Abstract
Objective(s):
The effect of litter size and suckling intensity on the expression of KiSS-1 mRNA in the arcuate nucleus (ARC) of rats were evaluated.
Materials and Methods:
Thirty two pregnant and four non-lactating ovariectomized (as control group) rats were used in this experiment. Lactating rats were allotted to eight equal groups. In three groups, litter size was adjusted to 5, 10, or 15 pups upon parturition and allowed to suckle their pups continuously by 8 days postpartum. In the other three groups, litter size was adjusted to five upon birth; the pups were separated from the dams for 6 hr on day 8 postpartum, after which the pups were allowed to suckle their dams for 2.5, 5, or 7.5 min prior to killing the dams. Two groups of lactating rats with either 10 or 15 pups were separated from their pups for 6 hr on day 8 postpartum, after which the pups were allowed to suckle their dams for 5 min before the dams were killed on day 8 postpartum. The ARC was removed and the expression of KiSS-1 mRNA was evaluated, using real-time PCR.
Results:
The expression of KiSS-1 mRNA in the ARC was decreased as the litter size and intensity of suckling stimulus were increased. The effect of suckling intensity on the expression of KiSS-1 mRNA was more pronounced than that of litter size.
Conclusion:
Increased litter size and suckling intensity decreased KiSS-1 mRNA expression in the ARC which may contribute to lactation anestrus in rat.
Keywords: Arcuate nucleus, KiSS-1 mRNA, Lactating rat, Litter size, Suckling intensity
Introduction
Follicular maturation and ovulation are inhibited during lactation in various mammals (1). Inhibition of estrous cycle in lactating rats mostly results from an inhibition of luteinizing hormone (LH) and gonadotropin releasing hormone (GnRH) secretion (2). Suckling is an important inhibitory cue of LH surge during the first 8 days of lactation in rats; separating pups from their dams restores LH secretion (3). Furthermore, the administration of bromocriptine (a dopamine agonist that is used in treatment of hyperprolactinemia) has been shown to have no effect on the inhibitory effect of suckling on LH pulse (4). Neuroendocrine mechanisms affecting the inhibition of LH surge during lactation are unknown (5). Suckling may be an appropriate model for studying the reproductive endocrine hormones involved during lactation.
Kisspeptins belong to a family of peptides, encoded by the KiSS-1 gene, mostly located in arcuate nucleus (ARC) and anteroventral periventricular (AVPV) region in rat brain (6). Kisspeptin has a fundamental role in controlling the gonadal axis (7), being a key regulator in LH secretion (8, 9). Kisspeptin neurons are located upstream of GnRH neurons and stimulate LH release by affecting these cells (10). Administration of kisspeptin stimulated LH secretion and induced ovulation in female rats (11, 12). Despite suppressed basal levels, LH secretory response to kisspeptin were preserved in pregnant and lactating female rats, although the magnitude of LH bursts and the sensitivity to kisspeptin were much higher in pregnant rats (13). Alterations in kisspeptin and KiSS-1 concentrations in the hypothalamus of rat during estrous cycle have been previously addressed (14-16). An increased KiSS-1 mRNA expression in ARC probably mediates the negative feedback effect of estrogen on gonadotropin secretion, which might be the GnRH pulse generating center (15). In contrast to ARC, KiSS-1 mRNA expression in AVPV and preoptic nucleus was reduced following ovariectomy and increased through estradiol implantation. It has been hypothesized that AVPV might be involved in preovulatory GnRH/LH surge (15). The level of KiSS-1 mRNA in ARC was highest during diestrus and lowest during proestrus in female rats (14).
Expression of kisspeptin neurons is down-regulated in lactating rats (17). Lactation is a proper model to investigate the neuroendocrine pathways regulating negative energy balance. Levels of LH pulsatile secretion are low in lactating rat (6) and human (18). Estrus and ovulation are delayed about 20 days in lactating rats suckling 6 to 10 pups. The delay period depends on the number of pups. If the number of pups is greater than 12 with a 2-day-long water and food withdrawal period, then the strength of suckling is increased (19). Therefore, the number of pups and strength of suckling may be critical stimulants in inhibiting the LH surge. The aims of the present study were to evaluate the effects of litter size and intensity of suckling on the expression of KiSS-1 mRNA in lactating rats. The findings would be beneficial to better clarifying the underlying mechanism(s) involved in the lower reproductive performance due to lactation anestrous in high-producing dairy cows or in prolific species (e.g. ovine and caprine).
Materials and Methods
Animals
Thirty two pregnant and 4 ovariectomized (3-4 months old) female Sprague-Dawley rats (Rattus norvegicus), weighing 205.9 ± 10.7g (mean ± SD), were housed in individual cages under controlled temperature (22 ± 2°C) and lighting (14 L: 10 D; lighting starting at 07:00 through 21:00) with free access to food and water in the Laboratory Animal Center of Shiraz University of Medical Sciences, Shiraz, Iran. The rats were fed 15 g/kg of food pellets diet which had 90% dry matter, 8% ash, 4.1% crude fat, 21.6% crude protein, 70.8% total digestible nutrients (TDN), 0.4% calcium, 0.3% potassium and 0.1% sodium. The rats were treated humanely and in compliance with the recommendations of the Animal Care Committee of our college.
Experimental groups
The rats were randomly assigned into 9 groups (n = 4 per group, Table 1). Four ovariectomized rats were used as the control group. The rats, assigned to the ovariectomized group, were anaesthetized by an intraperitoneal injection of ketamine (100 mg/kg; Woerden, Netherlands) and xylazine (7 mg/kg; Alfazyne, Woerden, Netherlands) and ovariectomized through ventral midline incision. Further procedures were carried out after a 2-week recovery period.
Table 1.
Experimental groups and procedure timeline for evaluation of the effect of increased litter size and suckling intensity on the relative expression of KiSS-1 gene in the rat (n=4)
| Groups | Litter size | Suckling before the dams killing on day 8 |
|---|---|---|
|
| ||
| Control | 0 | No suckling |
|
| ||
| 1 | 5 | Continuous |
| 2 | 10 | Continuous |
| 3 | 15 | Continuous |
| 4 | 5 | 6 hr separation, 2.5 min suckling |
| 5 | 5 | 6 hr separation, 5 min suckling |
| 6 | 5 | 6 hr separation, 7.5 min suckling |
| 7 | 10 | 6 hr separation, 5 min suckling |
| 8 | 15 | 6 hr separation, 5 min suckling |
Lactating rats were allotted to 8 groups (4 rats each) and they were allowed to suckle their pups until day 8 postpartum. In 3 groups, the litter size was adjusted to 5, 10, or 15 pups upon parturition and allowed to suckle their pups continuously. In further 3 groups of rats, the litter size was adjusted to 5 upon birth, the pups were separated from their dams on day 8 postpartum for 6 hr, after which the pups were allowed to suckle their dams for 2.5, 5, or 7.5 min before the dams were killed. The minimum of 2.5 min and 2.5 min intervals were selected according to the minimum time in increase in RNA levels was immediately detected after transcription stimulation of a single cell (20). Two groups of lactating rats with either 10 or 15 pups were similarly separated from their pups for 6 hr on day 8 postpartum, after which the pups were allowed to suckle their dams for 5 min, before the dams were killed.
Sampling
Rats were anaesthetized by ether and killed via cervical dislocation at 15:00 to 16:00 on day 8 postpartum. Brains were immediately removed and the diencephalon was dissected out by an anterior coronal section, anterior to the optic chiasm, and a posterior coronal cut at the posterior border of the mammillary bodies. To separate ARC, a third coronal cut was made through the middle of the optic tract, just rostral to infundibulum (21). The specimens were stored in liquid nitrogen until further analysis.
Real-time PCR
Total RNA was extracted, using the RNX-Plus buffer (Cinnagen, Tehran, Iran). Briefly, the tissue (100 mg) was ground in liquid nitrogen, transferred to RNX-Plus buffer (1 ml) in an RNase-free microtube, mixed thoroughly, and kept at room temperature for 5 min. Chloroform (0.2 ml) was added to the slurry and mixed gently. The mixture was centrifuged at 12,000 × g (4°C) for 20 min and the supernatant was transferred to another tube and precipitated with an equal volume of isopropanol for 15 min. The RNA pellet was washed with 75% ethanol and quickly dried and re-suspended in 50 µl RNase-free water. The purified total RNA was quantified by Nano-Drop ND 1000 spectrophotometer (Nano-Drop Techno-logies, Wilmington, DE, USA). The DNase treatment was carried out, using DNase kit (Fermentas, St. Leon-Roth, Germany) according to the manufacturer’s instructions. The DNase-treated RNA (3 µg) was used expression of the target mRNAs over the reference for the first strand cDNA synthesis, using 100 pmol oligo-dT, 15 pmol dNTPs, 20 U RNase inhibitor, and 200 U M-Mulv reverse transcriptase (Fermentas, St. Leon-Roth, Germany) in a 20 µl final volume. Primers were designed, using Allele ID 7 software (Premier Biosoft International, Palo Alto, USA) for reference gene and KiSS-1 (NM_181692). The rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (M32599) was used as a reference gene for data normalization (Table 2). Relative real-time PCR was performed in a 20 µl volume containing 1 µl cDNA, 1X Syber Green buffer and 4 pmol of primer. The amplification reactions were carried out in a Line-Gene K thermal cycler (BIOER Technology Co., Ltd, Hangzhou, China) under the following conditions: 2 min at 94°C, 40 cycles of 94°C 10 sec, 57°C 15 sec, and 72°C 30 sec. After 40 cycles, the specificity of the amplifications was tested by heating from 50°C to 95°C, resulting in melting curves. All amplification reactions were repeated 3 times under identical conditions, including a negative control and 5 standard samples. To ensure that the PCR products were generated from cDNA, but not the genomic DNA, proper control reactions were implemented in the absence of reverse transcriptase. For quantitative real-time PCR data, the relative expression of the KiSS-1 was calculated based on the threshold cycle (CT) method. The CT for each sample was calculated, using Line-gene K software (22). Accordingly, the fold values was calculated by the equation 2-ΔΔCT(23), where ΔCT is determined by subtracting the corresponding GAPDH CT value (internal control) from the specific CT of the target (KiSS-1). The ΔΔCT was obtained by subtracting the ΔCT of each experimental sample from that of the calibrator one (non-lactating ovariectomized rats).
Table 2.
Sequences of real time PCR primers for evaluation of the relative expression of KiSS-1 gene in the rat
| Primer | Sequence | Ampliconlength(bp) |
|---|---|---|
|
| ||
| KiSS 1-F | 5' TGCTGCTTCTCCTCTGTG 3' | 116 |
| KiSS 1-R | 5' CCAGGCATTAACGAGTTCC 3' | |
| GAPDH-F | 5' AAGAAGGTGGTGAAGCAGGCATC 3' | 112 |
| GAPDH-R | 5' CGAAGGTGGAAGAGTGGGAGTTG 3' | |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase
Statistical analysis
The data on relative expression of KiSS-1 gene were subjected to the test of normality and analyzed by one-way ANOVA (SAS 9.1SAS Institute Inc., Cary, NC), and mean separation was performed by Tukey's test at P = 0.01.
Results
Relative expression of KiSS-1 gene in rats continuously housed with 5 pups was greater than those suckling either 10 or 15 pups (P< 0.0001; Figure 1). Relative expression of KiSS-1 gene in the ARC of lactating rats with 5 pups which were separated from their pups for 6 hr but allowed suckling for 2.5 min was greater than in rats suckled for 5 or 7.5 min (P< 0.0001; Figure 2). Relative expression of KiSS-1 gene in the ARC of rats suckled for 2.5 min was lower than in rats continuously housed with the same number of pups (P = 0.0002). Relative expression of KiSS-1 gene in the ARC of lactating rats continuously housed 5, 10, or 15 pups were respectively greater (P< 0.0001) than in rats separated from their pups but allowed suckling for 5 min following 6 hr removal of the pups (Figure 3). There were no differences in the relative expression of KiSS-1 gene in the ARC of lactating rats with 5, 10, and 15 pups which were separated from their pups and the rats which were suckled for 5 min after 6 hr removal of pups (P> 0.05).
Figure 1.
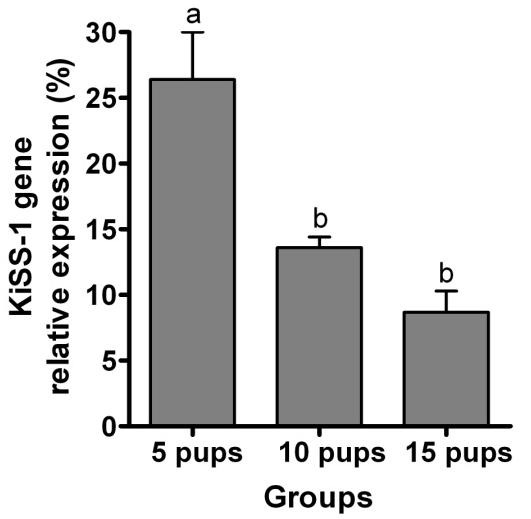
Effect of the litter size on the relative expression of KiSS-1 gene (mean ± SE) in the ARC of lactating rats (n = 4) on day 8 postpartum, continuously housed with their pups. a,b,c Different letters indicate significant difference (P< 0.01)
Figure 2.
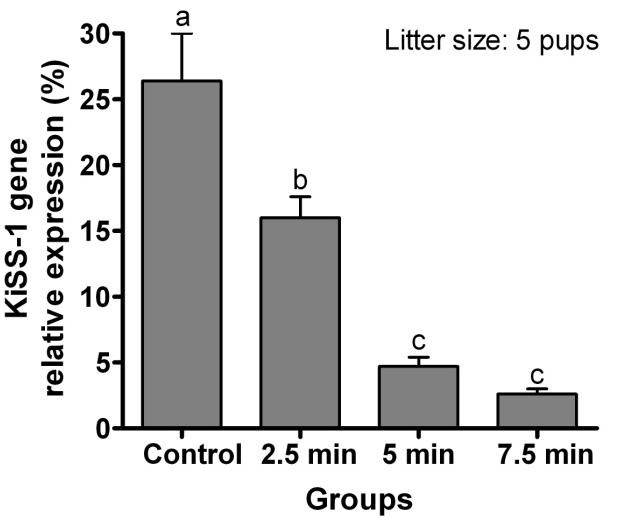
Effect of intensity of suckling on the relative expression of KiSS-1 gene (mean ± SE) in the ARC of lactating rats (n = 4) with 5 pups which were separated from their pups for 6 hr on day 8 postpartum, after which the pups were allowed to suckle their dams for 2.5, 5, or 7.5 min. Control lactating rats were not separated from their pups. a,b,c Different letters indicate significant difference (P< 0.01)
Figure 3.
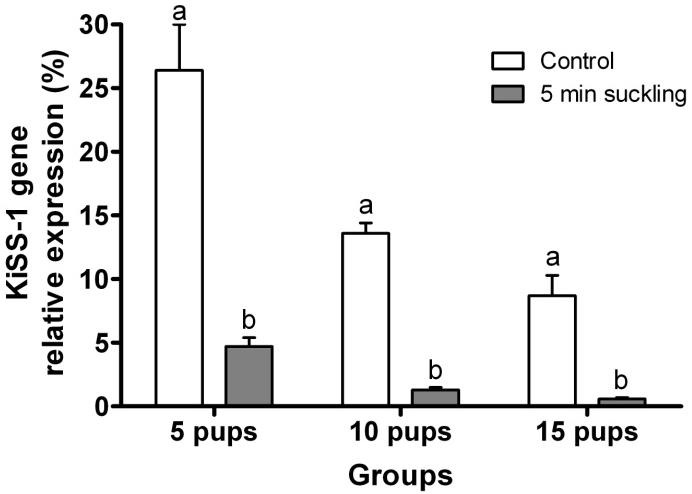
Effect of 5 min suckling duration (after 6 hr separation of dam and pup) on the relative expression of KiSS-1 gene (mean ± SE) in the ARC of lactating rats (n = 4) with 5, 10, 0r 15 pups. Control lactating rats were not separated from their pups. a,b Different letters indicate significant difference (P<0.01)
Discussion
Expression of KiSS-1 mRNA in the ARC of lactating rats was decreased as the number of suckling pups was increased. Consistent with our findings, Yamada et al(17) reported that suckling stimulus inhibited the expression of kisspeptin in ARC neurons. Adequate energy reserves are essential for reactivation of the reproductive axis. Increased number of pups per lactating rat results in higher milk production, and therefore the negative energy balance becomes more exacerbated (24). Regardless of the level of energy intake, the efficiency of energy use is increased substantially during lactation in rats. The mechanisms involved in negative energy balance play an important role in the change (24). KiSS-1 neurons are direct targets for regulation by leptin(25) and leptin levels were low in underfed rats (26). Decreased leptin production and action during lactation increases feed intake, necessary for successful rearing of the offspring (27). During periods of negative energy balance, GnRH release is suppressed (28). In rodents, GnRH neurons have no receptor for leptin(29). Therefore, during early lactation, low leptin levels may suppress kisspeptin and inhibit GnRH neuron.
The present study showed that increased intensity of suckling resulted in lower expression of KiSS-1 mRNA, with a greater influence of increased suckling intensity compared to the litter size. There is little data reporting the relationship between lactation stress and expression of kisspeptin, but it has been shown that corticotrophin-releasing factor decreased KiSS-1 expression in ARC of female rats (30). There are projections from corticotrophin-releasing factor neurons in the paraventricular nucleus to the regions where kisspeptin neurons are found (30). An inverse relationship was reported between the blood levels of adrenocorticotropin and corticosterone during lactation in rat (31). Moreover, cortisol treatment suppressed LH release in rats (32). Suckling is also an important inhibitor of LH surge in lactating rats (3). Therefore, increased glucocorticoid secretion due to increased litter size and/or suckling intensity may inhibit GnRH secretion through suppression of kisspeptin.
Results of the present study showed the negative effect of litter size on the expression of KiSS-1 gene. A relationship was reported between the height of the suckling-induced prolactin (PRL) rise and litter size in rat (33). There was a high degree of colocalization of PRL receptor mRNA in KiSS-1 mRNA-containing cells (34). Moreover, tuberoinfundibular dopaminergic (TIDA) neurons in ARC are known as the key regulator of PRL release (35). There is a close apposition between kisspeptin fibers and TIDA neurons in the ARC, suggesting kisspeptin may contribute to PRL release (35). Decreased kisspeptin was conversely correlated with LH secretion, and PRL inhibited LH secretion (36). One hypothesis is that kisspeptin neurons are inhibited as a result of suckling, and that some of kisspeptin fibers affect TIDA neurons and PRL secretion, with some kisspeptin neurons projecting to POA to stimulate GnRH release. Expression of neurokinin B (NKB) in ARC neurons were decreased in lactating rats and NKB neurons colocalise with kisspeptin neurons (37). NKB neurons in ARC are thought to stimulate LH release (37), therefore, the suckling stimulus may inhibit kisspeptin and NKB neurons.
Conclusion
An increased litter size and intensity of suckling decreased KiSS-1 mRNA expression in rat hypothalamic ARC which may suppress the pulsatile GnRH/LH secretion during lactation anestrus. The findings of the present study may help to characterize the mechanism(s) contributing to a decreased reproductive performance in high-producing dairy cows or in those twinning is a common phenomenon (e.g. ovine and caprine).
Acknowledgment
The results described in this paper were part of student thesis and this research was financially supported by the Shiraz University Vice-Chancellor for Research, Shiraz University, Shiraz, Iran.
Conflict of interest
There is no conflict of interest.
References
- 1.Butler WR. Nutritional interactions with reproductive performance in dairy cattle. Anim Reprod Sci. 2000;60:449–457. doi: 10.1016/s0378-4320(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 2.Fox SR, Smith MS. The suppression of pulsatile luteinizing hormone secretion during lactation in the rat. Endocrinology. 1984;115:2045–2051. doi: 10.1210/endo-115-6-2045. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K-I, Tsukamura H, Uchida E, Ohkura N, Ohkura S, Yokoyama A. Changes in the pulsatile secretion of LH after the removal of and subsequent resuckling by pups in ovariectomized lactating rats. J Endocrinol. 1989;121:277–283. doi: 10.1677/joe.0.1210277. [DOI] [PubMed] [Google Scholar]
- 4.Tsukamura H, Maeda KI, Ohkura S, Uchida E, Yokoyama A. The suppressing effect of the suckling stimulus on the pulsatile luteinizing hormone release is not mediated by prolactin in the rats at mid-lactation. Jpn J Anim Reprod. 1991;37:59–63. [Google Scholar]
- 5.Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol. 2002;23:225–256. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 6.Ohkura S, Uenoyama Y, Yamada S, Homma T, Takase K, Inoue N, et al. Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides. 2009;30:49–56. doi: 10.1016/j.peptides.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 8.Smith JT, Clarke IJ. Kisspeptin expression in the brain: catalyst for the initiation of puberty. Rev Endocr Metab Disord. 2007;8:1–9. doi: 10.1007/s11154-007-9026-4. [DOI] [PubMed] [Google Scholar]
- 9.Roa J, Tena-Sempere M. KiSS-1 system and reproduction: comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007;153:132–140. doi: 10.1016/j.ygcen.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Seminara SB. We all remember our first kiss: kisspeptin and the male gonadal axis. J Clin Endocrinol Metab. 2005;90:6738–6740. doi: 10.1210/jc.2005-2246. [DOI] [PubMed] [Google Scholar]
- 11.Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 13.Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, et al. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 14.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 15.Smith JT, Cunningham Cunningham, Rissman EF, Clifton DK, Steiner RA. Regulation of kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 16.Salehi MS, Jafarzadeh Shirazi MR, Zamiri MJ, Pazhoohi F, Namavar MR, Niazi A, et al. Hypothalamic expression of KiSS1 and RFamide-related peptide-3 mRNAs during the estrous cycle of rats. Int J Fertil Steril. 2013;6:304–309. [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, et al. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148:2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–376. doi: 10.1007/s10585-005-8186-4. [DOI] [PubMed] [Google Scholar]
- 19.Lindblom C, Södersten , Eneroth P. Effects of pup sucking behaviour on inhibition of sexual behaviour and ovulatory secretion of LH in lactating rats. J Endocrinol. 1985;104:419–425. doi: 10.1677/joe.0.1040419. [DOI] [PubMed] [Google Scholar]
- 20.Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, et al. From silencing to gene expression: real-time analysis in single cells. Cell. 2004;116:683–698. doi: 10.1016/s0092-8674(04)00171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehi MS, Namavar MR, Jafarzadeh Shirazi MR, Rahmanifar F, Tamadon A. A simple technique for separation of the anteroventral periventricular and arcuate nuclei in the rat hypothalamus. Anatomy. 2014. In press.
- 22.Larionov A, Krause A, Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livaka K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Roberts SB, Coward WA. Lactation increases the efficiency of energy utilization in rats. J Nutr. 1984;114:2193–2200. doi: 10.1093/jn/114.12.2193. [DOI] [PubMed] [Google Scholar]
- 25.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 26.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone LE, Higuchi T. Food intake and leptin during pregnancy and lactation. In: Russell JA, Douglas AJ, Windle RJ, Ingram CD, editors. Prog Brain Res. Vol. 133. Elsevier: 2001. pp. 215–227. [DOI] [PubMed] [Google Scholar]
- 28.True C, Kirigiti MA, Kievit P, Grove KL, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol. 2011;23:1099–1112. doi: 10.1111/j.1365-2826.2011.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab. 2001;12:65–72. doi: 10.1016/s1043-2760(00)00352-0. [DOI] [PubMed] [Google Scholar]
- 30.Kinsey-Jones JS, Li XF, Knox AMI, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2008;21:20–29. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 31.Fischer D, Patchev VK, Hellbach S, Hassan AH, Almeida OF. Lactation as a model for naturally reversible hypercorticalism plasticity in the mechanisms governing hypothalamo-pituitary- adrenocortical activity in rats. J Clin Investig. 1995;96:1208–1215. doi: 10.1172/JCI118153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vreeburg JTM, De Greef WJ, Ooms MP, Van Wouw P, Weber RFA. Effects of adrenocorticotropin and corticosterone on the negative feedback action of testosterone in the adult male rat. Endocrinology. 1984;115:977–983. doi: 10.1210/endo-115-3-977. [DOI] [PubMed] [Google Scholar]
- 33.Mattheij JAM, Gruisen EFM, Swarts JJM. The suckling-induced rise of plasma prolactin in lactating rats: its dependence on stage of lactation and litter size. Horm Res Paediatr. 1979;11:325–336. doi: 10.1159/000179070. [DOI] [PubMed] [Google Scholar]
- 34.Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152:526–535. doi: 10.1210/en.2010-0668. [DOI] [PubMed] [Google Scholar]
- 35.Sawai N, Iijima N, Takumi K, Matsumoto K, Ozawa H. Immunofluorescent histochemical and ultrastructural studies on the innervation of kisspeptin/neurokinin B neurons totubero-infundibular dopaminergic neurons in the arcuate nucleus of rats. Neurosci Res. 2012;74:10–16. doi: 10.1016/j.neures.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 36.McNeilly A. Suckling and the control of gonadotropin secretion. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd. Vol. 3. St. Louis, MO, USA: Elsevier Academic Press; 2006. pp. 2511–2536. [Google Scholar]
- 37.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23:52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


