitation of KGF-2 Chemical Degradation
Chemical degradation of KGF-2 was monitored by reverse phase HPLC (RP-HPLC). Triplicate, freeze-dried samples for each formulation, temperature and time point were reconstituted with distilled water, centrifuged at 13500 RPM to pellet potential insoluble aggregates, and the supernatant was collected for analysis. An Agilent 1100 HPLC system equipped with Chemstation™ software was used, with a C18 column (2.0 mm x 250 mm, 5 µm 300 Å, YMC, USA). A gradient reverse phase method was used with mobile phase A, 0.1% trifluoroacetic acid (TFA) in water and mobile phase B, 0.07% TFA in acetonitrile. The method consisted of two steps of organic phase gradient at a flow rate of 0.3ml/min. In the first step, a 5% per minute gradient of mobile phase B (B: 5% to 35%) is used. This was followed by protein elution with 0.3% per minute gradient of mobile phase (B: 35% to 42%). Approximately 20 µg of protein were loaded per injection. The total run time was 50 minutes. Absorbance was monitored at 215nm. The percent of chemical degradation of KGF-2 in the sample supernatants was calculated from the peaks areas for native and chemically-altered KGF-2 in the chromatograms with:
| Equation 1 |
To assess the ability of the method to detect oxidized KGF-2 species, a forced oxidation study of KGF-2 was conducted. KGF-2 was incubated with 0.06% v/v H2O2 at 37°C. Samples were collected at 0, 10, 20, 30 and 40 minutes and analyzed by RP-HPLC. The percent chemical degradation increased linearly with incubation time (R2 = 0.98).
Results
Residual moisture content
The lyophilized cakes post lyophilization appeared elegant and did not change over the storage period except for significant shrinkage observed for 5% sucrose-only formulation. The residual moisture content in the freeze-dried formulations was determined immediately after lyophilization (T0 samples). All samples had < 1% wt/wt residual moisture with an error less than 10% RSD. The stored samples were monitored for any change in Tg during the storage period of 16 weeks. DSC analysis of freeze-dried samples revealed no significant change in Tg was observed for all incubated samples relative to the T0 samples over the storage period and remained within an error of 5% RSD. This indicates that minimal or no change in moisture content occurred over the storage period.
Aggregation of KGF-2 after storage and reconstitution of lyophilized formulations
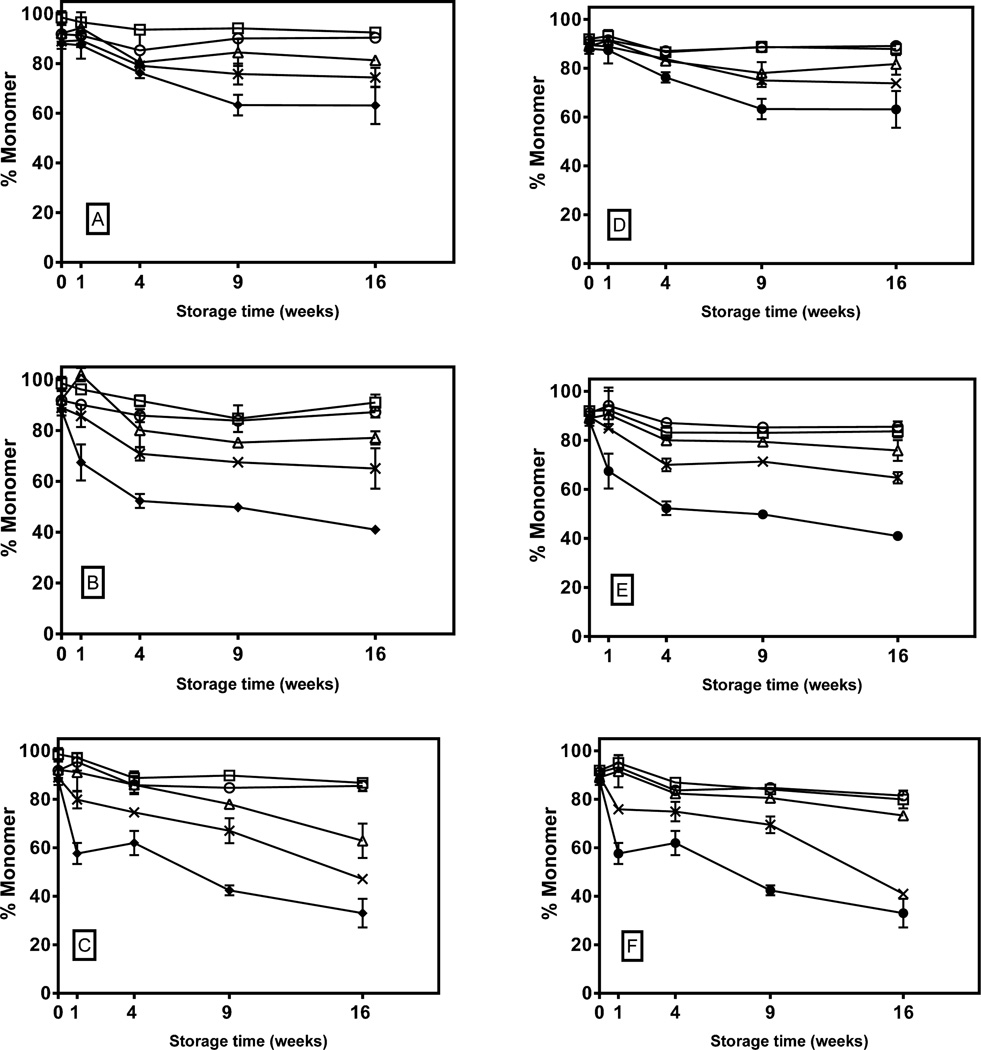
KGF-2 aggregated in all of the lyophilized samples tested, with the extent of aggregation generally increasing with increasing HES content (Figure 1). Aggregation was relatively rapid for the first 4 weeks of incubation, and then plateaued for all of the formulations during storage at 40 and 50 °C. During storage at 60°C, formulations with disaccharide alone or 4:1 disaccharide:HES mixtures also showed plateauing. The plateaus observed in disaccharide-containing formulations occurred after 10–30% of the monomer aggregated, with no apparent temperature dependence. During storage at all of the temperatures tested, KGF-2 in the HES-alone formulations exhibited markedly greater rates and extents of aggregation than formulations that contained disaccharides.
Figure 1. Percent monomeric KGF-2 in freeze-dried disaccharide-HES formulations as a function of storage time and temperature.
Left panel shows percent monomer in 5% trehalose (open circle), 4%trehalose-1% HES (open square), 2.5% trehalose-2.5% HES (open triangle) and 1% trehalose-4% HES (cross) stored and 5% HES (closed circle) at A) 40°C, B) 50°C and C) 60°C. Right panel shows results from formulations of 5% sucrose (open circle), 4%sucrose-1% HES (open square), 2.5% sucrose-2.5% HES (open triangle) and 1% sucrose-4% HES (cross) and 5% HES (closed circle) stored at D) 40°C, E) 50°C and F) 60°C.
Oxidative chemical degradation of KGF-2 after storage and reconstitution of lyophilized formulations
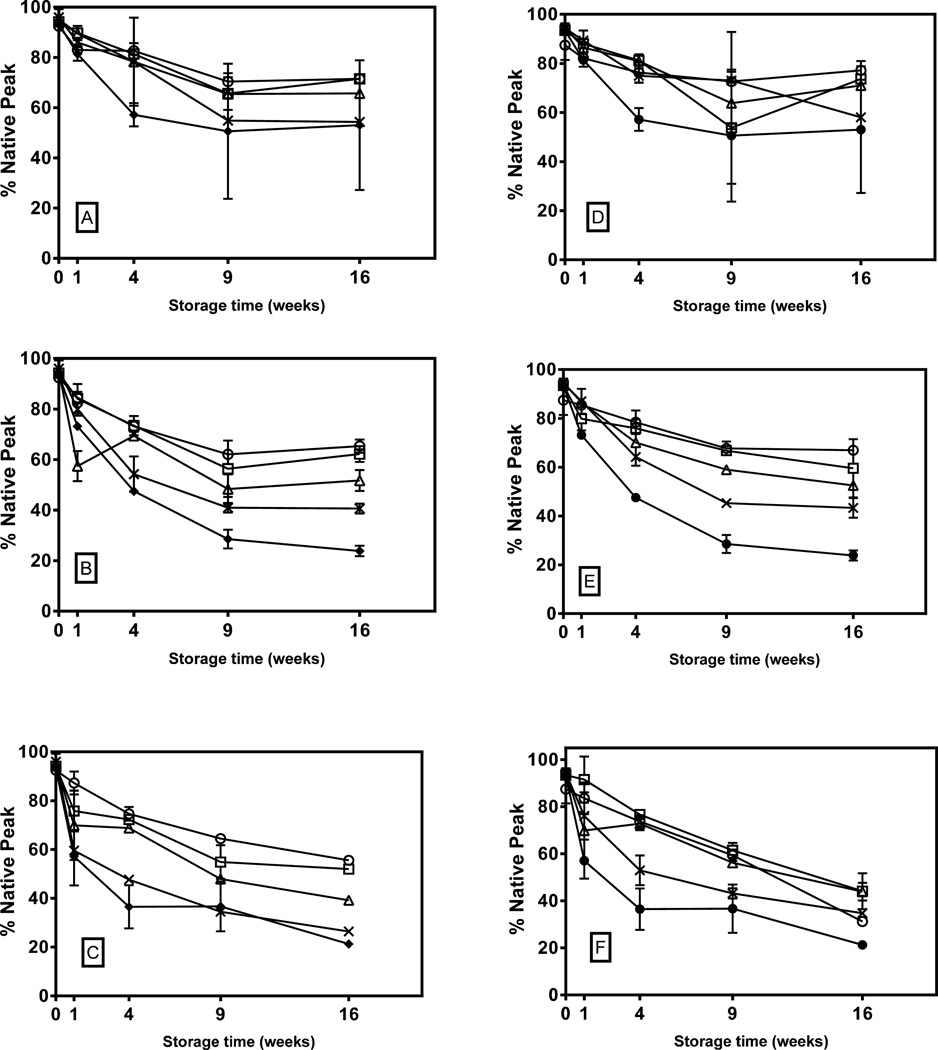
Oxidative chemical degradation of KGF-2 was measured in the fraction of the protein that did not aggregate (Figure 2). Within this fraction, the extent of degradation increased with increasing incubation temperature. And the extent of degradation generally increased with increasing fraction of HES in the formulations. At 40 and 50 °C, the rate of degradation appeared to plateau between 9 and 16 weeks of storage, but at 60°C, plateauing was not apparent. Even for the lowest storage temperature and the most stable formulations (5% sucrose or 5% trehalose), substantial (>20%) chemical degradation was detected. We note that this level, which is likely already unacceptable for a pharmaceutical formulation, may underestimate of total chemical degradation, because it does not include the potentially chemically-degraded molecules in the aggregated fraction.
Figure 2. Percent un-oxidized KGF-2 in freeze-dried disaccharide-HES formulations as a function of storage time and temperature.
Left panel shows percent un-oxidized in 5% trehalose (open circle), 4%trehalose-1% HES (open square), 2.5% trehalose-2.5% HES (open triangle) and 1% trehalose-4% HES (cross) and 5% HES (closed circle) stored at A) 40°C, B) 50°C and C) 60°C. Right panel shows results from formulations of 5% sucrose (open circle), 4%sucrose-1% HES (open square), 2.5% sucrose-2.5% HES (open triangle) and 1% sucrose-4% HES (cross) and 5% HES (closed circle) stored at D) 40°C, E) 50°C and F) 60°C.
Protein Secondary Structure in Lyophilized Formulations
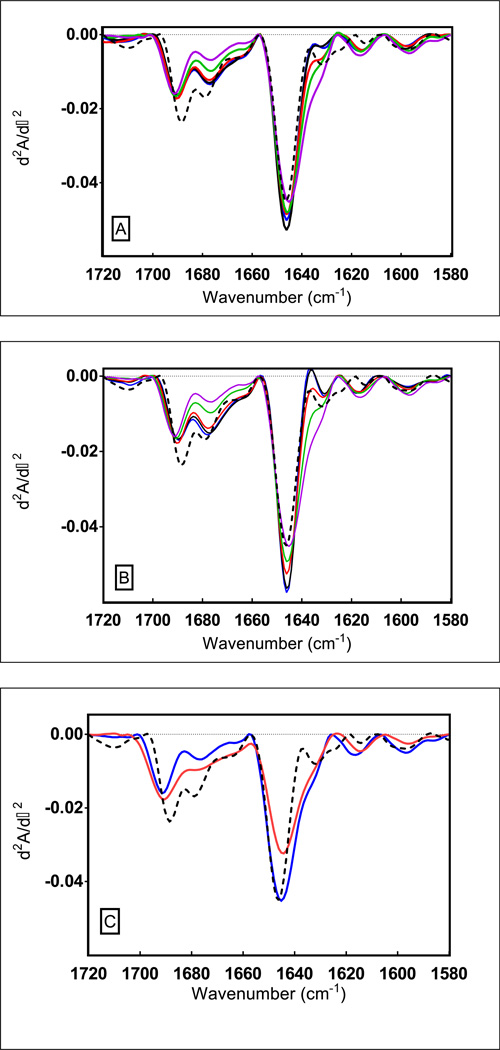
Infrared spectra of native KGF-2 in aqueous solution and for the protein in freeze-dried formulations (obtained immediately after freeze-drying) are shown in Figure 3. The main band at 1647 cm−1 in the second derivative spectrum of KGF-2 is attributed to random coil and β-sheet structure. The bands at ca. 1678 and 1685 cm−1 represent β-turns. [17] When spectra for lyophilized samples are compared with the spectrum for native KGF-2 measured in the liquid state prior to lyophilization (dashed line), significant differences are evident, reflecting perturbations of KGF-2 secondary structure. These differences are most clearly observed in the region of 1660–1700 cm−1 and in the region of 1620–1650 cm−1. The degree of structural perturbation is greatest for the formulation containing HES alone (Figure 3C), and the degree of perturbation decreases with increasing disaccharide content (Figures 3AB). To quantify the magnitude of changes in the infrared spectra relative to the spectrum for the native protein in aqueous solution, we calculated the change in width at half-height for the dominant band in the second derivative spectrum at ca. 1648 cm−1 (Table 2). In addition, the % area of overlap between the spectrum for the native protein in aqueous liquid solution and the spectra for the protein in lyophilized formulations was calculated (Table 2). In general, increases in spectral half-height widths and decreased areas of overlap were observed with increased HES content. KGF-2 secondary structure in HES without added disaccharides was noticeably more perturbed than in any of the formulations that contained disaccharides.
Figure 3. Second derivative FTIR absorbance (d2A/d-2) spectra of KGF-2 in disaccharide-HES freeze-dried formulations determined immediately after lyophilization.
The dashed trace belongs to the KGF-2 liquid control. Panel A (Sucrose-HES series) and B (Trehalose-HES series) shows increase in perturbation of native secondary structure when freeze-dried with increasing concentrations of HES; 0% HES (blue trace), 1% HES (black trace), 2.5% (red trace), 4% HES (green trace) and 5% HES (magenta trace). Panel C) shows comparison between native secondary structural perturbation of KGF-2 when freeze-dried with ‘HES alone’ (blue trace) and when freeze- dried in ‘buffer alone’ (red trace).
Table 2.
Lyophilized formulation glass dynamics properties and KGF-2 native secondary structure retention.
| Formulation | Tg °C |
1/<u2>, [Å]−2 |
ln τβ ln(hr) |
Change in half width for IR band at ca. 1648 cm−1 (ΔW1/2, cm−1) |
Area of overlap % |
|---|---|---|---|---|---|
| HES 5% | 203 | 2.44 | 3.8 | 12.42 | 78 |
| HES 4% + Trehalose 1% | 166 | 3,74 | 3.3 | 3.73 | 83 |
| HES 2.5% + Trehalose 2.5% | 119 | 5.05 | 3.2 | 2.51 | 87 |
| HES 1% + Trehalose 4% | 111 | ND | 3.1 | 1.79 | 87 |
| Trehalose 5% | 98 | 10.2 | 3.1 | 1.62 | 89 |
| HES 4% + Sucrose 1% | 158 | 3.43 | 3.1 | 3.22 | 83 |
| HES 2.5% + Sucrose 2.5% | 81 | 4.42 | 2.3 | 0.49 | 91 |
| HES 1% + Sucrose 4% | 70 | 5.85 | 1.9 | 0.44 | 90 |
| Sucrose 5% | 64 | 7.28 | 1.6 | 0.03 | 90 |
Infrared spectra were also monitored for KGF-2 in the lyophilized samples as a function of storage time and temperature. No significant changes were observed in the spectra after the 16-week storage period (data not shown).
Glassy State Physical Properties of Lyophilized Formulations
Glass transition temperatures (Tg) for the lyophilized formulations tested were obtained from a previous publication[1] and are shown in Table 2. Tg values were higher for trehalose-containing formulations than in corresponding sucrose-containing formulations. Tg increased with increasing HES content.
Moisture levels in formulations analyzed immediately after lyophilization were all below 1 wt %. Samples tested after storage at 40, 50 or 60 °C for up to 16 weeks showed no change in residual moisture levels (data not shown).
Specific surface areas of lyophilized formulations are reported in Table 2. Specific surface areas for the lyophilized formulations followed the same order as their Tg values, with specific surface areas increasing with increased HES content, and higher values observed for trehalose-containing formulations compared with sucrose-containing formulations.
Other glass dynamics parameters (Table 2) for the lyophilized formulations were obtained from published values[18, 19]. These include values for inverse mean square displacement <u2>−1 for hydrogen atoms as measured by neutron scattering, and the relaxation time τβ, which is a parameter that can be measured by isothermal calorimetry[18, 19] and that characterizes relaxation processes that correlate with fast Johari-Goldstein motions in the glass[20]. Since the weight fraction of the protein hGH is only 0.2% wt/wt of the formulations, it is unlikely that the glass dynamic parameters in these formulations would differ from those published for protein-free placebo formulations.
Discussion
To analyze the kinetics of KGF-2 degradation in lyophilized formulations, we first attempted to fit the data to two models: a model wherein the degradation was described by simple first-order kinetics, and a model with “square root of time” kinetics[7] , where P(t) is the purity as a function of time, P0 denotes the initial purity of KGF-2 and k is a rate constant. Both models produced poor fits with the experimental data sets (data not shown). Fits of the two models to the data for KGF-2 aggregation were especially poor, as neither model could capture adequately the plateauing of aggregation levels after the first four weeks of storage.
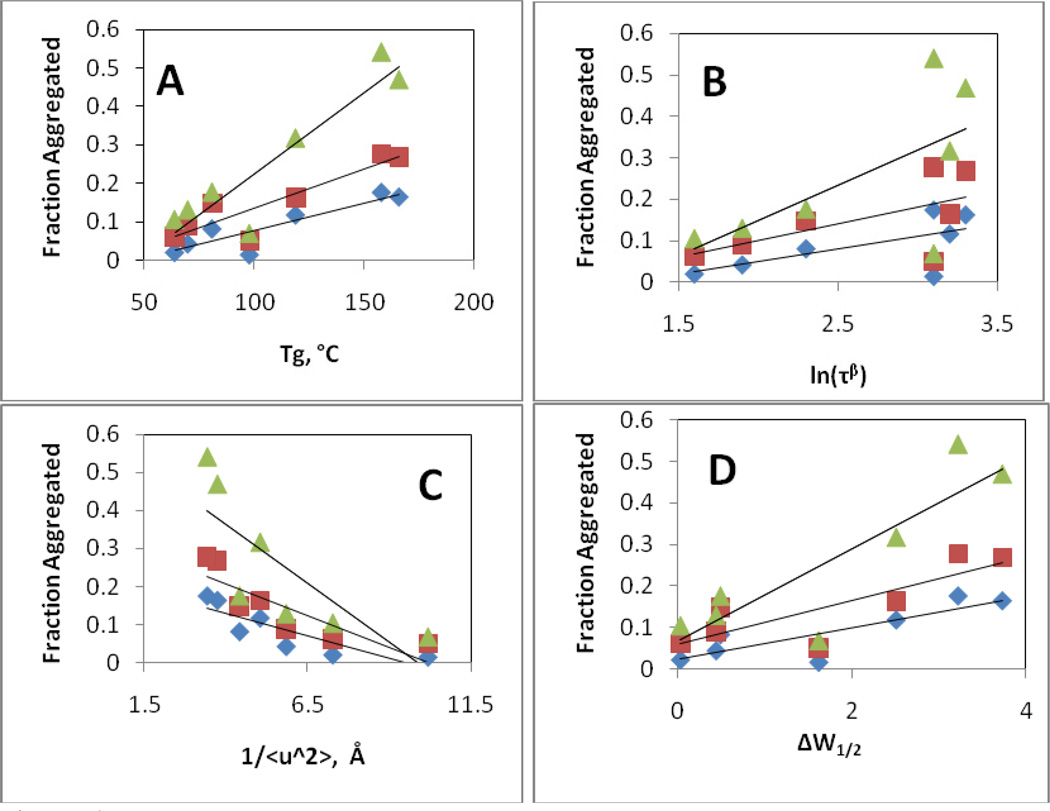
We anticipated that physical and chemical degradation of KGF-2 during storage in lyophilized formulations might be correlated with various parameters that reflect either: a) relaxation processes in the glass; or b) protein native structural retention. The parameters that we examined included the glass transition temperature Tg of the formulations, which is related to slow, large-scale, “α” relaxation processes in the glass, the relaxation time τβ which correlates with faster, Johari-Goldstein motions, and the inverse mean amplitude, <u2>−1 for hydrogen atoms, which provides an indication of the importance of very fast “β” relaxations. As measures of the degree of perturbation of protein secondary structure, we used the change in peak width at half height (ΔW1/2) for the dominant band at 1648 cm−1 in KGF-2’s second derivative infrared spectrum, and the percent overlap for infrared spectra of KGF-2 in lyophilized formulations compared with those for the native protein in liquid formulations. Plots of the fraction of KGF-2 that was oxidized or aggregated after 16 weeks of storage as a function of each parameter were examined for correlations (Figures 4–5, Table 4). The amount of aggregate formed during storage correlated only moderately (R2 values 0.6–0.78, Table 4) with increasing Tg, higher levels of rapid motion in the glass phase (smaller values of 1/<u2 >) and greater degrees of KGF-2 structural perturbation (either increased ΔW1/2 or decreased % spectral overlap). No correlation with aggregation was observed for values of τβ. For all of the parameters tested, the degree of correlation with the amount of aggregate formed during storage was independent of temperature.
Figure 4. Correlation of extent of aggregation of KGF-2 after 16 weeks of storage as a function of glassy state properties and protein secondary structure retention.
A) Fraction of KGF-2 aggregated as a function of Tg. B) Fraction of KGF-2 aggregated as a function of the natural logarithm of the characteristic Johari-Goldstein relaxation time ln(τβ). C) Fraction of KGF-2 aggregated as a function of inverse mean square displacement distance for hydrogen atoms (<u2>−1) as determined by neutron scattering. D) Fraction of KGF-2 aggregated as a function of retention of KGF-2 native secondary structure, as reflected in peak width at half height (ΔW1/2) for dominant band in the second derivative infrared spectrum. Diamonds, squares and triangles represent data taken after storage at 40, 50 and 60°C, respectively.
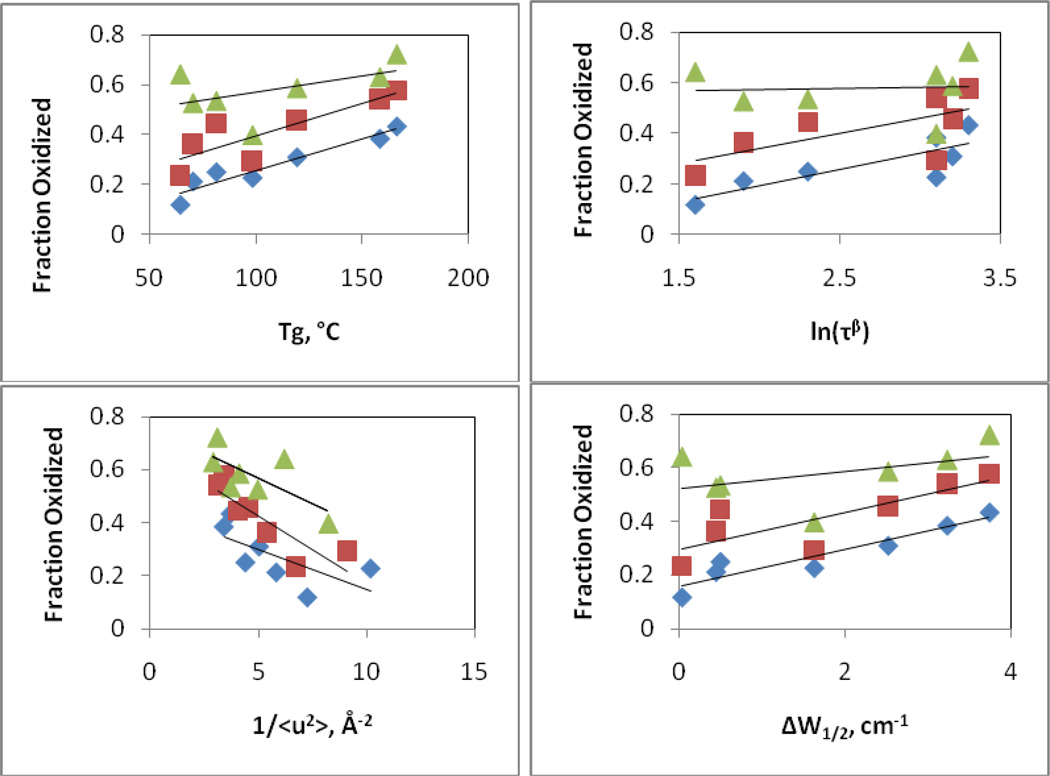
Figure 5. Correlation of the fraction of soluble KGF-2 that is oxidized after 16 weeks of storage as a function of glassy state properties and protein secondary structure retention.
A) Fraction of KGF-2 oxidized as a function of Tg. B) Fraction of KGF-2 oxidized as a function of the natural logarithm of the characteristic Johari-Goldstein relaxation time ln(τβ). C) Fraction of KGF-2 oxidized as a function of inverse mean square displacement distance for hydrogen atoms (<u2>−1) as determined by neutron scattering. D) Fraction of KGF-2 oxidized as a function of retention of KGF-2 native secondary structure, as reflected in peak width at half height (ΔW1/2) for dominant band in the second derivative infrared spectrum. Diamonds, squares and triangles represent data taken after storage at 40, 50 and 60°C, respectively.
Table 4.
Correlation coefficients R from linear regression plots of the fraction of KGF-2 that aggregated (top) or oxidized (bottom) in disaccharide and disaccharide:HES formulations during 16 weeks of storage, vs. formulation glass transition temperature, increase in infrared peak width, ln(τβ), and inverse mean square displacement of hydrogen atoms.
| Storage Temperature |
R2, Fraction KGF-2 Aggregated vs. Tg |
R2, Fraction KGF-2 Aggregated vs. ΔW1/2 |
R2, Fraction KGF-2 Aggregated vs. ln(τβ) |
R2, Fraction KGF-2 Aggregated vs. <u2>−1 |
|---|---|---|---|---|
| 40 °C | .78 | .70 | .34 | .72 |
| 50 °C | .72 | .62 | .24 | .74 |
| 60 °C | .77 | .69 | .28 | .65 |
| Storage Temperature |
R2, Fraction KGF-2 Oxidized vs. Tg |
R2, Fraction KGF-2 Oxidized vs. ΔW1/2 |
R2, Fraction KGF-2 Oxidized vs. ln(τβ) |
R2, Fraction KGF-2 Oxidized vs. <u2>−1 |
|---|---|---|---|---|
| 40 °C | .89 | .86 | .58 | .49 |
| 50 °C | .68 | .61 | .33 | .75 |
| 60 °C | .20 | .15 | .00 | .50 |
In contrast, at 40°C, the fraction of the protein molecules that were oxidized during 16 weeks storage was well-correlated with Tg and ΔW1/2, but poorly correlated with Johari-Goldstein relaxations (τβ) or fast β (<u2>−1) relaxations (Figure 4). Correlations for oxidation with Tg and ΔW1/2 decreased as temperature was increased to 50 and 60°C; at 60°C none of the four parameters showed significant correlation.
Overall, the parameter that was most correlated to KGF-2 damage (both aggregation and oxidation) was Tg. Interestingly, however, the correlation was the inverse of that which might intuitively have been expected. Protein aggregation requires large-scale diffusive motions which slow as Tg increases, yet protein degradation increased as Tg increased. Thus, it is not likely that KGF-2 aggregation occurs at a significant rate during storage within the glassy solid.
If aggregation does not occur in the glassy state, how might the time-dependent increases in aggregate levels be explained? One possible explanation is that small-scale motions, especially the fast β relaxations reflected in measurements of <u2>−1, might allow the protein to undergo time-dependent structural changes within the glassy formulations, creating populations with aggregation-prone structures that more rapidly aggregate upon reconstitution. This explanation seems to conflict with the observation that no additional changes in KGF-2 secondary structure could be observed as a function of storage time. However, structural changes in relatively small fractions of the protein population may not be detected by infrared spectroscopy.[7, 21, 22].
In general, the oxidation rates were higher than the aggregation rates indicating that not all of the oxidized species may have resulted in aggregation-prone species upon reconstitution. The pronounced plateau regions seen in the aggregation data (and in a more muted fashion in the oxidation data) suggest another explanation for the observed degradation kinetics: the possibility that the samples contained multiple environments wherein the protein degrades at different rates. In the freezing step of the lyophilization process, crystals of essentially pure ice are formed, and protein may accumulate at the ice-liquid interface[23]. During lyophilization, the ice is removed by sublimation, leaving the accumulated protein at the newly formed glassy solid-air interface[14]. Protein adsorbed at the surface may provide a population of molecules that are more susceptible to damage than those in the bulk glass.
Earlier studies showed that recombinant human interferon-γ adsorbs at ice/liquid interfaces during lyophilization[14], and bovine serum albumin and trypsin were found to be adsorbed at the surface of lyophilized disaccharide glasses[24]. A study on stability of a vaccine lyophilized in carbohydrate glasses found that the rate of degradation increased with the amount of protein present on the surface[25]. Of particular interest to the present study, Abdul-Fattah et al. lyophilized rhGH in various carbohydrate formulations and detected rhGH accumulated on the surface of the glassy powders[6]. In lyophilized sucrose formulations of an antibody, the fraction of the protein found at the glass-air interface increased with decreasing weight ratios of protein:sucrose; 1.0% of the antibody mass in the sample was found at the interface in formulations with a protein:sucrose ratio of 4:1, but this fraction increased from to 2.2% and 6.4% as the protein:sucrose ratio decreased to 1:4 and 1:19, respectively.[26] The protein:carbohydrate ratio in the current study is 1:50.
Proteins adsorbed at interfaces are often prone to structural damage and aggregation[27–29]. For example, lactate dehydrogenase, hemoglobin, Factor XIII and a monoclonal antibody unfolded and aggregated at ice-water interfaces[30–32]. Human growth hormone aggregation during freeze-thawing increased with faster cooling rates, presumably due to the greater amount of ice-water interface present at faster cooling rates.[33] Furthermore, for 6 different proteins, the formation of protein particles during freeze-thawing increased with increasing degree of surface denaturation.[4]
To explore whether accumulation of KGF-2 on surfaces of lyophilized powders could account for the observed degradation kinetics during storage, we first used BET analysis to measure specific surface areas of lyophilized formulations containing HES, trehalose, sucrose, and 1:1 weight ratio mixtures of HES:sucrose and HES:trehalose. Formulations with higher Tg values exhibited larger specific surface areas (Tables 2 and 3), a phenomenon that is explained by the slower rates and extents of Ostwald ripening of ice crystals in formulations with higher glass transition temperatures.[34] The amount of KGF-2 on the surface was then estimated by multiplying the specific surface area by an estimated surface coverage of 2 mg/m2 (Table 3). Estimated maximum ice-water surface coverage for interferon- γ, a similarly-sized protein, were reported as 4.5 mg/m2, and protein adsorption to a variety of surfaces such as glass[35, 36], silica[35, 36], polystyrene[37] and cellulose[36] has been reported to range from 2–4 mg/m2; thus our estimate of 2mg/m2 may yield a conservative estimate of the amount of KGF-2 on the surface of lyophilized samples.
Table 3.
Specific surface areas for lyophilized formulations determined from BET analysis of krypton adsorption, and estimated fraction of KGF-2 on the surface of the lyophilized powders.
| Formulation | Specific Surface Area, m2/g |
Estimated Fraction of KGF-2 on Surface, % |
|---|---|---|
| 5% HES | 2.08±0.01 | 21.2 |
| 5% trehalose | 1.55±0.01 | 15.8 |
| 5% sucrose | 1.17±0.06 | 11.9 |
| 2.5% HES and 2.5% trehalose | 1.93±0.03 | 19.6 |
| 2.5% HES and 2.5% sucrose | 1.63±0.01 | 16.6 |
| 4% HES and 1% trehalose | 2.02±0.06 | 20.6 |
| 4% HES and 1% sucrose | 1.96±0.11 | 20.0 |
| 1% HES and 4% trehalose | 1.66±0.08 | 16.9 |
| 1% HES and 4% sucrose | 1.43±0.05 | 14.6 |
We fit the data for KGF-2 aggregation and oxidation during storage to a simple model that assumes that protein molecules on the surface and in the bulk glass degrade with first order kinetics:
| Equation 2 |
where P(t) is the mass of native protein in the sample as a function of storage time t, P0 is the amount of native protein in the sample immediately after lyophilization, f is the fraction of the protein in the sample found on the surface of the glass, and ksurface and kbulk are apparent first order rate constants for aggregation or oxidation in on the surface and in the bulk, respectively, of the glassy powders.
For the samples that contained disaccharides or disaccharide:HES mixtures, the best-fit values of ksurface and kbulk for both aggregation and oxidation were temperature-dependent, but were essentially independent of formulation. A global fit to the data for these eight sample types was then used to determine a single value of ksurface and kbulk at each temperature (Table 5). Samples containing only HES as an excipient were analyzed separately; values of ksurface for aggregation in the HES-only formulations were larger than those obtained for the disaccharide-containing formulations, although the rate constants for degradation of KGF-2 on the surface were still much larger than those in the bulk glass (Table 5). This is consistent with the observation that the structure of KGF-2 was much more perturbed after lyophilization in HES samples than in any of the samples containing disaccharides, as evidenced by values of ΔW1/2 that ranged from 0.03 to 3.73 cm−1 in formulations containing disaccharide, whereas ΔW1/2 in HES samples was 12.42 cm−1 (Table 2).
Table 5.
Apparent first-order rate constants for KGF-2 aggregation and oxidation at the surface and in the bulk glass phase of various lyophilized formulations.
|
Degradation type |
Lyophilized Formulation | T=40°C | T=50°C | T=60°C | |||
|---|---|---|---|---|---|---|---|
|
ksurface, wk−1 |
kbulk, wk−1 |
ksurface, wk−1 |
kbulk, wk−1 |
ksurface, wk−1 |
kbulk, wk−1 |
||
| Aggregation |
All disaccharide and disaccharide:HES mixtures |
0.07 | ~0 | 0.39 | ~0 | 0..26 | ~0 |
| HES | .21 | .01 | 3.4 | 0.04 | >10 | 0.05 | |
| Oxidation |
All disaccharides and disaccharide:HES mixtures |
0.54 | 0.01 | 1.35 | 0.03 | >10 | 0.04 |
| HES | 0.93 | 0.03 | 1.3 | 0.09 | >10 | 0.09 | |
Reported values are combined overall best fits for all formulations that contained disaccharides, and for the HES-only formulation, which was analyzed separately.
Interestingly, for both aggregation and oxidation, the best-fit rate constants suggest that degradation is much more rapid in the population of protein molecules found on the surface as compared to those found in the bulk glass. In fact, kbulk for aggregation was essentially zero. For aggregation the small value for kbulk is intuitive, because the very high viscosities found in the glassy state should effectively eliminate the large-scale translational motions required for proteins to aggregate. Likewise, because aggregation typically requires protein unfolding, and protein molecules at interfaces are more likely to be conformationally perturbed, the larger values of ksurface are expected for aggregation. A similar argument can be made to rationalize the values of ksurface and kbulk obtained for oxidation, because protein oxidation requires diffusion of an oxidizing species (likely O2) to the protein, and rates of oxidation are increased for exposed amino acid residues. Both the diffusion of O2 and the exposure of (normally buried) amino acid residues are expected to be higher in the conformationally-perturbed surface layer, yielding larger values of ksurface as compared kbulk for oxidation.
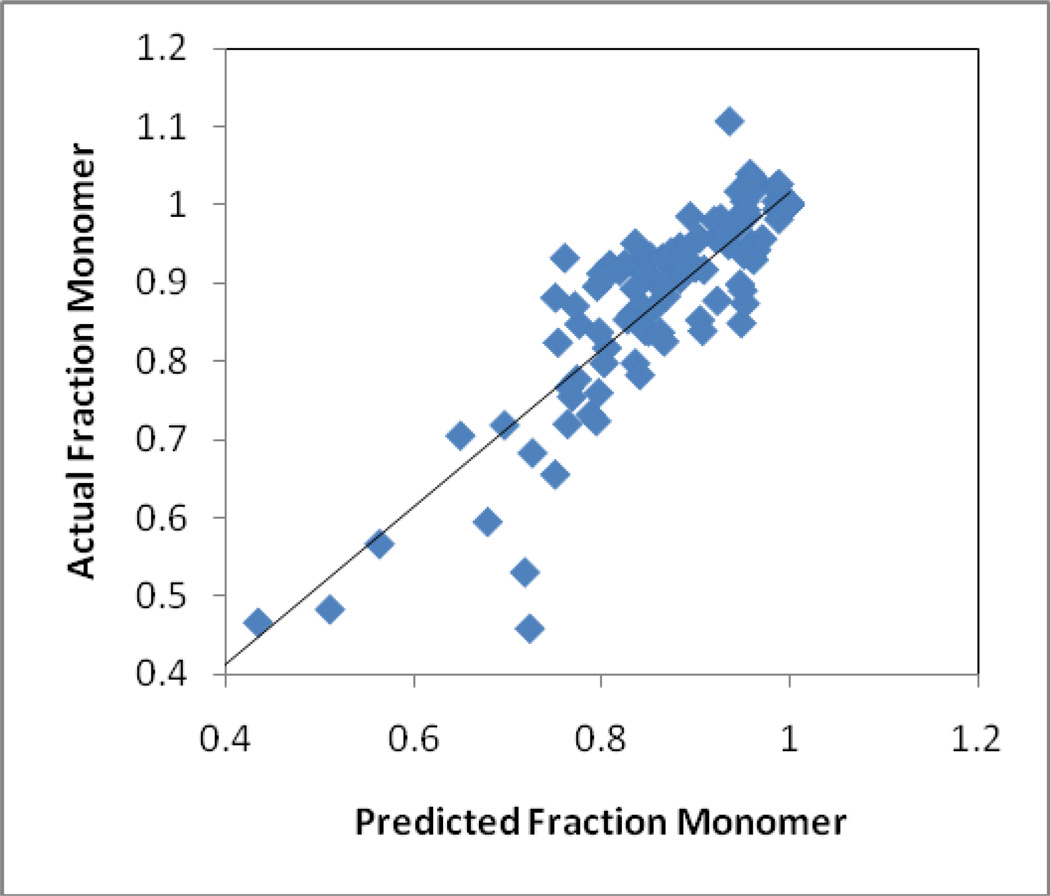
Figure 6 shows the observed amount of monomeric KGF-2 remaining in each of the nine sample types samples at each of the storage times and temperatures tested, plotted versus the amount predicted based on Equation 2 and the best fit rate constants from Table 5. The simple model provided a satisfactory fit to the data, as evidenced by the correlation coefficient R2=0.82.
Figure 6. Measured levels of monomeric KGF-2 compared with predictions from the model described by Equation 2 and the associated parameters found in Table 5.
Data are for all monomer fractions recorded after storage at 1, 4, 9 and 16 weeks and temperatures of 40, 50, and 60 °C, for each of the formulations.
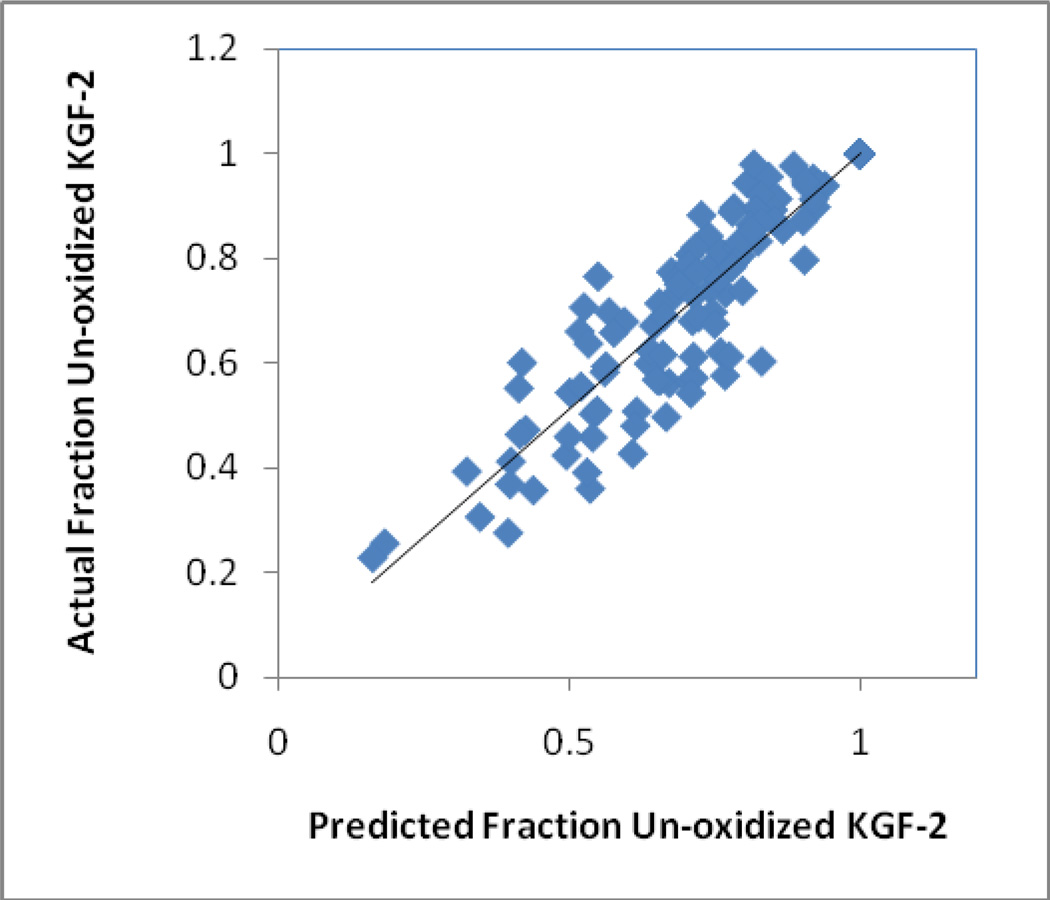
Similar results were obtained from the analysis of KGF-2 oxidation using Equation 2. Figure 7 shows the measured fraction of un-oxidized KGF-2 remaining in solution in each of the nine sample types at each of the storage times and temperatures tested, plotted against the fraction predicted from equation 2 and the best fit rate constants from Table 5. The simple model provides a reasonable fit to the data (correlation coefficient R2 =0.84), especially considering the fact that the measured oxidation levels only account for oxidation in the soluble fraction of KGF-2, and likely reflect degradation at multiple oxidation sites.
Figure 7. Measured levels of un-oxidized KGF-2 in the soluble fraction compared with predictions from the model described by Equation 2 and the associated parameters found in Table 5.
Data are for all un-oxidized fractions recorded after storage at 1, 4, 9 and 16 weeks and temperatures of 40, 50, and 60 °C, for each of the formulations.
Conclusions
Although lyophilized formulations of therapeutic proteins provide effective and relatively stable dosage forms, degradation of the dried protein can occur at rates that are still unacceptable for a pharmaceutical product. Therefore, to optimize stability of proteins in dried formulations, it is important to understand the factors that govern stability during long-term storage. In the current study, we found as expected[1] that degradation rates were generally decreased in formulations with greater native state structural retention and with reduced fast β relaxations. But these two factors could not account quantitatively for the aggregation and chemical degradation rates of KGF-2 observed. Rather, it appears that a dominant factor governing protein degradation in freeze-dried formulations is the fraction of protein found at the glass solid-air interface. We suggest that future mechanistic studies on lyophilized protein formulations and practical efforts to optimize stability of freeze-dried proteins consider this factor, and develop means by which to reduce surface area of dried formulations and/or minimize protein adsorption at the interface. Such mitigation strategies could include annealing during the freeze-drying process[13, 34] and the inclusion of nonionic surfactants, respectively.
Highlights.
Storage stability of KGF-2 was correlated to key physical properties of formulations
The correlation of KGF-2 stability with most physical properties was only moderate
Strong correlation was found with the fraction of the protein at solid-air interface
Abbreviations
- KGF-2
keratinocyte growth factor-2
- HES
hydroxyethyl starch
- BET
Brunauer–Emmett–Teller
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu Y, Carpenter JF, Cicerone MT, Randolph TW. Contributions of local mobility and degree of retention of native secondary structure to the stability of recombinant human growth hormone (rhGH) in glassy lyophilized formulations. Soft Matter. 2013;9:7855–7865. [Google Scholar]
- 2.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: Some practical advice. Pharmaceutical Research. 1997;14:969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JF, Prestrelski SJ, Dong AC. Application of infrared spectroscopy to development of stable lyophilized protein formulations. European Journal of Pharmaceutics and Biopharmaceutics. 1998;45:231–238. doi: 10.1016/s0939-6411(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang BS, Kendrick BS, Carpenter JF. Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. Journal of Pharmaceutical Sciences. 1996;85:1325–1330. doi: 10.1021/js960080y. [DOI] [PubMed] [Google Scholar]
- 5.Allison SD, Chang B, Randolph TW, Carpenter JF. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Archives of Biochemistry and Biophysics. 1999;365:289–298. doi: 10.1006/abbi.1999.1175. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Fattah A, Lechuga-Ballesteros D, Kalonia D, Pikal M. The impact of drying method and formulation on the physical properties and stability of methionyl human growth hormone in the amorphous solid state. Journal of Pharmaceutical Sciences. 2008;97:163–184. doi: 10.1002/jps.21085. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Tchessalov S, Warne NW, Pikal MJ. Impact of sucrose level on storage stability of proteins in freeze-dried solids: I. Correlation of protein-sugar interaction with native structure preservation. J Pharm Sci. 2009;98:3131–3144. doi: 10.1002/jps.21621. [DOI] [PubMed] [Google Scholar]
- 8.Heller M, Carpenter J, Randolph T. Effects of Phase Separating Systems on Lyophilized Hemoglobin. J. Pharm. Sci. 1996;85:1358–1362. doi: 10.1021/js960019t. [DOI] [PubMed] [Google Scholar]
- 9.Heller M, Carpenter J, Randolph T. Protein Phase Separation During Freeze-Drying: Implications for Pharmaceutical Proteins. Biotechnology Progress. 1997;13:590–596. doi: 10.1021/bp970081b. [DOI] [PubMed] [Google Scholar]
- 10.Izutsu K, Yoshioka S, Terao T. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm Res. 1993;10:1232–1237. doi: 10.1023/a:1018988823116. [DOI] [PubMed] [Google Scholar]
- 11.Izutsu K, Kojima S. Freeze-concentration separates proteins and polymer excipients into different amorphous phases. Pharm Res. 2000;17:1316–1322. doi: 10.1023/a:1026412107574. [DOI] [PubMed] [Google Scholar]
- 12.Katayama DS, Carpenter JF, Menard KP, Manning MC, Randolph TW. Mixing Properties of Lyophilized Protein Systems: A Spectroscopic and Calorimetric Study. Journal of Pharmaceutical Sciences. 2009;98:2954–2969. doi: 10.1002/jps.21467. [DOI] [PubMed] [Google Scholar]
- 13.Webb SD, Cleland JL, Carpenter JF, Randolph TW. Effects of annealing lyophilized and spray-lyophilized formulations of recombinant human interferon-gamma. J Pharm Sci. 2003;92:715–729. doi: 10.1002/jps.10334. [DOI] [PubMed] [Google Scholar]
- 14.Webb SD, Golledge SL, Cleland JL, Carpenter JF, Randolph TW. Surface adsorption of recombinant human interferon-gamma in lyophilized and spray-lyophilized formulations. Journal of Pharmaceutical Sciences. 2002;91:1474–1487. doi: 10.1002/jps.10135. [DOI] [PubMed] [Google Scholar]
- 15.Murzin AG, Lesk AM, Chothia C. [3-Trefoil fold: Patterns of structure and sequence in the Kunitz inhibitors interleukins-1β and 1α and fibroblast growth factors. Journal of Molecular Biology. 1992;223:531–543. doi: 10.1016/0022-2836(92)90668-a. [DOI] [PubMed] [Google Scholar]
- 16.Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. Journal of the American Chemical Society. 1938;60:309–319. [Google Scholar]
- 17.Dong A, Huang P, Caughey WS. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry. 1990;29:3303–3308. doi: 10.1021/bi00465a022. [DOI] [PubMed] [Google Scholar]
- 18.Chieng N, Cicerone MT, Zhong Q, Liu M, Pikal MJ. Characterization of dynamics in complex lyophilized formulations: II. Analysis of density variations in terms of glass dynamics and comparisons with global mobility, fast dynamics, and Positron Annihilation Lifetime Spectroscopy (PALS) European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2013;85:197–206. doi: 10.1016/j.ejpb.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chieng N, Mizuno M, Pikal M. Characterization of dynamics in complex lyophilized formulations: I. Comparison of relaxation times measured by isothermal calorimetry with data estimated from the width of the glass transition temperature region. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2013;85:189–196. doi: 10.1016/j.ejpb.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngai KL. Relaxation and diffusion in complex systems. New York: Springer; 2011. [Google Scholar]
- 21.DAntonio J, Murphy BM, Manning MC, Al-Azzam WA. Comparability of protein therapeutics: Quantitative comparison of second-derivative amide I infrared spectra. Journal of Pharmaceutical Sciences. 101:2025–2033. doi: 10.1002/jps.23133. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Li C, Nguyen X, Muzammil S, Towers E, Gabrielson J, Narhi L. Qualification of FTIR Spectroscopic Method for Protein Secondary Structural Analysis. Journal of Pharmaceutical Sciences. 2011;100:4631–4641. doi: 10.1002/jps.22686. [DOI] [PubMed] [Google Scholar]
- 23.Twomey A, Less R, Kurata K, Takamatsu H, Aksan A. In Situ Spectroscopic Quantification of Protein-Ice Interactions. The Journal of Physical Chemistry B. 2013;117:7889–7897. doi: 10.1021/jp403267x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millqvist-Fureby A, Malmsten M, Bergenstahl B. Surface characterisation of freeze-dried protein/carbohydrate mixtures. International Journal of Pharmaceutics. 1999;191:103–114. doi: 10.1016/s0378-5173(99)00285-9. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Fattah A, Truong-Le V, Yee L, Pan E, Ao Y, Kalonia D, Pikal M. Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on stability II: Stability of a vaccine. Pharmaceutical Research. 2007;24:715–727. doi: 10.1007/s11095-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 26.Abdul-Fattah A, Truong-Le V, Yee L, Nguyen L, Kalonia D, Cicerone M, Pikal M. Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on stability (I): Stability of a monoclonal antibody. Journal of Pharmaceutical Sciences. 2007;96:1983–2008. doi: 10.1002/jps.20859. [DOI] [PubMed] [Google Scholar]
- 27.Sluzky V, Tamada JA, Klibanov AM, Langer R. Kinetics of Insulin Aggregation in Aqueous-Solutions Upon Agitation in the Presence of Hydrophobic Surfaces. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:9377–9381. doi: 10.1073/pnas.88.21.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sluzky V, Klibanov AM, Langer R. Mechanism of Insulin Aggregation and Stabilization in Agitated Aqueous-Solutions. Biotechnology and Bioengineering. 1992;40:895–903. doi: 10.1002/bit.260400805. [DOI] [PubMed] [Google Scholar]
- 29.Bee JS, Randolph TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of Surfaces and Leachables on the Stability of Biopharmaceuticals. Journal of Pharmaceutical Sciences. 2011;100:4158–4170. doi: 10.1002/jps.22597. [DOI] [PubMed] [Google Scholar]
- 30.Schwegman J, Carpenter J, Nail S. Evidence of Partial Unfolding of Proteins at the Ice/Freeze-Concentrate Interface by Infrared Microscopy. Journal of Pharmaceutical Sciences. 2009;98:3239–3246. doi: 10.1002/jps.21843. [DOI] [PubMed] [Google Scholar]
- 31.Kreilgard L, Jones L, Randolph TW, Frokjaer S, Carptenter J. Tween 20 Prevents Freeze-Thawing and Shaking-Induced Denaturation: A Mechanistic Study on Protein Surfactant Interactions, in. Presented at 10th Annual AAPS Meeting; Seattle. 1996. [Google Scholar]
- 32.Kerwin BA, Heller MC, Levin SH, Randolph TW. Effects of tween 80 and sucrose on acute short-term stability and long-term storage at −20 degrees C of a recombinant hemoglobin. Journal of Pharmaceutical Sciences. 1998;87:1062–1068. doi: 10.1021/js980140v. [DOI] [PubMed] [Google Scholar]
- 33.Eckhardt BM, Oeswein JQ, Bewley TA. Effect of freezing on aggregation of human growth hormone. Pharm. Res. 1991;8:1360–1364. doi: 10.1023/a:1015888704365. [DOI] [PubMed] [Google Scholar]
- 34.Searles JA, Carpenter JF, Randolph TW. Annealing to optimize the primary drying rate, reduce freezing-induced drying rate heterogeneity, and determine T-g ‘ in pharmaceutical lyophilization. Journal of Pharmaceutical Sciences. 2001;90:872–887. doi: 10.1002/jps.1040. [DOI] [PubMed] [Google Scholar]
- 35.Hoehne M, Samuel F, Dong A, Wurth C, Mahler H, Carpenter J, Randolph T. Adsorption of Monoclonal Antibodies to Glass Microparticles. Journal of Pharmaceutical Sciences. 2011:123–132. doi: 10.1002/jps.22275. [DOI] [PubMed] [Google Scholar]
- 36.Bee J, Chiu D, Sawicki S, Stevenson J, Chatterjee K, Freund E, Carpenter J, Randolph T. Monoclonal Antibody Interactions With Micro- and Nanoparticles: Adsorption, Aggregation, and Accelerated Stress Studies. Journal of Pharmaceutical Sciences. 2009;98:3218–3238. doi: 10.1002/jps.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel MFM, van Mierlo CPM, Visser A. Kinetic and structural characterization of adsorption-induced unfolding of bovine alpha-lactalbumin. Journal of Biological Chemistry. 2002;277:10922–10930. doi: 10.1074/jbc.M106005200. [DOI] [PubMed] [Google Scholar]