Abstract
Purpose:
The purpose of this study was to compare the impact of continuous and pulsed energy deliveries on microwave ablation growth and shape in unperfused and perfused liver models.
Methods:
A total of 15 kJ at 2.45 GHz was applied to ex vivo bovine liver using one of five delivery methods (n = 50 total, 10 per group): 25 W continuous for 10 min (25 W average), 50 W continuous for 5 min (50 W average), 100 W continuous for 2.5 min (100 W average), 100 W pulsed for 10 min (25 W average), and 100 W pulsed for 5 min (50 W average). A total of 30 kJ was applied to in vivo porcine livers (n = 35, 7 per group) using delivery methods similar to the ex vivo study, but with twice the total ablation time to offset heat loss to blood perfusion. Temperatures were monitored 5–20 mm from the ablation antenna, with values over 60 °C indicating acute cellular necrosis. Comparisons of ablation size and shape were made between experimental groups based on total energy delivery, average power applied, and peak power using ANOVA with post-hoc pairwise tests.
Results:
No significant differences were noted in ablation sizes or circularities between pulsed and continuous groups in ex vivo tissue. Temperature data demonstrated more rapid heating in pulsed ablations, suggesting that pulsing may overcome blood perfusion and coagulate tissues more rapidly in vivo. Differences in ablation size and shape were noted in vivo despite equivalent energy delivery among all groups. Overall, the largest ablation volume in vivo was produced with 100 W continuous for 5 min (265.7 ± 208.1 cm3). At 25 W average, pulsed-power ablation volumes were larger than continuous-power ablations (67.4 ± 34.5 cm3 versus 23.6 ± 26.5 cm3, P = 0.43). Similarly, pulsed ablations produced significantly greater length (P ≤ 0.01), with increase in diameter (P = 0.09) and a slight decrease in circularity (P = 0.97). When comparing 50 W average power groups, moderate differences in size were noted (P ≥ 0.06) and pulsed ablations were again slightly more circular.
Conclusions:
Pulsed energy delivery created larger ablation zones at low average power compared to continuous energy delivery in the presence of blood perfusion. Shorter duty cycles appear to provide greater benefit when pulsing.
Keywords: microwave ablation, pulsed power, continuous power, temperature monitor
1. INTRODUCTION
Radiofrequency (RF) and microwave ablation are alternative treatment options for patients with hepatocellular carcinoma (HCC) and colorectal liver metastasis, who are not candidates for surgery.1–6 Early studies have demonstrated the potential efficacy and safety of ablative modalities; however, some studies have noted high local tumor recurrence rates, especially for tumors greater than 3 cm in diameter.1–3,5–7 In addition, RF ablations are susceptible to the “heat-sink” effect, where incomplete tumor heating may occur from the cooling effect of nearby vessels.8–11 Microwave energy delivery may reduce the heat-sink effect of local blood flow and be able to create larger ablation zones that can provide a more complete ablative margin.8–13
Despite encouraging early results with microwave energy, the techniques for energy delivery continue to evolve. Existing systems utilize continuous energy delivery or modulate delivery based on antenna temperature.14 These techniques rely on a mixture of direct microwave heating and passive heat transfer via conductive and convective mechanisms. However, maximizing the contribution from direct heating by increasing peak power delivery may help to overcome heat sinks and provide greater control over the ablation zone geometry.15–19 Previous studies have demonstrated that pulsing a high peak power can create larger and more circular ablations than continuous delivery of a lower continuous power, despite equivalent total energy delivery.15–17 Those studies were limited to a single energy and power, were performed in kidneys, and had a small sample size for comparison. The aim of this study was to evaluate the effect of continuous power and pulsed power on ablation zone size and shape during microwave ablation in both unperfused and perfused liver models. We hypothesize that a greater peak power may overcome blood perfusion more effectively than a lower peak power.
2. MATERIALS AND METHODS
The study was performed in two different liver tissue models: no blood flow (ex vivo) and normal blood flow (in vivo). Both models were evaluated to cover the range of physiological conditions in humans with liver pathologies such as compromised blood flow from cirrhosis, hypovascular tumors, and normal perfusion.13,20–22
2.A. Ex vivo liver
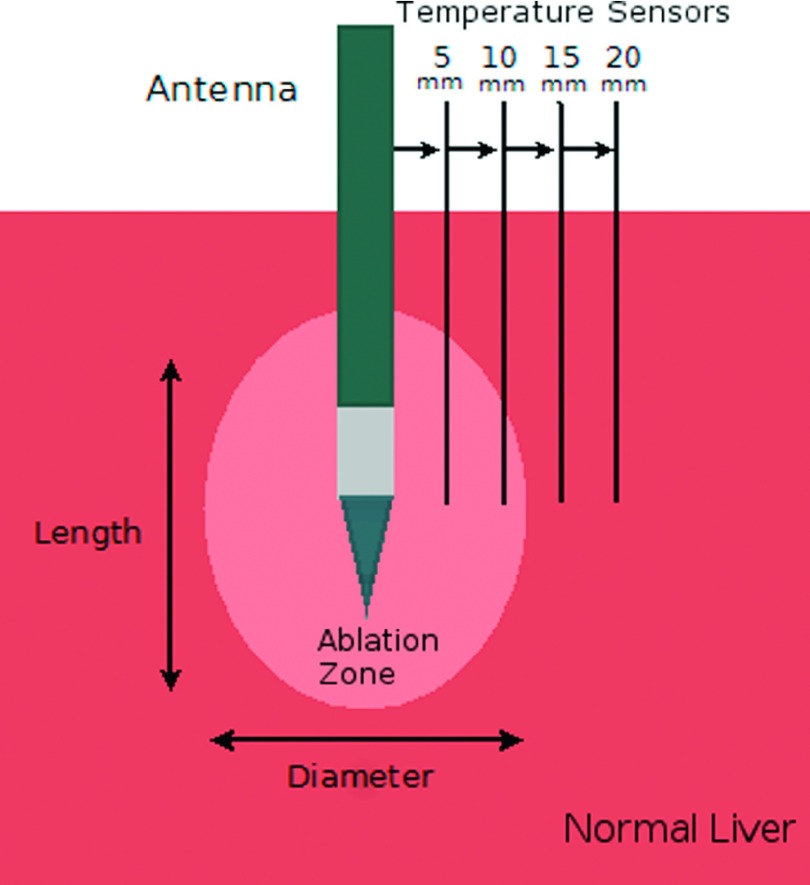
Microwave ablation experiments were performed in ex vivo bovine livers obtained from an abattoir. The tissue was cut into approximately 10 × 10 × 5 cm blocks at room temperature, avoiding major vessels whenever possible. An uncooled monopole antenna constructed from a 17-gauge coaxial cable and a 2 cm radiating segment was inserted 5 cm into each tissue block. Microwave energy was then delivered through a commercially available 2.45 GHz source (MG300, CouberMuegge, Norwalk, CT). A total of 15 kJ were applied to the ex vivo liver samples (n = 50 total) using one of five delivery cycles: 25 W for 10 min (n = 10), 50 W for 5 min (n = 10), 100 W for 2.5 min (n = 10), 100 W pulsed 30 s on and 90 s off (25% duty) over 10 min (n = 10), and 100 W pulsed 50 s on and 50 s off (50% duty) over 5 min (n = 10; Fig. 1 and Table I). These peak powers are commensurate with current clinical systems. Tissue temperature was monitored during three ablations in each group by four fiber-optic temperature probes (Neoptix, Inc., Quebec, Canada) 5, 10, 15, and 20 mm away from the radiating segment of the antenna (Fig. 2). Temperatures were recorded every second. Temperature profiles and time to necrosis were plotted against time to informally assess differences between pulsed and power groups. The time to achieve a lethal increase in temperature was determined based on the difference between the average body temperature of 37 °C and the temperature necessary for acute cellular necrosis 60 °C (23 °C).23–25 Tissue desiccation was also assessed informally based on a brown appearance in the white inner zone of coagulation.
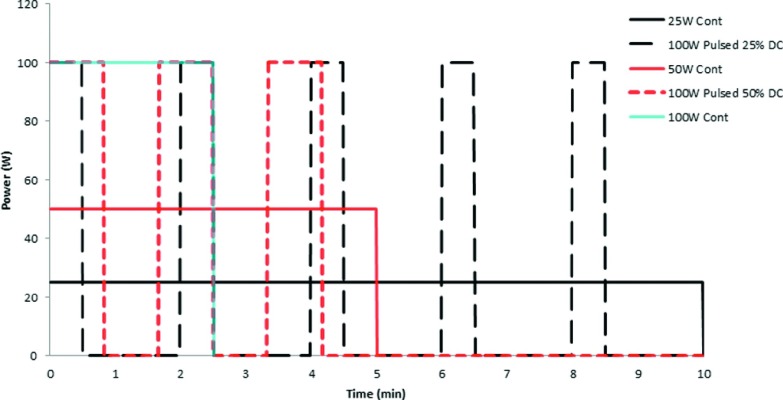
FIG. 1.
Experimental power delivery protocols, each delivering a total of 15 kJ: 25 W continuous for 10 min (solid black), 100 W pulsed 30 s on and 90 s off for 10 min (dashed black), 50 W for 5 min (solid red or dark gray), 100 W pulsed 50 s on and 50 s off (dotted red or dark gray) and 100 W continuous for 2.5 min (solid blue or light gray).
TABLE I.
Experimental groups for both ex vivo and in vivo studies.
| Group | Input power (W) | Time on (s) | Time off (s) | Duty (%) | Total time (min) | Average power (W) | Total energy (kJ) |
|---|---|---|---|---|---|---|---|
| Ex vivo | |||||||
| 1 | 25 | 600 | — | 100 | 10 | 25 | 15 |
| 2 | 100 | 30 | 90 | 25 | 10 | 25 | 15 |
| 3 | 50 | 300 | — | 100 | 5 | 50 | 15 |
| 4 | 100 | 50 | 50 | 50 | 5 | 50 | 15 |
| 5 | 100 | 150 | — | 100 | 2.5 | 100 | 15 |
| In vivo | |||||||
| 1 | 25 | 1200 | — | 100 | 20 | 25 | 30 |
| 2 | 100 | 30 | 90 | 25 | 20 | 25 | 30 |
| 3 | 50 | 600 | — | 100 | 10 | 50 | 30 |
| 4 | 100 | 75 | 75 | 50 | 10 | 50 | 30 |
| 5 | 100 | 300 | — | 100 | 5 | 100 | 30 |
FIG. 2.
Ex vivo experimental setup. Fiber-optic temperature sensors were placed 5–20 mm from the antenna. Ablation zone length and diameter were measured along and transverse to the antenna insertion tract, respectively.
After each ablation was completed, the tissue was sliced along the antenna tract, revealing a cross section of the ablation zone that was optically scanned and saved digitally for analysis. The ablation zone transverse diameter and length were measured using ImageJ (v1.46r, U.S. National Institutes of Health, Bethesda, MD). The ablation aspect ratio was calculated as the diameter divided by the length. An ablation volume was estimated based on the cross-sectional ablation diameter and length (4/3 π⋅diameter2⋅length).
2.B. In vivo liver
All animal care and procedures performed in this study were approved by our Institutional Animal Care and Use Committee and complied with National Research Council guidelines.26
Microwave ablations were performed in six female domestic swine (mean 45 kg; Arlington Farms, Arlington, WI). Each animal was sedated with 7 mg/kg of intramuscularly tiletamine hydrochloride (Telazol; Wyeth, Fort Dodge, IA) and 2.2 mg/kg of xylazine hydrochloride (Xyla-Ject; Phoenix Pharmaceutical, St Joseph, MO), then intubated. The anesthesia was maintained with 2% inhaled isoflurane (Halocarbon Laboratories, River Edge, NJ). Lactated ringers solution was administered intravenously throughout the procedure. The liver was then surgically exposed to enable placement of the microwave antenna.
A total of 30 kJ was applied to swine livers in vivo (n = 35) using five delivery methods, similar to the ex vivo study: 25 W for 20 min (n = 7), 50 W for 10 min (n = 7), 100 W for 5 min (n = 7), 100 W pulsed 30 s on and 90 s off (25% duty) over 20 min (n = 7), and 100 W pulsed 75 s on/75 s off (50% duty) over 10 min (n = 7). Total energy was increased compared to ex vivo experiments to offset heat loss due to blood perfusion. To minimize bias, swine liver lobes were randomly assigned to the five experimental groups. All ablations were performed using a clinical microwave ablation system (Certus 140; Neuwave Medical, Madison, WI) and a gas-cooled triaxial antenna (Certus 140 LK; Neuwave Medical).
After ablations were completed, animals were euthanized with an intravenous injection of 0.2 ml/kg pentobarbital sodium 390 mg/ml and phenytoin sodium 50 mg/ml (Beuthanasia-D; Schering-Plough, Kenilworth, NJ). The liver was then removed and sliced along the antenna insertion tract, where the cross section of the ablation zone was scanned as described for ex vivo experiments.
2.C. Statistical analysis
The mean and standard deviation (SD) of each metric (length, diameter, aspect ratio, and volume) were calculated and are reported as mean ±1 SD. We standardized the comparison of groups between the ex vivo and in vivo datasets. A one-way fixed effect ANOVA was used to assess differences between experimental groups within each dataset for each response. Direct comparisons of pulsed and continuous energies were performed between groups with equivalent average power using a Holm–Sidak post hoc test. P-values less than 0.05 were considered significant. There was no adjustment of p-values for multiplicity. All analyses were performed using GraphPad Prism v6.05.27
3. RESULTS
3.A. Ex vivo liver
No significant differences were found in ablation diameter, length, aspect ratio, or volume among groups in ex vivo liver (ANOVA P ≥ 0.07). Pulsed-power groups tended to have slightly smaller and more elongated ablations compared to continuous-power groups. Direct comparison between groups with equivalent average power showed that 25 W continuous created significantly greater aspect ratio (0.6 ± 0.0 versus 0.5 ± 0.1, P = 0.03) compared to 100 W pulsed at a 25% duty cycle. Differences in length, diameter, aspect ratio, and volume were not significant between 50 W average power groups.
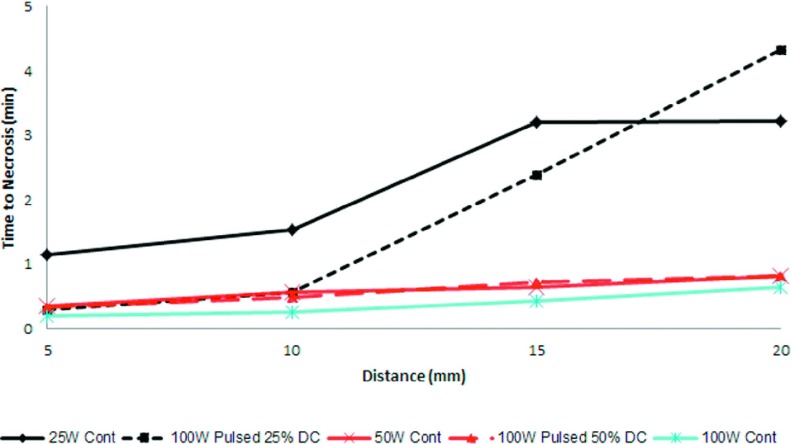
Temperatures collected during ex vivo ablations illustrated different growth patterns in each group (Fig. 3). Overall, the greatest temperatures were achieved nearest to the antenna, with successively lower temperatures at more distant radial locations as expected. Pulsed delivery provided more rapid heating and achieved higher temperatures than continuous heating for equivalent average power (Fig. 3). As a result, the time to achieve a lethal increase in temperature was shorter in the pulsed groups (Fig. 4). For instance, at 10 mm from the antenna, 100 W achieved the lethal temperature in 35 s, compared to 1 min 32 s for 25 W.
FIG. 3.
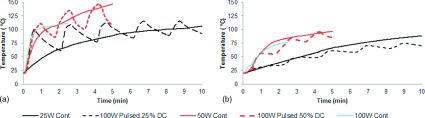
Temperature profile over time at two different distances: (a) 5 mm from the antenna shaft: pulsed delivery of higher peak power created faster heating. While power was on, pulsed ablations had greater heating rates at all points than the continuous ablation. (b) 20 mm from the antenna shaft: the heat rate decreases with distance. The heating profile was similar for both pulsed and continuous groups.
FIG. 4.
Expected time required to reach a temperature increase and achieve necrosis during ablation with respect to distance from the antenna shaft. Distances less than 15 mm, pulsed power reached the lethal threshold temperature faster than continuous power with an average power of 25 W. As average power increases these differences diminished.
3.B. In vivo liver
In contrast to ex vivo results, differences were found between power delivery groups for diameter, length, and volume in vivo (ANOVA P ≤ 0.001 in length, diameter, and volume). The largest ablation volumes were produced with 100 W continuous-power delivery (265.7 ± 208.1 cm3), while the smallest ablations were produced with 25 W continuous-power delivery (23.7 ± 26.5 cm3). There was no significant difference between aspect ratios among groups (ANOVA, P = 0.62). The most spherical ablation was created with 50 W pulsed (0.7 ± 0.1) while the most elongated ablation was found with 25 W pulsed at 25% duty (0.6 ± 0.1; Table II).
TABLE II.
Experimental results.
| Group | Pulsed | Average power (W) | Length (cm) | P-value | Diameter (cm) | P-value | Aspect ratio | P-value | Volume (cm3) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Ex vivo | ||||||||||
| A | No | 25 | 5.5 ± 0.5 | 0.84 | 3.3 ± 0.3 | 0.12 | 0.6 ± 0.0a | 0.03 | 246.8 ± 63.3 | 0.46 |
| B | Yes | 25 | 5.6 ± 0.5 | 2.9 ± 0.2 | 0.5 ± 0.1 | 203.1 ± 31.0 | ||||
| C | No | 50 | 5.5 ± 0.4 | 0.84 | 3.1 ± 0.4 | 0.84 | 0.6 ± 0.0 | 0.58 | 224.1 ± 65.8 | 0.80 |
| D | Yes | 50 | 5.6 ± 0.5 | 3.0 ± 0.2 | 0.5 ± 0.1a | 214.7 ± 32.4 | ||||
| E | No | 100 | 5.8 ± 0.8 | 3.3 ± 0.4 | 0.6 ± 0.1 | 267.5 ± 90.7 | ||||
| In vivo | ||||||||||
| A | No | 25 | 2.3 ± 0.7b | 0.01 | 1.4 ± 0.5a | 0.09 | 0.6 ± 0.1 | 0.97 | 23.6 ± 26.5c | 0.43 |
| B | Yes | 25 | 3.5 ± 0.6 | 2.1 ± 0.4 | 0.6 ± 0.1 | 67.6 ± 34.5 | ||||
| C | No | 50 | 3.8 ± 0.9b | 0.06 | 2.4 ± 0.7a | 0.09 | 0.6 ± 0.1 | 0.97 | 105.4 ± 78.3 | 0.38 |
| D | Yes | 50 | 4.6 ± 0.6 | 3.0 ± 0.4 | 0.7 ± 0.1 | 176.7 ± 45.9 | ||||
| E | No | 100 | 5.2 ± 0.8b | 3.3 ± 0.9a | 0.6 ± 0.1 | 265.7 ± 208.1c | ||||
P < 0.03 for the difference in diameter obtained in vivo between A–C, A–D, A–E, B–D, and B–E.
P < 0.01 for the difference in length obtained in vivo between groups A–C, A–E, and C–E.
P < 0.03 for the difference in volume obtained in vivo between A–E and C–E.
Notable differences were observed between continuous and pulsed deliveries of equivalent average powers. For 25 W average power, pulsed delivery created a significantly longer ablation (3.5 ± 0.6 cm versus 2.3 ± 0.7 cm, P = 0.02) when compared to continuous delivery. Pulsed delivery also produced a larger ablation diameter (2.1 ± 0.4 cm versus 1.4 ± 0.5 cm, P = 0.09) and volume (67.4 ± 34.5 cm3 versus 23.6 ± 26.5 cm3, P = 0.43) than continuous power. No difference in aspect ratio was noted between 25 W average power groups. A similar trend of greater diameter, length, and volume was observed between pulsed and continuous deliveries of 50 W average power, but comparisons failed to meet the criteria for statistical significance.
Qualitative differences in tissue desiccation were noted within the ablation zone (Fig. 5). The 25 W continuous-power group showed little desiccation inside the ablation zone while 100 W pulsed at 25% duty (25 W average) showed greater tissue desiccation and necrosis. Substantial charring was noted in 100 W continuous ablations.
FIG. 5.
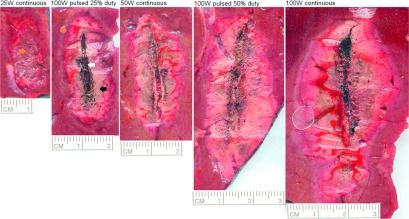
Cross-sectional area of the ablation zones from each experimental group in vivo. Different stages of tissue desiccation were observed within the ablation zone: little desiccation (orange or dark gray arrow), more complete desiccation or tissue necrosis (black arrow), and charring (white arrow).
4. DISCUSSION
This study was designed to determine the efficacy of pulsed- and continuous-power deliveries on ablation size and shape. Both ex vivo and in vivo models were used to evaluate a range of perfusion rates encountered in clinical settings. The ex vivo results showed no practical difference between ablation zone geometries after pulsed- and continuous-power deliveries. However, the lack of differences identified ex vivo tissue was not surprising. In tissue without a heat sink from parenchymal blood perfusion, equivalent energy delivery should produce similar ablation sizes. Differences between groups may actually have been more attributable to differences in peak temperature and total heating time, which are hypothesized to impact vapor generation and associated heat-mass transfer, as well as tissue contraction.28 In vivo testing seems most appropriate for future studies of pulsed-power delivery.
More substantial differences were identified in the in vivo study. Pulsed delivery ablations were characterized by greater length and diameter and slightly more elongation compared to continuous delivery ablations with similar average power. The effect of pulsing also appeared to diminish as average power increased from 25 to 50 W. In fact, the largest ablations were created when using the highest average power (100 W) and shortest ablation time (2.5 min). These results are consistent with the hypothesis that greater peak power may more effectively overcome blood perfusion. For instance, 25 W seemed unable to generate heat fast enough to overcome heat dissipation due to blood perfusion, while 100 W pulsed or continuous coagulated a greater volume of tissue and created a larger ablation zone. Similarly, 50 W continuous ablations were more able to overcome perfusion when compared to 25 W continuous. At the 25–50 W average power levels, peak power had a greater impact on ablation size than total energy delivery.
The heating and cooling time associated with each individual energy pulse had an effect on the ablation zone size and shape. A 25% duty cycle had shorter heating time and longer cooling time than a 50% duty cycle. As a result, the 25% duty cycle groups produced smaller ablations than the 50% duty cycle groups (volume 6% smaller ex vivo, 162% smaller in vivo). Lower duty cycle also created slightly more elongated ablations (aspect ratio 4% lower ex vivo, 14% lower in vivo). The shorter heating time may have minimized radial growth and instead allowed heat dissipation during the longer cooling time period. Higher powers might have also affected the ablation zone shape by causing greater desiccation. Ji and Brace demonstrated that changes in the dielectric properties of tissue exposed to high temperatures lead to changes in ablation growth, including elongation of the heating pattern.29 The greater desiccation and slight elongation noted in high-power ablations in this study are consistent with those previous results.
The ablation zones in this study were commensurate with those of previous studies using similar energy delivery.16,30,31 Brace et al.15 demonstrated that pulsed delivery created larger and more circular ablations compared to continuous delivery of 200 W in porcine kidney in vivo. However, that study utilized a relatively high 67% duty cycle and interpretation of the results was complicated by uncooled applicators and inconsistency in the power generation system. Chiang et al.32 also reported microwave ablation at similar energy levels. In that ex vivo bovine liver study using 50 W for 5 min and 100 W for 2 min, the ablation diameter (2.32 ± 0.18 cm and 1.86 ± 0.24 cm, respectively) and length (3.83 ± 0.92 cm and 4.44 ± 0.44 cm) produced more elongated shapes as the power increased. Hines-Peralta et al. also compared the differences of ex vivo and in vivo ablation with different applied microwave powers and found that higher peak powers created larger lesions, and ex vivo ablations were larger than in vivo ablations.30
Treatment of larger tumors remains a clinical challenge. When the thermal dose does not overcome the temperature threshold for tissue necrosis, there is a higher risk of leaving viable tumor cell untreated.25,33 Incomplete or inadequate ablation margins are known predictors of local tumor recurrence.34 Therefore, ablating an adequate tumor margin in addition of delivering the appropriate thermal dose is critical to eliminate changes of recurrence after an ablation treatment. In clinical practice, there is a wide variety of power delivery protocols depending on the microwave ablation system (maximum powers of 32–180 W), institution, or physician preferences.3,35–37 The results of this study suggest that in most clinical settings, but particularly those with normal blood perfusion, delivering low powers for long periods of time is suboptimal for creating sufficient thermal coagulation necrosis. Higher peak powers were more effective at treating large ablation volumes, whether delivered via pulsing or during a shorter overall ablation time.
Additional optimization of pulsing protocols may yield more effective ablations. Previous studies have used pulsed-power delivery more as a tool to minimize system drawbacks such as tissue charring, which can decrease the efficacy of the applicator.38,39 In the case of radiofrequency ablation, some systems utilize pulsed power, while others have explored ramped or low-power delivery with temperature control to offset the increased impedance associated with rapid temperature elevations and tissue charring.39,40 Solazzo et al. found optimal “on” and “off” times of 21–35 s and 22–38 s, respectively, depending on the electrode type and size.39 Due to the charring limitations during RF ablation, power output algorithms are utilized to maintain tissue conductance. This is not as relevant during microwave ablation. In addition, it has been demonstrated that microwave ablation is less affected by changes in blood perfusion than other ablation modalities such as RF ablation.22 Our results suggest that pulsed microwave power may also be an effective approach to creating larger ablation volumes in perfused tissue.
5. CONCLUSION
This study compared the effects of continuous and pulsed energy deliveries during microwave ablation in ex vivo and in vivo models. While no differences in ablation size were noted in the ex vivo model, significantly larger and slightly more elongated ablations were created when using pulsed-power delivery in the in vivo model. The potential benefits of pulsing diminished with increased average power. Pulsing was most effective at a duty cycle less than 50%. Further investigation is needed to optimize pulsed energy delivery parameters.
ACKNOWLEDGMENTS
The authors thank Lisa Sampson, BS, for her assistance in the experimental setup. This work was supported by the National Institutes of Health 1R01CA14273701 and the National Institute of General Medical Sciences of the National Institutes of Health R25GM085232. The author, Christopher Brace, Ph.D., also wishes to disclose potential conflicts of interest: paid consultant, patent author, and shareholder with NeuWave Medical, Inc., Madison, WI.
REFERENCES
- 1.Boutros C., Somasundar P., Garrean S., Saied A., and Espat N. J., “Microwave coagulation therapy for hepatic tumors: Review of the literature and critical analysis,” Surg. Oncol. 19(1), e22–e32 (2010). 10.1016/j.suronc.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Groeschl R. T.et al. , “Recurrence after microwave ablation of liver malignancies: A single institution experience,” HPB 15(5), 365–371 (2013). 10.1111/j.1477-2574.2012.00585.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H.-X.et al. , “Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: A survival analysis of 137 consecutive patients,” Clin. Radiol. 60(9), 1018–1025 (2005). 10.1016/j.crad.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Lloyd D. M.et al. , “International multicentre prospective study on microwave ablation of liver tumours: Preliminary results,” HPB (Oxford) 13, 579–585 (2011). 10.1111/j.1477-2574.2011.00338.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M.-D.et al. , “Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study,” J. Gastroenterol. 40(11), 1054–1060 (2005). 10.1007/s00535-005-1671-3 [DOI] [PubMed] [Google Scholar]
- 6.Izumi N.et al. , “Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation,” Cancer 91(5), 949–956 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Seifert J. K. and Morris D. L., “Indicators of recurrence following cryotherapy for hepatic metastases from colorectal cancer,” Br. J. Surg. 86(2), 234–240 (1999). 10.1046/j.1365-2168.1999.00995.x [DOI] [PubMed] [Google Scholar]
- 8.Brace C. L., “Microwave tissue ablation: Biophysics, technology and applications,” Crit. Rev. Biomed. Eng. 38(1), 65–78 (2010). 10.1615/CritRevBiomedEng.v38.i1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brace C. L., “Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences?,” Curr. Prob. Diagn. Radiol. 38(3), 135–143 (2009). 10.1067/j.cpradiol.2007.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schramm W., Yang D., and Haemmerich D., “Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation,” Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 5013–5016 (2006). 10.1109/IEMBS.2006.259288 [DOI] [PubMed] [Google Scholar]
- 11.Wright A. S., Sampson L. A., Warner T. F., Mahvi D. M., and Lee F. T. J., “Radiofrequency versus microwave ablation in a hepatic porcine model,” Radiology 236(1), 132–139 (2005). 10.1148/radiol.2361031249 [DOI] [PubMed] [Google Scholar]
- 12.Yu N. C., Raman S. S., Kim Y. J., Lassman C., Chang X., and Lu D. S. K., “Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model,” J. Vasc. Interv. Radiol. 19(7), 1087–1092 (2008). 10.1016/j.jvir.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 13.Chiang J., Hynes K., and Brace C. L., “Flow-dependent vascular heat transfer during microwave thermal ablation,” in 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (2012), pp. 5582–5585 10.1109/EMBC.2012.6347259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer C. M.et al. , “Microwave ablation of porcine kidneys in vivo: Effect of two different ablation modes (‘temperature control’ and ‘power control’) on procedural outcome,” Cardiovasc. Intervent. Radiol. 35(3), 653–660 (2012). 10.1007/s00270-011-0171-5 [DOI] [PubMed] [Google Scholar]
- 15.Brace C., Andreano A., Laeseke P. F., Hinshaw J. L., and F. T. Lee, Jr., “Microwave kidney ablation: High power pulsing creates larger ablations compared to low continuous power,” in Radiological Society of North America Annual Meeting (RSNA), Chicago, IL(2009). [Google Scholar]
- 16.Andreano A., Huang Y., Meloni M. F., F. T. Lee, Jr., and Brace C. L., “Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue,” Med. Phys. 37(6), 2967–2973 (2010). 10.1118/1.3432569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer C. M.et al. , “Microwave ablation in porcine livers applying 5-minute protocols: Influence of deployed energy on extent and shape of coagulation,” J. Vasc. Interv. Radiol. 23(12), 1692–1699 (2012). 10.1016/j.jvir.2012.08.029 [DOI] [PubMed] [Google Scholar]
- 18.Andreano A. and Brace C. L., “A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver,” Cardiovasc. Intervent. Radiol. 36(2), 505–511 (2013). 10.1007/s00270-012-0405-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubner M. G., Hinshaw J. L., Andreano A., Sampson L., F. T. Lee, Jr., and Brace C. L., “High-powered microwave ablation with a small-gauge, gas-cooled antenna: Initial ex vivo and in vivo results,” J. Vasc. Interv. Radiol. 23(3), 405–411 (2012). 10.1016/j.jvir.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg S., Meloni F., Torosatti N., Nissenbaum I., Appelbaum L., and Agnello L., “Parameter characterization for microwave tumor ablation: Comparison of experimental and HCC data,” in World Conference on Interventional Oncology, New York, NY(2011). [Google Scholar]
- 21.Orsi M. D.et al. , “In vitro blood-perfused bovine liver model a physiologic model for evaluation of the performance of radiofrequency ablation devices,” J. Vasc. Interv. Radiol. 22(10), 1478–1483 (2011). 10.1016/j.jvir.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 22.Dodd G. D., Dodd N. A., Lanctot A. C., and Glueck D. A., “Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model,” Radiology 267(1), 129–136 (2013). 10.1148/radiol.12120486 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg S. N., Gazelle G. S., and Mueller P. R., “Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance,” Am. J. Roentgenol. 174(2), 323–331 (2000). 10.2214/ajr.174.2.1740323 [DOI] [PubMed] [Google Scholar]
- 24.Knavel E. M. and Brace C. L., “Tumor ablation: Common modalities and general practices,” Tech. Vasc. Interv. Radiol. 16(4), 192–200 (2013). 10.1053/j.tvir.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapareto S. A. and Dewey W. C., “Thermal dose determination in cancer therapy,” Int. J. Radiat. Oncol., Biol., Phys. 10(6), 787–800 (1984). 10.1016/0360-3016(84)90379-1 [DOI] [PubMed] [Google Scholar]
- 26.National Research Council, Guide for the Care and Use of Laboratory Animals, 8th ed. (National Academies Press, Washington, DC, 2011). [Google Scholar]
- 27.Prism G. P., GraphPad prism version 6.00 for Windows, GraphPad Software, La Jolla California, 1992.
- 28.Brace C. L., Diaz T. A., Hinshaw J. L., and Lee F. T., “Tissue contraction caused by radiofrequency and microwave ablation: A laboratory study in liver and lung,” J. Vasc. Interv. Radiol. 21(8), 1280–1286 (2010). 10.1016/j.jvir.2010.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Z. and Brace C. L., “Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation,” Phys. Med. Biol. 56(16), 5249–5264 (2011). 10.1088/0031-9155/56/16/011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hines-Peralta A. U.et al. , “Microwave ablation: Results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver,” Radiology 239(1), 94–102 (2006). 10.1148/radiol.2383050262 [DOI] [PubMed] [Google Scholar]
- 31.Strickland A. D.et al. , “Experimental study of large-volume microwave ablation in the liver,” Br. J. Surg. 89(8), 1003–1007 (2002). 10.1046/j.1365-2168.2002.02155.x [DOI] [PubMed] [Google Scholar]
- 32.Chiang J., Hynes K. A., Bedoya M., and Brace C. L., “A dual-slot microwave antenna for more spherical ablation zones: Ex vivo and in vivo validation,” Radiology 268(2), 382–389 (2013). 10.1148/radiol.13122128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhardwaj N., Strickland A. D., Ahmad F., Atanesyan L., West K., and Lloyd D. M., “A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver,” Pathology 41(2), 168–172 (2009). 10.1080/00313020802579292 [DOI] [PubMed] [Google Scholar]
- 34.Livraghi T., Meloni F., Solbiati L., and Zanus G., “Complications of microwave ablation for liver tumors: Results of a multicenter study,” Cardiovasc. Intervent. Radiol. 35(4), 868–874 (2012). 10.1007/s00270-011-0241-8 [DOI] [PubMed] [Google Scholar]
- 35.Iannitti D. A.et al. , “Hepatic tumor ablation with clustered microwave antennae: The US phase II trial,” HPB 9(2), 120–124 (2007). 10.1080/13651820701222677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubner M. G., Brace C. L., Ziemlewicz T. J., Hinshaw J. L., and Lee F. T., “Microwave ablation of hepatic malignancy,” Sem. Intervent. Radiol. 30(1), 56–66 (2013). 10.1055/s-0033-1333654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubner M. G., Brace C. L., Hinshaw J. L., and Lee F. T., “Microwave tumor ablation: Mechanism of action, clinical results, and devices,” J. Vasc. Interv. Radiol. 21(Suppl. 8), S192–S203 (2010). 10.1016/j.jvir.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D., Converse M. C., Mahvi D. M., and Webster J. G., “Measurement and analysis of tissue temperature during microwave liver ablation,” IEEE Trans. Biomed. Eng. 54(1), 150–155 (2007). 10.1109/TBME.2006.884647 [DOI] [PubMed] [Google Scholar]
- 39.Solazzo S. A., Ahmed M., Liu Z., Hines-Peralta A. U., and Goldberg S. N., “High-power generator for radiofrequency ablation: Larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings,” Radiology 242(3), 743–750 (2007). 10.1148/radiol.2423052039 [DOI] [PubMed] [Google Scholar]
- 40.Pereira P. L.et al. , “Radiofrequency ablation: In vivo comparison of four commercially available devices in pig livers,” Radiology 232(2), 482–490 (2004). 10.1148/radiol.2322030184 [DOI] [PubMed] [Google Scholar]