Abstract
Objective(s):
Buthionine sulfoximine (BSO) inhibits synthesis of glutathione as the main intracellular antioxidant. The aim of the present study is to investigate the effect of BSO-induced oxidative stress on histological structure of testis, testosterone secretion and semen parameters.
Materials and Methods:
Thirty male BALB/c mice were divided into 3 groups. In control group, the mice did not receive any chemical. In the experimental group, the mice received 2 mmol/kg BSO for 35 days. In the sham group, the mice received the solvent of BSO (0.9% saline). After the treatment, the mice were sacrificed. Their testes were fixed in Buein's fixative, embedded in paraffin and prepared for histological studies. To assess semen parameters, the sperms were collected from cauda epididymis. Blood samples were used for determination of super oxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GPX), glutathione (GSH), catalase (CAT) and the serum testosterone level. The data analyzed using ANOVA and Dunnett's tests and SPSS software, version11.5. P- values at 0.05 level considered significant.
Results:
Data showed that in experimental group in comparison to control group; the concentration of CAT, GPX, SOD,GSH and the total level of testosterone is reduced while MDA level is increased significantly. The number of sperms with progressive motility were decreased (P<0.001) but sperms with abnormal morphology were increased (P<0.001). Histological studies revealed that the values for tubal differentiation index and spermatogenic index in experimental group were reduced (P<0.001).
Conclusion:
It is concluded that exposure to oxidative stress induced by BSO could affect testicular structure and semen parameters.
Keywords: Buthionine sulfoximine, Glutathione, Oxidative Stress Markers, Testis
Introduction
Infertility is a major problem that affects the involved couples medically and psycho-socially. Several factors are involved in infertility, among them oxidative stress (OS) plays an important role. Therefore, in recent years, numerous studies have been conducted to evaluate the involvement of the effect of oxidative stress on infertility (1, 2).
Oxidative stress is an imbalance between the free radicals and antioxidant defense system of the body. The oxygen-centered free radicals are referred as reactive oxygen species (ROS) and are the main cause of OS in biological systems. Oxidative stress is involved in pathogenesis of several diseases such as CNS diseases, Parkinson's disease, AIDS, cataract and premature birth (1). The severity of OS induced damages mainly depend on the amount of ROS production, exposure time to ROS and some extracellular factors such as oxygen pressure and temperature (3-5).
ROS are produced by numerous metabolic and physiologic processes in the body, but the increased level of ROS is toxic to cells including spermatozoa (1, 2, 6). It is reported that ROS in a balanced concentration is necessary for capacitation, acrosome reaction, sperm motility and fertility (3, 7). It is also shown that low concentration of ROS result in changes of sperm membrane fluidity which is necessary for fusion of sperm and oocyte membrane (8). Excess levels of ROS can damage proteins, lipids and DNA, altering cellular structure and function (9). Human semen, in addition to mature sperm, may contain immature sperms and leukocytes which are two main cells to produce ROS (10). Studies have shown that leukocytospermia is associated with sperm DNA damage (11, 12). Another study has demonstrated that immature sperms can affect significantly spermiogenesis and result in production of cytoplasmic droplets which are the main sources of ROS production (13). In addition to ROS, nitrogen-derived molecules, known as reactive nitrogen species (RNS) are another group of free radicals (4, 14).
Glutathione (GSH) is a tripeptide which is synthesized in the body from amino acids: cysteine, glutamic acid and glycin. The sulfhydryl group (SH) of cysteine keeps glutathione in a reduced state. The oxidized form of glutathione is called glutathione disulfide (GSSG) (15-17). Glutathione is the main and most common intracellular antioxidant which protects the cell against OS (18). The studies have shown that the intracellular amount of GSH is important and the increased biosynthesis of glutathione increases the protection of cells against ROS and toxicants. There are evidences that GSH is used for combating oxidative agents in the seminiferous tubules and spermatogenic and sertoli cells are actively involved in GSH synthesis (19, 20). BSO is a synthetic amino acid that inhibits synthesis of cysteine-glutamate and result in decline of intracellular amount of glutathione in vivo and in vitro (21, 22). Since the effect of oxidative stress resulting from low level of GSH on spermatogenesis have not been studied, the aim of the present study is to investigate the effect of BSO-induced oxidative stress on histological structure of testes, testosterone secretion and semen parameters.
Materials and Methods
Materials
All chemicals were purchased from Sigma Aldrich (St Louis, MO) or Fisher Scientific (Pittsburgh, PA), unless otherwise noted. All kits for assessment of oxidative markers were purchased from Ransel, Randox Company, Antrim, United Kingdom.
Methods
In the present study, 30 male BALB/c mice aging 8 weeks were divided into 3 groups. In control group, the mice did not receive any chemical. In the experimental group, the mice received 2 mmol/kg BSO for 35 days as IP injection. The third group as sham group received 0.9% saline as the solvent of BSO in a similar volume used for experimental group. BSO was purchased as powder from Sigma Company and dissolved in 0.9% saline. After the experimental period the mice were sacrificed with cervical dislocation, their testes were dissected apart and fixed in Buein’s fixative. For histological studies, the specimens were embedded in paraffin and 5 µm thick sections stained with H&E and studied with light microscope. For histomorphometric studies, tubal differentiation index (TDI) and spermatogenic index (SI) were determined. TDI assessment was carried out according to previous studies (23, 24), briefly from each testicular specimen, in 20 randomly selected microscopic fields, a total of 200 cross sectioned seminiferous tubules were analyzed and the percentage of tubules which contained; primary spermatocyte, secondary spermatocyte and spermatid were considered as TDI value and averaged for each group. For evaluating SI according to Stash et al (25), in 20 randomly selected fields, a total of 200 tubules were analyzed and the percentage of tubules contained mature spermatozoa, were considered as SI value and averaged for each group.
In order to examine semen parameters, sperms were collected from male mice from the cauda epididymis. Firstly, the left cauda epididymis in each mouse was dissected, cut into small pieces in petri dish with PBS and incubated for 45 min in Ham's F-10 media at 37°C to allow sperms to be released. For assessment of the sperm morphology, 20 µl of sperm suspension was diluted with distilled water and one drop of diluted sperm (dead sperms), from each group, was placed on a neobar slide studied under light microscope and percent of normal and abnormal morphology was determined. For assessment of sperm motility one drop of sperm suspension was placed on a microscopic slide and their motility was determined as rapid progressive, slow progressive, in situ and non-motile levels using 40X objective lens.
Blood samples were obtained from the heart and used for determinations of concentration of GSH, SOD, MDA, GPX, CAT and testosterone level.
Biochemical analysis
Glutathione (GSH) activity was assayed using the Tietze recycling assay that GSH was determined using a slight variation of Griffith's (26) modification of Tietze's (27) assay, based on the principle that GSH can be measured by an enzymatic recycling procedure in which it is sequentially oxidized by 5, 5'- dithiobis-(2-nitrobenzoic acid; DTNB) and reduced by NADPH in the presence of glutathione reductase. The rate of formation of 2-nitro-5-thiobenzoic acid (TNB) can be followed using a spectrophotometer and GSH quantitated by reference to a standard curve. A stock buffer of 143 mm sodium phosphate and 6.3 mm sodium-EDTA (pH 7.5) was made up in distilled water, and used to prepare separate solutions of 0.3 mm NADPH, 6 mm DTNB and 50 units ml-1 GSH reductase (type HI, from Saccharomyces cerevisae, Sigma). For each lysate, a final tube was made up containing 700 µl NADPH solutions, 100 µl DTNB, 100 µl of GSH standard or sample and 100 p1 of water. This mixture was warmed at 30°C for 10 min before being transferred to a cuvette containing 10 µl of the GSH reductase, and the rate of absorbance at 412 nm measured on a spectrophotometer (Kontron Uvikon). Standards of known GSH content were made up by serial dilution in 0.6% 5-SA and the samples assayed by reference to a standard curve, all points being repeated in triplicate. MDA activity was assayed using the thiobarbituric acid (TBA) reaction, described by Ohkawa et al (28). This method was used to obtain a spectrophotometric measurement of the color produced during the reaction to thiobarbituric acid (TBA) with monodialdehyde (MDA) at 535 nm. Super oxide dismutase (SOD) activity was assayed using the xezantin-xezantine oxidase system that leads to production of superoxide radicals (29). This method carried out by kit from Randox Company. Gluthatione peroxidase (GPx) activity was measured according to the method described by Paglia and Valentine (30), in which GPx activity is coupled with the oxidation of NADPH by glutathione reductase. The oxidation of NADPH was spectrophotometrically followed up at 340 nm at 37°C. The absorbance at 340 nm was recorded for 5 min. The activity was the slope of the lines as mmol of NADPH oxidized per minute. Catalase activity was assayed by incubating with excess substrate (hydrogen peroxide, H2O2) for 5 min on ice. The reaction will then be quenched (stopped) by the addition of H2SO4. The amount of H2O2 remaining in the reaction mixture (enzyme + substrate + cofactor + buffer) after 5 min of catalase action will be determined by titration with potassium permanganate (KMnO4), a very strong oxidizing reagent. The amount of substrate remaining in the mixture is inversely proportional to the activity of the enzyme. Catalase activity is defined as the number of micromoles of hydrogen peroxide decomposed in 5 min at 0°C per ml (31).
Testosterone level in the sera from different groups was determined using Elisa kit (Oxford Bio- Inovation LTD).
Statistical analysis
Statistical analysis was made using the ANOVA and Dunnett's post hoc tests for comparison of data in the experimental group with the control and sham group. All analyses were performed using SPSS software, version 11.5 (SPSS Inc, IL, Chicago, USA). P- values at 0.05 level considered significant.
Results
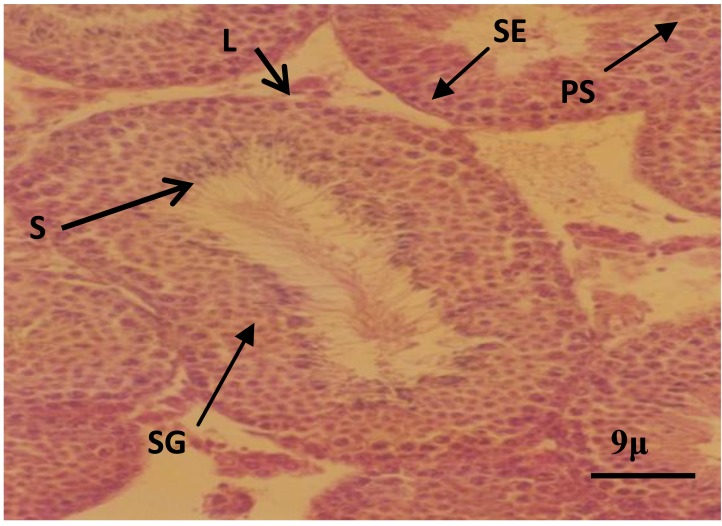
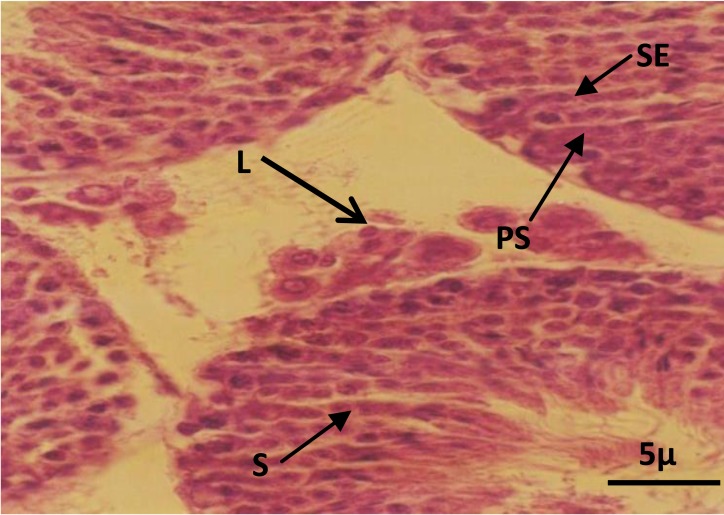
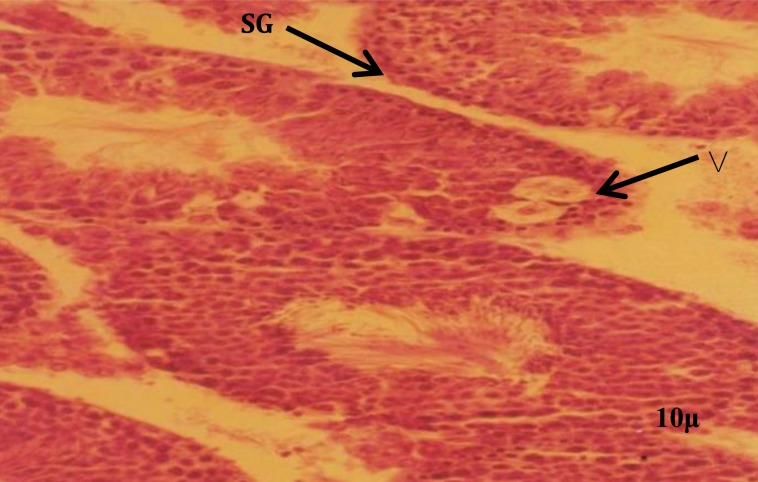
The histologic study in control and sham groups showed that seminiferous tubules have normal structure as evidenced by well-organized distribution of spermatogenic and leydig cells (Figures 1 and 2). While in the experimental group, which was received BSO, the architecture of spermatogenic epithelium was disturbed and vacuolar degeneration appeared in the spermatogenic epithelium (Figure 3). Spermatogenic cells in this group contained small and dense nuclei (Figure 3).
Figure 1.
Photomicrograph of seminiferous tubules in control group. Note the spermatogenic cells at different stages. Leydig cells (L), spermatogonia (SG), primary spermatocyte (PS), spermatid (S), sertoli cell (SE).H&E staining
Figure 2.
Photomicrograph of seminiferous tubules in sham group. Note the spermatogenic and leydig cells. Leydig cells (L), primary spermatocyte (PS), spermatid (S), sertoli cell (SE). H&E staining
Figure 3.
Photomicrograph of seminiferous tubules in experimental group. Note the spermatogenic cells with dense nucleus and vacuoles. Spermatogonia (SG), vacuoles (V). H&E staining
Based on seminal analysis, degrees of sperm motility in the semen is summarized in Table 1. As it is shown in the Table, the percent of sperms with rapid progressive motility is significantly (P<0.001) decreased in experimental group in comparison to control and sham groups. While, the percent of sperms with in situ motility and immotile sperms is significantly (P<0.001) increased in experimental group in comparison to control and sham groups. However, the percent of sperms with slow progressive motility did not change between different groups.
Table 1.
The percent of sperm motility in control, sham and experimental groups
| Groups | Exp-Cont | Exp-Sham | |||
|---|---|---|---|---|---|
| Cont | Sham | EXP | P-Value | P-Value | |
|
| |||||
| Rapid Progresive | 54.53±14.26 | 54.21±21.87 | 18.87±9.82 | <.001 | <.001 |
| Slow Progressive | 24.79±8.59 | 19.56±9.77 | 23.92±11.62 | <.001 | <.001 |
| Insitu | 9.78±6.17 | 12.33±6.29 | 21.04±5.84 | .007 | 0.001 |
| Non-Motile | 9.19±6.25 | 13.90±15.28 | 36.19±7.04 | <.001 | <.001 |
Data are presented as mean±SD
Cont: Control; EXP: Experimental
P-Value based on ANOVA and Dunnet Post Hoc tests for comparing EXP vs Cont and comparing Exp vs Sham
The morphological feature of sperms is shown in Table 2. As it is shown in the table, in experimental group, the percent of sperms with abnormal morphology is increased significantly (P<0.001) in comparison to control and sham groups.
Table 2.
The percent of normal and abnormal sperms in control, sham and experimental groups
| Groups | Exp-Cont | Exp-Sham | |||
|---|---|---|---|---|---|
| Cont | Sham | EXP | P-Value | P-Value | |
|
| |||||
| Normal | 89.22±8.77 | 81.03±23.35 | 28.93±8.31 | <.001 | <.001 |
| Abnormal | 10.79±8.77 | 19±23.34 | 71.07±8.31 | <.001 | <.001 |
Data are presented as mean ±SD
Cont: Control; EXP: Experimental
P-value based on ANOVA and Dunnet Post Hoc tests for comparing EXP vs Cont and comparing Exp vs Sham
Histomorphometric study revealed spermatogenic index (SI) and tubular differentiation index (TDI) in control and experimental groups. The values for SI and TDI are shown in Table 3. As it is shown in the Table, both indices are significantly (P<0.001) decreased in experimental group in comparison to control and sham groups.
Table 3.
The number of seminiferous tubules, containing spermatozoa and population of spermatogenic cells in control, sham and experimental groups
| Groups | Exp-Cont | Exp-Sham | |||
|---|---|---|---|---|---|
| Cont | Sham | EXP | P-Value | P-Value | |
|
| |||||
| SI | 3.1150±0.28 | 2.9450±0.34 | 1.3800±0.22 | <.001 | <.001 |
| TDI | 3.1400±0.21 | 2.9700±0.28 | 1.3050±0.26 | <.001 | <.001 |
Data are presented as mean±SD
Cont: Control; Exp: Experimental
P-value based on ANOVA and Dunnet Post Hoc tests for comparing Exp vs Cont and comparing Exp vs Sham
SI and TDI were computed based on mean over all related items
Table 4 is showing that the concentration of GSH, catalase, GPX and SOD in experimental group which was received BSO, in comparison to control and sham groups, is reduced significantly (P<0.001). While, the concentration of MDA in experimental group in comparison to control and sham groups, is increased significantly (P<0.001).
Table 4.
The effect of BSO on mean (±SD) measures of oxidative stress in mice (n=10/group)
| Groups | |||||
|---|---|---|---|---|---|
|
| |||||
| Cont | Sham | EXP | Exp-Cont | Exp-Sham | |
| P-Value | P-Value | ||||
|
| |||||
| Catalase U/mg of /protein | 53.5 ±1.1 | 53.3±1.1 | 37.3±0.5 | <0.001 | <0.001 |
| GPX U/grHg | 140.6±1.9 | 140.5±0.9 | 121.9±2.6 | <0.001 | <0.001 |
| GSH mmol/l | 3/90±0.8 | 3/50±0. 6 | 1/65±0.52 | <0.001 | <0.001 |
| SOD U/grHb | 1988±8 | 1990±3 | 1282±30 | <0.001 | <0.001 |
| MDA nmol/ml | .98±0.15 | .94±0.05 | 3.77±0.55 | <0.001 | <0.001 |
#: P-value based on one way ANOVA for overall comparisons
Determination of testosterone level in the serum showed that its level in control and sham groups was respectively, 3.35±0.15 and 3.37±0.10, while in experimental group, it was 0.98±0.15 which is significantly (P<0.001) lower than the values in control and sham groups.
Discussion
The present study was carried out to evaluate the effect of BSO-induced oxidative stress on male reproduction. The result of the study revealed that the level of oxidative stress markers have changed after BSO administration and represented an oxidative stress status. That is, levels of GSH, CAT, GPX and SOD were decreased and the level of MDA was increased (Table 4). Regarding the mechanism of the effect of BSO, it is known that BSO is a strong and specific inhibitor of glutathione (GSH) synthesis (32). Glutathione is the major intracellular antioxidant which works against produced ROS (33). Our results showed that in the BSO-administrated group progressive motility of sperms were decreased but the percent of non-motile and morphologically abnormal sperms were increased (Tables 1 and 2).
These findings are well correlate with the BSO-induced oxidative stress, i.e. decreasing of antioxidant levels and increasing of oxidant levels. Previous studies have also demonstrated similar changes in the level of antioxidant enzymes and lipid peroxidation during OS induced by low oxygen delivery in man (34) and ethanol injection in rat (35). The other finding of the present study showed that, histological architecture of testes was affected by BSO administration and cellular damage was obvious. As it is known, testosterone is a hormone which is secreted by leydig cells and is necessary for spermatogenesis. Reducing of testosterone level in the present study well correlate with the findings that indicate a spermatogenesis disturbance. In agreement with our findings, structural and functional damages of the cells under the influences of oxidative stress has previously been reported (36, 37).
GSH has an important role in biological processes and its usage in infertile men would result in improvement of infertility (38, 39). On the other hand it is shown that increasing of the level of antioxidants such as vitamin E, C, glutathione, SOD and catalase could increase sperm motility. Conversely it is reported that increasing of the ROS in the sperm preparation media would result in DNA damage, changes in acrosome reaction and failure of sperm attachment to zona pellucida (40). It is believed that most of the alterations are produced by lipid peroxidation in the sperm membrane (41). Accordingly, it has been reported that a large proportion of infertile men have an elevated levels of seminal ROS (37, 42). In general, ROS production is highest in immature spermatozoa from men with abnormal semen values. Production of ROS positively correlates with the sperm deformity index and high level of MDA has a negative correlation with sperm count (43).
Decreasing of SI and TDI in experimental group, indicate that BSO-induced OS affect spermatogenesis. This finding is in agreement with a previous study showing that morphology and sperm count is decreased with increasing of MDA level (44), and glutathione deficiency lead to instability of mid piece and decreasing of sperm motility (45, 46). In another study, glutathione treatment in infertile men with unilateral varicocele led to a statistically significant improvement in the sperm quality (47).
Conclusion
The results of the present study indicate that BSO-induced OS in mouse can lead to spermatogenesis instability, decreasing of sperm quality. Therefore, it can be suggested that in any condition that there is a possibility of the involvement of oxidative stress in infertility, intake of cystein rich diet , an amino acid which is essential for GSH synthesis, would be helpful.
Acknowledgment
This article is resulted from a research proposal leading to thesis of Fakhrosadat Sajjadian, PhD student of Anatomical Sciences and was approved and financially supported by Research Deputy, Tabriz University of Medical Sciences, Tabriz-Iran.
References
- 1.de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10:15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- 2.Aitken RJ. Molecular mechanisms regulating human sperm function. Mol Hum Reprod. 1997;3:169–173. doi: 10.1093/molehr/3.3.169. [DOI] [PubMed] [Google Scholar]
- 3.Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl. 2003;5:231–242. [PubMed] [Google Scholar]
- 4.Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Prabakaran SA. Mechanism, measurement and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- 6.Rodriguez H, Cutler RG. Advances in Basic Science, Diagnostics and Interventions. Singapore: World Scientific Pub Co Inc; 2002. Critical reviews of oxidative stress and aging; pp. 170–181. [Google Scholar]
- 7.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 8.Kodama H, Kuribayashi Y, Gagnon C. Effect of sperm lipid peroxidation on fertilization. J Androl. 1996. pp. 151–157. [PubMed]
- 9.Agarwal A, Prabakaran SA. Mechanism, measure-ment, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–974. [PubMed] [Google Scholar]
- 10.Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl. 2004;6:59–65. [PubMed] [Google Scholar]
- 11.Alvarez JG, Sharma RK, Ollero M, Saleh RA, Lopez MC, Thomas AJ, Jr, et al. Increased DNA damage in sperm from leukocytospermics semen samples as determined by the sperm chromatin structure assay. Fertil Steril. 2002;78:319–329. doi: 10.1016/s0015-0282(02)03201-6. [DOI] [PubMed] [Google Scholar]
- 12.Shekarriz M, Sharma RK, Thomas AJ, Jr, Agarwal A. Positive myeloperoxidase staining (Endtz test) as an indicator of excessive reactive oxygen species formation in semen. J Assist Reprod Genet. 1995;12:70–74. doi: 10.1007/BF02211372. [DOI] [PubMed] [Google Scholar]
- 13.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, Aitken RJ. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress and sperm function. J Androl. 1996;17:276–287. [PubMed] [Google Scholar]
- 14.Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 15.Ford WC, Whittington K. Antioxidant treatment for male subfertility: a promise that remains unfulfilled. Hum Reprod. 1998;13:1416–1419. doi: 10.1093/oxfordjournals.humrep.a019707. [DOI] [PubMed] [Google Scholar]
- 16.Tarin JJ, Brines J, Canno A. Antioxidants may protect against infertility. Hum Reprod. 1998;13:1415–1416. doi: 10.1093/humrep/13.6.1415. [DOI] [PubMed] [Google Scholar]
- 17.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–353. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 18.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod. 1998;13:1240–1247. doi: 10.1093/humrep/13.5.1240. [DOI] [PubMed] [Google Scholar]
- 19.Irvin S. Glutathione as a treatment for male infertility. Rev Reprod. 1996;1:6–12. doi: 10.1530/ror.0.0010006. [DOI] [PubMed] [Google Scholar]
- 20.Chaudher AR, Das P, Singh R. Study of oxidative stress and reduced glutathione levels in seminal plasma of human subjects with different fertility potential. Biomed Res. 2008;19:207–210. [Google Scholar]
- 21.Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11:573–585. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 22.Wroblewski N, Schill WB, Henkel R. Metal chelators change the human sperm motility pattern. Fertil Steril. 2003;79:1584–1589. doi: 10.1016/s0015-0282(03)00255-3. [DOI] [PubMed] [Google Scholar]
- 23.Niakani A, Farrokhi F, Hasanzadeh S. Decapeptyl ameliorates cyclophosphamide-induced reproductive toxicity in male BALB/c mice: histomorphometric, steriologic and hormonal evidences. Iran J Reprod Med. 2013;11:791–800. [PMC free article] [PubMed] [Google Scholar]
- 24.Porter KL, Shetty G, Meistrich ML. Testicular edema is associated with spermatogonial arrest in irradiated rats. Endocrinology. 2006;147:1297–1305. doi: 10.1210/en.2005-0890. [DOI] [PubMed] [Google Scholar]
- 25.Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A Novel Potent Indazole Carboxylic Acid Derivative Blocks Spermatogenesis and Is Contraceptive in Rats after a Single Oral Dose. Biol Reprod. 2008;78:1127–1138. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- 26.Griffith OW. Examination of glutathione and glutathione disulfide using glutathione reductaseand 2- vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 27.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 28.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 29.Fairbanks V, Klee GG. Biochemical aspects of haematology. In: Burtis CA, Ashwood ER, editors. Tietze textbook of clinical chemistry. Philadelphia: WB Saunders; 1994. pp. 2020–2021. [Google Scholar]
- 30.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 31.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 32.Ortega AL, Mena S, Estrela JM. Glutathione in cancer cells death. Cancer. 2011;3:1285–1310. doi: 10.3390/cancers3011285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis KJ. Oxidative stress, antioxidant defenses and damage removal, repair and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 34.Emokpae AM, Ojiefo UP, Aisha KG. Antioxidant enzymes and acute phase proteins correlate with marker of lipid peroxide in adult nigerian sickle cell disease patients. Iran J Basic Med Sci. 2010;13:177–182. [Google Scholar]
- 35.Ramezani A, Goudarzi I, Lashkarboluki T, Ghorbanian MT, Abrari K, Elahdadi Salmani M. Role of oxidative stress in ethanol-induced neurotoxicity in the developing cerebellum. Iran J Basic Med Sci. 2012;15:965–974. [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualotto FF, Sharma RK, Nelson DR, Thomas AJ, Agarwal A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril. 2000;73:459–464. doi: 10.1016/s0015-0282(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 38.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 39.Nudell DM, Monoski MM, Hipshultz LI. Common medications and drugs: how they affect male fertility. Urol Clin North Am. 2002;29:965–973. doi: 10.1016/s0094-0143(02)00079-4. [DOI] [PubMed] [Google Scholar]
- 40.Fingerova H, Oborna I, Novotny J, Svobodova M, Brezinova J, Radova L. The measurement of reactive oxygen species in human neat semen and in suspended spermatozoa. A comparison. Reprod Biol Endocrinol. 2009;7:118. doi: 10.1186/1477-7827-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amorim EA, Torres CA, Graham JK, Amorim LS, Santos LV. The hypoosmotic swelling test in fresh rabbit spermatozoa. Anim Reprod Sci. 2009;111:338–343. doi: 10.1016/j.anireprosci.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Jr, Agarwal A. The reactive oxygen species–total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–2807. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 43.Aziz N, Saleh RA, Sharma RK, Lewis-Jones I, Esfandiari N, Thomas AJ, Jr, et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril. 2004;81:349–354. doi: 10.1016/j.fertnstert.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Zunjarrao GB, Kavita MM, Jayshree GN, Vandana ZB, Birendra KY. Human seminal oxidative stress: correlation with antioxidants and sperm quality parameters. Ann Biol Res. 2011;2:351–359. [Google Scholar]
- 45.Zubkova EV, Robaire B. Effect of glutathione depletion on antioxidant enzymes in the epididymis, seminal vesicles, and liver and on spermatozoa motility in the aging brown norway rat. Biol Reprod. 2004;71:1002–1008. doi: 10.1095/biolreprod.104.028373. [DOI] [PubMed] [Google Scholar]
- 46.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Bhardwaj A, Verma A, Majumdar S, Khanduja KL. Status of vitamin E and reduced glutathione in semen of Oligozoospermic and azoospermic patients. Asian J Androl. 2000;2:225–228. [PubMed] [Google Scholar]