Abstract
Objective This study presents a group of patients experiencing recurrent cerebrospinal fluid (CSF) leakage associated with ipsilateral anacusis who underwent subtotal petrosectomies with the goal of stopping the CSF leak and preventing meningitis.
Materials and Methods Eight patients with CSF leakage were enrolled: three patients with giant vestibular schwannomas had CSF leakage after gamma knife failure and subsequent removal via a retrosigmoid approach; two patients had malformations at the level of the inner ear with consequent translabyrinthine fistulas; two had posttraumatic CSF leakages; and one had a CSF leakage coexisting with an encephalocele. Two patients developed meningitis that resolved with antibiotic therapy. Each patient had preoperative anacusis and vestibular nerve areflexia on the affected side.
Results The patients with congenital or posttraumatic CSF leaks had undergone at least one unsuccessful endaural approach to treat the fistula. All eight patients were treated successfully with a subtotal petrosectomy. The symptoms disappeared within 2 months postoperatively. No meningitis, signs of fistula, or other symptoms occurred during the follow-up.
Conclusion A subtotal petrosectomy should be the first choice of treatment in patients with recurrent CSF leakage whenever there is associated unilateral anacusis.
Keywords: CSF leakage, subtotal petrosectomy, unilateral anacusis
Introduction
Cerebrospinal fluid (CSF) leakage from the lateral skull base involves an abnormal communication between the sterile subarachnoid space and the tympanomastoid cavity.1 Its etiology can be spontaneous, posttraumatic, or postsurgical.2 3 It usually presents with nonspecific symptoms such as aural fullness, tinnitus, headache, and vertigo. In some cases, rhinorrhea, otorrhea, or a middle ear effusion can hide a CSF leak.4 5
A CSF leakage is a rare but potentially life-threatening condition.1 2 3 4 5 The estimated risk of meningitis associated with this condition is 4 to 50%.3 6 7 Due to the serious complications, it is essential to make an early diagnosis to start appropriate treatment.8
Numerous surgical approaches have been proposed for stopping CSF leakages and minimizing the risk of intracranial infection. The choice depends on the fistula location, the patient's comorbidities, and the hearing level.1 3 5
In 1986, Coker et al9 proposed the subtotal petrosectomy (SP) as an alternative treatment for CSF leakage. They stated that it is mandatory not to delay surgical obliteration of all accessible pneumatized spaces in the petrous bone to close all possible fistulas and to prevent meningitis. An SP allows good exposure of both the middle and posterior fossa plates, avoids opening the dura, and has a low CSF leakage recurrence rate.10 Unfortunately, this surgical technique is not used widely because obliteration of the petrous bone and middle ear causes conductive deafness of ∼ 60 dB.9 11 12
Nevertheless, this approach may be considered the first option in patients with recurrent temporal bone CSF leakage (greater risk of meningitis) or anacusis on the affected side. To our knowledge, few reports have examined this specific topic in the treatment of CSF leakage with SP.10 11 12 13 14
This article presents our results in eight patients with recurrent CSF leakages caused by different etiologies with preoperative ipsilateral anacusis.
Materials and Methods
This study enrolled eight patients (five women and three men; average age 43.7 [range: 19–66] years) with CSF leakages referred to our department between 2000 and 2011.
Preoperatively, all patients were evaluated using pure tone audiometry and caloric vestibular testing, with cervical and ocular vestibular-evoked myogenic potentials from 2005 and the video Head Impulse Test after 2009.
In the study group were patients with three different CSF leakage etiologies: congenital, posttraumatic, and postsurgical (Tables 1–3).
Table 1. Cases 1, 2, and 3.
| Spontaneous CSF leak | |||
|---|---|---|---|
| Case | 1 | 2 | 3 |
| Age, y/sex | 49/F | 20/F | 66/M |
| History | Left congenital hearing loss Meningitis episode 2 y earlier |
Right congenital hearing loss | Middle ear encephalocele meningitis episode 3 y earlier |
| Preoperative symptoms | Left anacusis Orthostatic headache Positional vertigo |
Left anacusis Orthostatic headache Positional vertigo |
Right anacusis Headache Positional vertigo |
| CT and MRI | Absence of the platen of stapes, defect of its mucosal lining, and cochlea malformation | IAC, stapes platen, oval window, and cochlea malformations | Mastoid encephalocele, CSF leak into the epitympanic recess and tympanic antrum |
| Preoperative audiological test | Left anacusis | Right anacusis | Right sensorineural hearing loss |
| Preoperativevestibular test | Complete left vestibular areflexia | Complete right vestibular areflexia | Complete right vestibular areflexia |
| Site of fistula | Oval window | IAC | Erosion of the tegmen tympani |
| Failed treatment | None | Oval window closure with fat and temporalis fascia through endaural approach | None |
| Postoperative complications | None | None | None |
| Postoperative symptoms | Disappeared at 30 d | Disappeared at 40 d | Disappeared at 20 d |
| Follow-up | 1 y | 9 y | 10 mo |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; IAC, internal auditory canal; MRI, magnetic resonance imaging.
Table 2. Cases 4 and 5.
| Posttraumatic CSF leak | ||
|---|---|---|
| Case | 4 | 5 |
| Age, y/sex | 55/F | 31/F |
| History | Successful left stapedotomy 10 y earlier, barotrauma, sudden hearing loss and vertigo | Head injury without temporal bone fracture: sudden hearing loss and vertigo |
| Preoperative symptoms | Left anacusis Positional vertigo |
Left anacusis Orthostatic headache Positional vertigo |
| Radiologic evaluation | Left pneumolabyrinth | Left stapediovestibular dislocation |
| Preoperative audiological test | Left anacusis | Left anacusis |
| Preoperative vestibular test | Complete left vestibular areflexia | Complete left vestibular areflexia |
| Site of fistula | Oval window at the level of the stapedotomy (prosthesis dislocation) | Oval window (stapes dislocation) |
| Failed treatment | Oval window closure with fat through endaural approach | Oval window closure with fat and temporalis fascia through endaural approach |
| Postoperative complications | None | None |
| Postoperative symptoms | Disappeared at 40 d | Disappeared at 55 d |
| Follow-up | 2 y | 4 y |
Abbreviation: CSF, cerebrospinal fluid.
Table 3. Cases 6, 7, and 8.
| Postsurgical CSF leak | |||
|---|---|---|---|
| Case | 6 | 7 | 8 |
| Age, y/sex | 61/M | 48/F | 50/F |
| History | Right CSF leakage following removal of a giant VS by retrosigmoid approach after gamma knife failure | Left CSF leakage following removal of a giant VS by retrosigmoid approach after gamma knife failure | Right CSF leakage following removal of a giant VS by retrosigmoid approach after gamma knife failure |
| Preoperative symptoms | Right rhinorrhea | Left rhinorrhea and subcutaneous accumulation of CSF | Right rhinorrhea |
| Preoperative audiological test | Right anacusis | Left anacusis | Right anacusis |
| Preoperativevestibular test | Complete right vestibular areflexia | Complete left vestibular areflexia | Complete right vestibular areflexia |
| Site of fistula | Posterior fossa dura | Posterior fossa dura | Posterior fossa dura |
| Failed treatment | Conservative management retrosigmoid approach | Conservative management retrosigmoid approach | Conservative management retrosigmoid approach |
| Postoperative complications | None | None | None |
| Postoperative symptoms | Disappeared at 10 d | Disappeared at 15 d | Disappeared at 21 d |
| Follow-up | 2 y | 12 y | 5 y |
Abbreviations: CSF, cerebrospinal fluid; VS, vestibular schwannoma.
Of those with congenital CSF leakages, one patient had an absent stapes foot plate with a defect in the mucosal lining and a cochlear malformation. He had a previous episode of bacterial meningitis treated successfully with intravenous antibiotics. The second patient had internal auditory canal (IAC), stapes footplate, oval window, and cochlear malformations (Fig. 1). The third patient had mastoid encephaloceles and a break in the continuity of the tegmen tympani, diagnosed 3 years after an episode of meningitis that resolved with medical therapy. In this patient, magnetic resonance imaging (MRI) suggested the presence of CSF leakage into the epitympanic recess and tympanic antrum (Fig. 2).
Fig. 1.
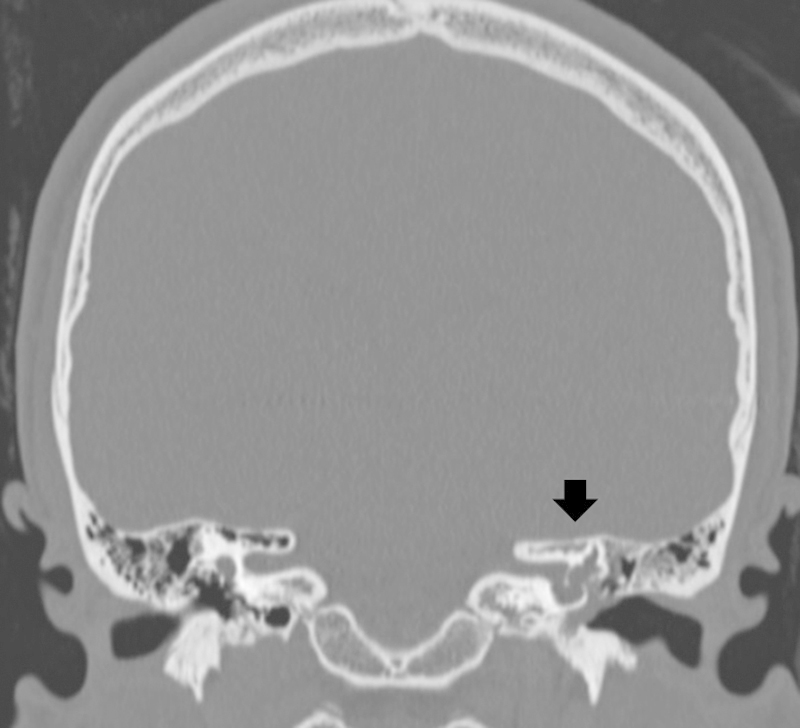
Coronal plane computed tomography showing internal auditory canal, stapes footplate, oval window, and cochlear malformations (arrow).
Fig. 2.
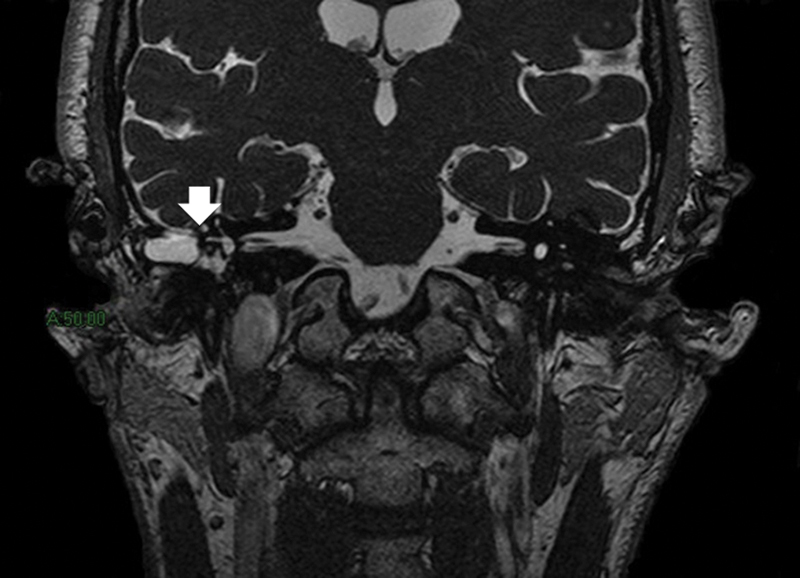
Coronal T2-weighted magnetic resonance image showing mastoid encephaloceles and break in continuity of the tegmen tympani (arrow). Note the presence of a cerebrospinal fluid leak into the epitympanic recess and tympanic antrum.
Two patients had posttraumatic CSF leakages: one had barotrauma 10 years after a successful stapedotomy, with prosthesis dislocation, enlargement of the platinectomy hole, and subsequent pneumolabyrinth; and the other had head trauma without a temporal bone fracture but with stapedo-vestibular dislocation.
The three patients with postsurgical CSF leakages were referred after surgical removal of huge vestibular schwannomas. Initially, they were treated radiosurgically with unsuccessful outcomes. Therefore, a retrosigmoid approach was used to remove the growing masses in the neurosurgery department of another hospital. In these patients, the postoperative CSF leakage was managed conservatively (bed rest, head elevation, lumbar drainage, and 500 mg acetazolamide once/day for 4 days) and surgically (retrosigmoid approach) with unsatisfactory results.
Each patient analyzed had preoperative anacusis and vestibular nerve areflexia on the affected side.
Preoperatively, the patients were counseled regarding the serious complications of this pathology. The possibility of attempting to manage the CSF leakage using a subtotal petrosectomy, as described by Fisch, to close the fistula and to prevent the risk of meningitis, was explained to the patients. With this technique, the external auditory canal is closed as a blind sac, the eustachian tube is cauterized, and the tube is obliterated with bone wax reinforced by a small piece of temporal muscle plus fibrin glue. Finally, the middle ear and mastoid space are obliterated with abdominal fat, completing the procedure.
An alternative wait-and-see policy and other surgical options were also discussed with the patients.
All of the patients who opted to undergo this surgical treatment were operated on by one of the authors (G.M.).
Each patient was evaluated using computed tomography and MRI 6 months postoperatively and then annually for 5 years. The average follow-up has been 8.5 years (range: 1–12 years).
Results
The patients with congenital or posttraumatic CSF leakage had undergone at least one endaural approach to treat the fistula at other hospitals. In two of these patients, a transmastoid (TM) approach to close the fistula with temporalis fascia was also attempted. None of these procedures resolved the CSF leakage and improved the symptoms. In one of the two patients with a previous episode of meningitis, during the second endaural approach, we decided to perform an SP immediately because of the huge volume of CSF leaking from the oval window.
The main symptoms of the patients with congenital and posttraumatic CSF leaks were severe orthostatic headaches and positional vertigo, whereas rhinorrhea was the main symptom of postsurgical CSF leakage (Tables 1–3).
All eight patients were treated successfully with SP. None of them developed postsurgical complications or subsequent meningitis.
The symptoms of the five patients with congenital and posttraumatic CSF leaks improved within 20 days and disappeared within 2 months. In the three patients with CSF leaks following the removal of a vestibular schwannoma via a retrosigmoid approach, the rhinorrhea disappeared within 10 to 21 days following the SP.
During the follow-up period, none of the patients had further postoperative symptoms or signs indicative of CSF leakage.
Discussion
The incidence of CSF leakage involving the temporal bone is not clear, although it is believed to be less common than leaks involving the anterior skull base.1 CSF leakage usually occurs in the context of traumatic temporal bone fracture or as a result of surgery.1 6 12 13
Spontaneous fluid leakage is a rare condition seen in children with congenital labyrinthine malformations (hearing-impaired children with enlargement of existing bony pathways such as the cochlear aqueduct, petromastoid canal, Hyrtl fissure, or facial canal) and in adults as a result of tegmen dehiscence or progressive middle cranial fossa bony erosion (arachnoid granulations or encephalocele).1 2 3 4 Spontaneous CSF leakage is associated with the highest rate of clinical recurrence.4 5 6 7 8 9 10 11 12 13 14 15
Posttraumatic leakage is prevalent after petrous bone fractures, whereas the most common acquired cause is the surgical excision of a vestibular schwannoma.12 16 17
Retrospective reviews revealed intraoperative CSF leaks in 42.2% of petrous bone fractures violating the otic capsule and 6 to 30% of patients after removing a vestibular schwannoma.11 12 13 18 The most common anatomical sites of fistulas are the IAC (44%)13 and mastoid air cell.11 19 Drilling the roof of the IAC can lead to a communication with the middle ear. Moreover, a dural defect > 2 cm in diameter cannot seal spontaneously.11 13 In a review, 20 of 156 patients who underwent vestibular schwannoma resections via a retrosigmoid approach had postoperative CSF leakage.19
To our knowledge, few studies have analyzed the incidence of CSF leakage in patients initially treated with radiation therapy and then by surgery for the progressive growth of a vestibular schwannoma.20 21 22 Radiotherapy can impair the dura repair mechanism, causing recurrent CSF leakage.20 21 Gerganov et al22 found no significant difference in the CSF leakage rates of patients who underwent radiation therapy before or after surgery; the only leak was in a patient who had undergone radiosurgery following subtotal tumor removal.
In our series, three patients with a giant vestibular schwannomas had CSF leakage after gamma knife failure and subsequent removal via a retrosigmoid approach. Two patients had malformations at the level of the inner ear with consequent translabyrinthine fistulas, whereas the CSF leakage in a third patient coexisted with an encephalocele. Two patients had posttraumatic CSF leakages.
Temporal bone CSF leakage is a diagnostic challenge due to the difficulty connecting the symptoms, signs, and radiologic findings.3 5 The main symptoms of patients with congenital and posttraumatic CSF leakages of our series were orthostatic headache and vertigo. None of these patients had CSF otorrhea or rhinorrhea. This is presumably due to the small amount of CSF that leaked into the middle ear cavity and to an intact tympanic membrane. By contrast, the three patients who underwent removal of a vestibular schwannoma presented with unilateral CSF rhinorrhea. The tympanic membrane was normal, and the eustachian tube provided a pathway for fluid leakage from the middle ear cavity to the nasal fossa.13 16
All patients had anacusis and complete vestibular areflexia on the affected side.
Lateral skull base CSF leakage is of great clinical interest because of the potential risk of meningitis; the incidence is 40 to 50% if not treated promptly.4 5 6 7 Some studies closely connected the risk of meningitis to the etiology (nontraumatic 30%, surgical 21%, and cranial trauma 3.5%) and the number of recurrences.3 4
Most otologists and neurosurgeons suggest initial conservative treatment for postsurgical CSF leakage.2 18 Prophylactic treatment with antibiotics is not effective at preventing meningitis in patients with CSF leakage. Consequently, surgical repair of CSF fistulas demands careful planning because of the high long-term risk of morbidity with a persistent CSF leakage.3 6 7 8 Hamilton et al10 reported that the cumulative risk of meningitis for patients managed conservatively reached 85% over an observation period of 10 years.
Various approaches for CSF leakage repair have been proposed. Many authors champion the TM approach as the initial choice for repairing posterior fossa or mastoid tegmen defects.2 5 Multiple bony defects in the tegmen might not be seen due to the limited exposure through the mastoid.23 24 Therefore, TM approaches alone are not always effective for closing the fistula, and the rate of CSF leak recurrence is not negligible.1 2 The middle cranial fossa approach is an alternative technique but is chosen less frequently because of the greater risk of morbidity associated with craniotomy and temporal lobe retraction.1 24
In a retrospective review, Savva et al6 analyzed the efficacy of these treatments in 92 patients with CSF leakage. In 65 cases using a TM approach with occasional middle fossa exposure, the successful closure rate was 79.4%, 77.7%, and 75.8% at 1, 2, and 3 years, respectively. Isolated tegmen tympani defects were repaired successfully with a TM approach in 8 of 11 patients and with a middle cranial fossa or combined approach in the other 3.5 Postoperative CSF leakage remained in one case. Falcioni et al17 proposed performing an SP when nonsurgical measures in patients treated for vestibular schwannoma were unsuccessful after 3 days.
This article presents eight cases of CSF leakage of the temporal bone associated with unilateral anacusis. All of our patients had undergone previous unsuccessful surgery via endaural or retrosigmoid approaches. Two patients had experienced episodes of meningitis that resolved with antibiotic therapy.
To manage CSF fistulas and reduce the risk of meningitis, we decided to perform a subtotal petrosectomy as described by Coker et al.9 In this technique, the external auditory canal is closed as a blind sac, the eustachian tube is cauterized and obliterated with bone wax, and reinforced using a small piece of temporal muscle plus fibrin glue. Finally, the middle ear space is obliterated with abdominal fat, completing the procedure. Therefore, the primary aim of an SP is to exclude the pneumatized portion of the temporal bone from the upper airway to reduce the long-term risk of meningitis.10 11 12 13 Unfortunately, the middle ear obliteration required with an SP results in conductive deafness of ∼ 60 dB.9 12 This side effect is the main reason that many authors do not recommend this type of surgery.5 6
There are few reports on treating CSF leakage using a SP.10 11 12 13 14 In 1986, Coker et al9 were the first to suggest an SP for closing persistent CSF leaks secondary to trauma, congenital lesions, iatrogenic injury of the dura, and early and late CSF leakage due to surgical procedures at the cerebellopontine angle. The performed SP in 13 cases of CSF leakage and no postoperative recurrence was reported. Kronenberg et al11 proposed SP with middle ear obliteration as an effective solution for a large CSF leak following surgery; four of the nine patients analyzed developed CSF leakage after suboccipital removal of a vestibular schwannoma.
Few patients with lateral skull base CSF leakage experience associated ipsilateral anacusis, which would suggest treatment with an SP. In a retrospective review, Magliulo et al12 proposed using an SP to prevent CSF leakage and the related risk of meningitis in patients with a posttraumatic CSF leak following a petrous bone fracture violating the otic capsule. It is known that these lesions cause profound hearing loss in most patients. Intraoperatively, they found obvious CSF leakage in 42.2% of the cases. None of their patients reported other episodes of CSF leakage or meningitis after the SP.
Our series included other causes of CSF leakage in a group of patients with preoperative anacusis. SP was confirmed to be an ideal choice when this audiological condition coexists. The CSF leakage was treated successfully in all of our patients. The symptoms disappeared within 2 months postoperatively, and the patients' quality of life clearly improved. No meningitis, signs of fistula, or other symptoms occurred during the follow-up.
Conclusion
A subtotal petrosectomy with obliteration of the eustachian tube and middle ear is a relatively safe method of closing temporal bone defects. The eradication of all accessible air cell tracts and mucosa in the petrous pyramid, obliteration of the eustachian tubal orifice, closure of the external auditory canal, and fat obliteration of the middle ear are essential steps in this procedure. A subtotal petrosectomy should be the first choice of treatment in patients with recurrent CSF leakage whenever there is associated unilateral anacusis.
Footnotes
Conflict of Interest/Disclaimer The authors have no conflict of interest, grant, or other fund support to disclose.
References
- 1.Pelosi S, Bederson J B, Smouha E E. Cerebrospinal fluid leaks of temporal bone origin: selection of surgical approach. Skull Base. 2010;20(4):253–259. doi: 10.1055/s-0030-1249249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown N E, Grundfast K M, Jabre A, Megerian C A, O'Malley B W Jr, Rosenberg S I. Diagnosis and management of spontaneous cerebrospinal fluid-middle ear effusion and otorrhea. Laryngoscope. 2004;114(5):800–805. doi: 10.1097/00005537-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 3.De Paula Vernetta C Ramírez Sabio J B García Callejo J Serrano Carañana M N Marco Algarra J Cerebrospinal fluid (CSF) leaks in the ear: revision of 5 cases [in Spanish]. Acta Otorrinolaringol Esp 2005566273–279. [DOI] [PubMed] [Google Scholar]
- 4.Toh A, De R. Spontaneous cerebrospinal fluid otorrhoea presenting as otitis externa. Eur Arch Otorhinolaryngol. 2007;264(6):689–691. doi: 10.1007/s00405-006-0235-3. [DOI] [PubMed] [Google Scholar]
- 5.Oliaei S, Mahboubi H, Djalilian H R. Transmastoid approach to temporal bone cerebrospinal fluid leaks. Am J Otolaryngol. 2012;33(5):556–561. doi: 10.1016/j.amjoto.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Savva A, Taylor M J, Beatty C W. Management of cerebrospinal fluid leaks involving the temporal bone: report on 92 patients. Laryngoscope. 2003;113(1):50–56. doi: 10.1097/00005537-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 7.MacGee E E, Cauthen J C, Brackett C E. Meningitis following acute traumatic cerebrospinal fluid fistula. J Neurosurg. 1970;33(3):312–316. doi: 10.3171/jns.1970.33.3.0312. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson B J, Wilkins R H, Hudson W, Farmer J Jr. Spontaneous CSF otorrhea from tegmen and posterior fossa defects. Laryngoscope. 1986;96(6):635–644. doi: 10.1288/00005537-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Coker N J, Jenkins H A, Fisch U. Obliteration of the middle ear and mastoid cleft in subtotal petrosectomy: indications, technique, and results. Ann Otol Rhinol Laryngol. 1986;95(1 Pt 1):5–11. doi: 10.1177/000348948609500102. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton J W, Foy P M, Lesser T H. Subtotal petrosectomy in the treatment of cerebrospinal fluid fistulae of the lateral skull base. Br J Neurosurg. 1997;11(6):496–500. doi: 10.1080/02688699745646. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg J, Findler G, Braham J. Cerebrospinal fluid otorrhea treated by extended subtotal petrosectomy with obliteration. Skull Base Surg. 1991;1(3):168–170. doi: 10.1055/s-2008-1057001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magliulo G, Ciniglio Appiani M, Iannella G, Artico M. Petrous bone fractures violating otic capsule. Otol Neurotol. 2012;33(9):1558–1561. doi: 10.1097/MAO.0b013e31826bf135. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg J, Bendet E, Findler G, Roth Y. Cerebrospinal fluid (CSF) otorhinorrhoea following vestibular schwannoma surgery treated by extended subtotal petrosectomy with obliteration. J Laryngol Otol. 1993;107(12):1122–1124. doi: 10.1017/s0022215100125459. [DOI] [PubMed] [Google Scholar]
- 14.Teo D T, Tan T Y, Eng S P, Chan Y M. Spontaneous cerebrospinal fluid otorrhoea via oval window: an obscure cause of recurrent meningitis. J Laryngol Otol. 2004;118(9):717–720. doi: 10.1258/0022215042244804. [DOI] [PubMed] [Google Scholar]
- 15.Kutz J W Jr, Husain I A, Isaacson B, Roland P S. Management of spontaneous cerebrospinal fluid otorrhea. Laryngoscope. 2008;118(12):2195–2199. doi: 10.1097/MLG.0b013e318182f833. [DOI] [PubMed] [Google Scholar]
- 16.Magliulo G, Sepe C, Varacalli S, Fusconi M. Cerebrospinal fluid leak management following cerebellopontine angle surgery. J Otolaryngol. 1998;27(5):258–262. [PubMed] [Google Scholar]
- 17.Falcioni M, Romano G, Aggarwal N, Sanna M. Cerebrospinal fluid leak after retrosigmoid excision of vestibular schwannomas. Otol Neurotol. 2008;29(3):384–386. doi: 10.1097/MAO.0b013e31816021e3. [DOI] [PubMed] [Google Scholar]
- 18.Fishman A J, Marrinan M S, Golfinos J G, Cohen N L, Roland J T Jr. Prevention and management of cerebrospinal fluid leak following vestibular schwannoma surgery. Laryngoscope. 2004;114(3):501–505. doi: 10.1097/00005537-200403000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Nutik S L Korol H W Cerebrospinal fluid leak after acoustic neuroma surgery Surg Neurol 1995436553–556.; discussion 556–557 [DOI] [PubMed] [Google Scholar]
- 20.Shuto T Inomori S Matsunaga S Fujino H Microsurgery for vestibular schwannoma after gamma knife radiosurgery Acta Neurochir (Wien) 20081503229–234.; discussion 234 [DOI] [PubMed] [Google Scholar]
- 21.Iwai Y Yamanaka K Yamagata K Yasui T Surgery after radiosurgery for acoustic neuromas: surgical strategy and histological findings Neurosurgery 200760(2, Suppl 1): ONS75–ONS82; discussion ONS82 [DOI] [PubMed] [Google Scholar]
- 22.Gerganov V M, Giordano M, Samii A, Samii M. Surgical treatment of patients with vestibular schwannomas after failed previous radiosurgery. J Neurosurg. 2012;116(4):713–720. doi: 10.3171/2011.12.JNS111682. [DOI] [PubMed] [Google Scholar]
- 23.Charalampakis S, Koutsimpelas D, Gouveris H, Mann W. Post-operative complications after removal of sporadic vestibular schwannoma via retrosigmoid-suboccipital approach: current diagnosis and management. Eur Arch Otorhinolaryngol. 2011;268(5):653–660. doi: 10.1007/s00405-010-1480-z. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal A, Baisakhiya N, Deshmukh P T. Combined middle cranial fossa and trans-mastoid approach for the management of post-mastoidectomy CSF otorrhoea. Indian J Otolaryngol Head Neck Surg. 2011;63 01:142–146. doi: 10.1007/s12070-011-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


