Abstract
Objective(s):
Sea cucumber derived bioactive compound is considered efficient in treatment of bone disorders. This study was performed to evaluate the effect of this extract on differentiation of rat bone marrow mesenchymal stem cells (rBMMSc) into osteogenic lineage.
Materials and Methods:
Isolated rBMMSc were grown in DMEM supplemented with 10% FBS. The cells were exposed to different concentration of extract. After 21 days, Alizarin red staining, alkaline phosphatase assay and RT-PCR were performed. The results were analyzed by ANOVA software and P value <0.05 was considered significant.
Results:
Morphological methods revealed that appropriate concentrations of extract increased osteogenic differentiation in a dose-dependent manner. RT-PCR revealed that extract without or with osteogenic medium due to osteopontin expression had a potential role in osteogenesis.
Conclusion:
Based on our data it concluded that S. cucumber extract stimulated Bone marrow mesenchymal cells to differentiate into osteogenic lineage without existence of osteogenic medium.
Keywords: Bone marrow, Differentiation, Marine, Osteogenic
Introduction
Bone marrow mesenchymal stem cells (BMSCs) are well known as population of multipotent stem cells with capacity of self renewal and differentiation into multiple cell lineages. They have been considered as one of the most appreciable source of osteoprecursor cells and they can provide exclusive source for tissue engineering strategies, cell based therapies and regenerative medicine specially for bone defects repairing (1).
A great part of research in the field of pharmacology and therapeutics has focused on natural products as secondary metabolite. Natural products have considerable diversity and they can be collected from a terrestrial and marine vertebrates and invertebrates (2). Marine organisms both flora and fauna are strong basis for establishment of new drug compounds containing very promising biological activity (3). Sea cucumber (S. cucumber) has been produced various bioactive therapeutic elements such as anti cancer, anti inflammatory, anti angiogenic and antimicrobial agents that can be utilized as pharmacological complex for treating bone defects (4). The presence of variety of bioactive elements such as triterpene glycosides (saponins), chondroitin sulfates, glycosaminoglycan (GAGs), sulfated polysac-charides, mucopolysaccharide, glucosamine, vitamins and minerals especially calcium is responsible for biomedicine properties of sea cucumbers which have valuable effects in treatment of arthritis disorders (5). Previous research studies have shown that sea cucumber is favorable in healing of muscular skeletal inflammatory defects by maintaining prostaglandins balance (6). Fucan sulfates isolated from body wall of S. cucumber (Stichopus japonicus) suppressed the osteoclastogenesis in vitro which suggests that these constituents derived from S. cucumber are powerful inhibitors of osteoclastogenesis and functional for remedy of arthritis (7). The aim of this study was to evaluate the effects of crude alcoholic extract of S. cucumber on capacity of rat BMSCs to induce osteogenic lineage.
Materials and Methods
Reagents
DMEM Medium, FBS (fetal bovine serum) trypsin-EDTA and antibiotic (Penicilin-Stereptomycin) were obtained from (Gibco, USA). Osteogenic medium containing dexamethasone, L-ascorbic acid 2-phosphate, β-glycerol phosphate were purchased from (Idezist, Iran). Alizarin red (Bio Idea, Iran), alkaline phosphatase kit (Pars Azmoon, Iran), RNA isolation kit from (Roshe, Germany) were purchased. C-DNA synthesis kit and RT-PCR kit were purchased from (Pars Tous, Iran). CD44, CD29 antibody (Abcam, England), IgG2a-PE antibody (Abcam, England) were prepared. Specimens of the S. cucumber were obtained from the coastal areas of Persian Gulf waters (Gheshm, Iran). The experiments were performed at Research Center Applied Biology of Mashhad Branch Islamic Azad University in 2013.
Preparation of sea cucumber methanol extract
At first, morphometric evaluation of S. cucumber was performed at Research Center Applied Biology of Mashhad Islamic Azad University. Then, Specimens of sea cucumber were washed, body wall of samples were isolated and stored at -80 °C. For preparing of extract, body wall of S. cucumber samples (about 20 g) were dried, ground and mixed with 200 ml methanol. Then, the extract was shaked in orbital shaker (72 hr) at room temperature, filtered through an 11 µm Whatman filter and concentrated under vacuum evaporator and stored at 4°C before use.
Isolation, characterization and cell cultivation of wistar rat mesenchymal stem cells
Male wistar rats were purchased from Razi Institute in Mashhad, Iran. Bone marrow mesenchymal stem cells were obtained from 6 weeks old male wistar rats (approximately 100 g). Briefly, femurs and tibias were detached, trimmed of excess soft tissues and washed with PBS. Then both ends were cut and flushed with DMEM medium containing 10% FBS and 1% penicillin streptomycin supplemented with L glutamine. Bone marrow cells were then collected from diaphysis by centrifugation (Sigma, USA) at 2000 rpm for 5 min. The collected cells were resuspended in 5 ml DMEM medium plus 15% FBS, plated in a 25 cm2 tissue culture flask and cultured at 37 °C in a humidified 5% CO2 incubator (Memmert, Germany). The medium was changed every 2 days and floating cells were removed. After monolayer of adherent cells had reached enough confluency, cells were passaged by trypsinization and sub cultured at a concentration of 2000 cells/cm2 for subsequent experiments. For most of the experiments, we used bone marrow mesenchymal stem cells at the 3rd–4th passages. For characterization of BMSCs, 2 weeks after isolation of bone marrow mesenchymal stem cells, cells were seeded at 96 well plate at 2×104. After 24h, medium removed and washed 3 times with cold PBS. In next stage to performance flow cytometery, the cells were harvested, centrifuged and rat’s monoclonal antibody CD44 and CD29 conjugated to phycoerythrin (PE) were added. Then the cells were rinsed with PBS and fixed with 1% formalin. IgG2a-PE was used as isotope. Eventually, the cells were observed by fluorescence microscopy and flow cytometery.
Treatment of bone marrow mesenchymal stem cells by sea cucumber methanol extract
To confirm the osteogenic potential of the BMSCs used, after three times passages, 103 bone marrow mesenchymal stem cells were seeded into 96- well plate and incubated overnight. Experimental groups were divided into 2 classes. For the first experimental group (sea cucumber methanol extract plus osteogenic induction medium), The bone marrow mesenchymal stem cells were exposed to osteogenic media containing DMEM, 10% FBS, 10mM glyceraldehydes 3 phosphate, 60 mM ascorbic acid and 10 nM dexamethasone (Idezist, Iran) along with different concentration (3.5, 6.25, 12.5 and 25 µg/ml) of alcoholic S. cucumber extract. In this group, the osteogenic medium was replaced every 3 days. In second experimental group, bone marrow mesenchymal stem cells were plated and cultured in DMEM media supplemented with 1% antibiotics, 10% FBS containing different concentration of sea cucumber methanol extract.
Effect of sea cucumber methanol extract on mineralization
To measure mineralization induced by S. cucumber extract, the bone marrow mesenchymal stem cells were plated in 96-well plates and cultured for 21 days (sea cucumber extract plus osteogenic media and sea cucumber extract alone). After 21 days treatment, the medium was removed; the cells were washed twice with PBS and fixed in 70% methanol at room temperature for 30 min. After two washes with PBS, to stain the calcium deposits, BMSCs were stained with Alizarin red solution for 20 min. To remove the nonspecific stained cells, the cells were then rinsed with PBS, allowed to dry completely and evaluated under invert microscopy.
Measurement of alkaline phosphatase activity
The bone marrow mesenchymal stem cells were cultured in 48 well plates at density of 2×104 cells per well as monolayer. The medium was changed every three days. To detect osteogenic differentiation of BMSCs, Alkaline Phosphatase (ALP) assay was performed 14 days after treatment. Culture medium was aspirated and the cell monolayer was washed with PBS and cell lysis was performed with NP-40. ALP activity of supernatant was determined using a colorimetric ALP activity assay kit (Pars Azmoon, Iran).
Isolation of RNA and RT-PCR analysis
Total cellular RNA extraction from BMSCs was performed using the High Pure RNA Isolation kit (Roche, Germany). Two µg RNA was reverse transcribed and cDNA synthesized using Easy cDNA Synthesize (ParsTous, Iran) Kit. cDNA was obtained in the presence of random hexamer or oligo dT, incubated at 65°C for 5 min, followed by addition RT premix, incubation 25°C for 10 min, 50°C for 60 min and 70°C for 10 min. Expression levels of osteopo-ntin mRNA were examined using RT-PCR. The produced cDNA (2 µl) was added to 10x buffer, Mgcl2 25 mM, dNTP, Taq DNA polymerase, the appropriate forward and reverse primers, and Polymerase chain reaction was performed as 1 cycle at 95°C/4 min, 35 cycles at 94°C/30 sec for denaturation, 57°C 30 sec for annealing, 72°C/30 sec for extension and 1 cycles 5 min at 72°C. The primers used were as follows: B2M Forward 5’ TGGTGCTTGGCTCACTGACC 3’, Reverse 5’ TATGTTCGGCTTCCCATTCT 3’ was used as housekeeping gene. Forward primer osteopontin was designed as 5’ ACAGCCAGGACTCCATTGAC 3’, reverse primer osteopontin was designed as 5’ ACACTATCACCTCGGCCATC 3’. Following amplification, PCR products were analyzed by electrophoresis on a 2% agarose gel and visualized by green viewer staining. DNA ladder (50 bp) was used as size markers.
Statistical analysis
Data were analyzed as mean ± standard error of the mean (SEM). Statistical comparisons were made by SPSS software and analyzed with two -way variance (ANOVA) and when significant differences were observed, P-value of 0.05 was considered as statistically significant.
Results
Immunocytochemistry studies for characterization of bone marrow mesenchymal stem cells
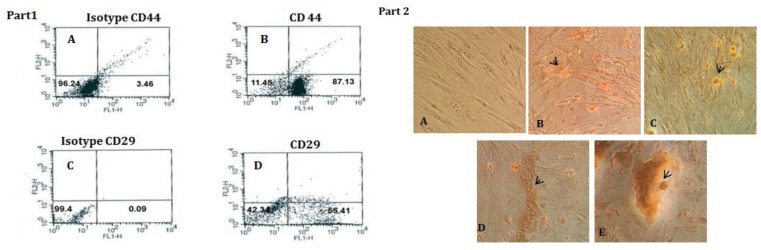
Flow cytometrical assessments of bone marrow mesenchymal stem cells were revealed that the presences of CD44 and, CD29 on surface of BMSCs were potent markers of stemcellness of BMSCs (Figure 1, part 1).
Figure 1.
Part 1- Obtained results from flowcytometry. A) Isotype CD44:IgG2a-PE. B) 87. 13% Expression of CD44 marker in BMSCs. C) Isotype CD29:IgG2a-PE. D) Approximately 55.41% BMSCs expressed CD29 marker. Part 2- The dose dependent effect of Sea cucumber methanol extraction the presence of osteogenic mediumon BMSCs: the morphology of BMSCs 21 days after treatment period were assessed by Alizarin red staining, As shown in the Figures, suitable concentrations of Sea cucumber alcoholic extract (6.25, 12.5, 25 µg/ml) formed red mineral amorphous deposits that it was indicating one of the prominent marker differentiation to osteoblasts. A) Control. B - E) (3.5, 6.25, 12.5, 25 µg/ml). Magnification 200 µm
Dose dependent effect of crude sea cucumber methanol extraction mineralization in bone marrow mesenchymal stem cells
To visualize mineralization (nodule formation) induced by S. cucumber extract, we used Alizarin red staining qualitative in BMSCs cultures. Figure 1 part 2 illustrated that S. cucumber extract clearly (21 days after treatment) produced a larger red color spots in the treated group than untreated group in response to the concentration that it was indicating calcium deposits (6.25, 12.5 and 25 µg/ml). The mineralization assay showed that S. cucumber extraction caused osteoblast differentiation in a dose-dependent manner and it could significantly stimulate mineralization that was showed by Alizarin red staining.
Sea cucumber alcoholic extract stimulates alkaline phosphatase activity in bone marrow mesenchymal stem cells
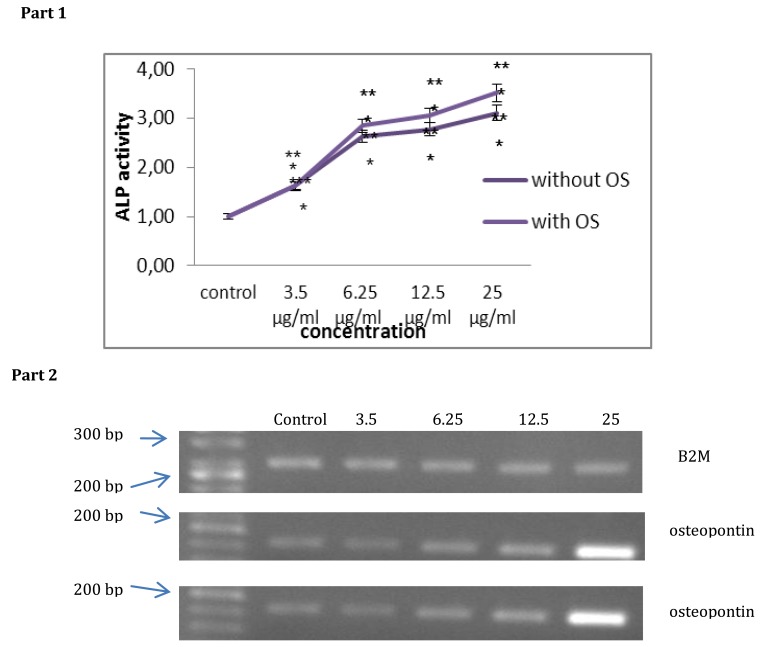
Alkaline phosphatase activity (as an osteoblastic marker) were significantly increased at the appropriate concentration (6/25 to 25 µg/ml) compared to the control group on day 14th. ALP activity was greater in the osteogenic group as compared to the experimental group (P<0.001). As it was shown the ALP activity of all S. cucumber extract-treated groups was significantly higher than control group (Figure 2, Part 1).
Figure 2.
Part 1- ALP activity assay. ALP activity was evaluated in BMSCs cells treated with sea cucumber alcoholic extract along with osteogenic medium and with treated with Sea cucumber alcoholic extract alone. Mean value was different from control group (*P<0.05, **P<0.01, ***P<0.001). Statistical analysis was performed using a two-way ANOVA. Part 2- Expression levels of mRNAs for osteopontin in bone marrow mesenchymal stem cells. Total RNA was isolated from bone marrow mesenchymal stem cells 21 days after treatment with S. cucumber alcoholic extract alone (5A) or with osteogenic medium (5B). Levels of mRNAs for rat osteopontin (196 bp) were determined by RT-PCRwith specific primers. Amplification of B2 MmRNA was used as a housekeeping gene (230 bp). Lane left to right: 50 bp marker, control, 3.5, 6.25 µg/ml, 12.5 µg/ml and 25 µg/ml, B2M
Assessment of osteopontin expression (as osteoblast differentiation marker gene) influenced by sea cucumber methanol extract in bone marrow mesenchymal stem cells
To analyze the expression level of osteopontin, which is one of the osteoblast marker, we were examined osteogenic differentiation in the absence or presence of S. cucumber methanol extract with RTPCR analysis. RT-PCR analysis indicated that S. cucumber extract alone coud induce the expression of mRNAs for osteopontin in a dose-dependent manner. On day 21 (end of differentiation period), osteopontin mRNA was expressed in control BMSCs and its level of expression enhanced to 12.5 and 25 µg/ml. In contrast, treatment with 3.5 and 6.25 µg/ml S. cucumber extract induced low expression of osteopontin mRNA in bone marrow mesenchymal stem cells as observed in control group. Expression of this marker in bone marrow mesenchymal stem cells indicated S. cucumber methanol extract had osteogenic potential (Figure 2, Part 2).
Discussion
Previous research have been exhibited that mesenchymal stem cells by differentiation toward osteogenic and chondrogenic lineage had ability to repair both bony and cartilaginous defects, respectively (8). While mesenchymal stem cells isolated from bone marrow could differentiate into an osteogenic lineage when cultured in the presence of dexamethasone, ascorbic acid, and β glycerolphosphate (osteogenic supplements); also other trophic factors or drugs have been proposed for osteogenic induction either in vitro or in animal models (9). It is known that Osteoblast differentiation is a complicated process regulated by many endocrine, paracrine and autocrine factors. Glucocorticoids in human and rat bone marrow stromal cells are vital factors for formation of a mineralized extracellular matrix (10). Although, cell culture conditions applied to differentiation to adipocytes and osteocytes in bone marrow mesenchymal stem cells are well established, discovery of natural efficient source due to minimal side effects of chemical materials for induction of differentiation is very appealing and has attracted great attention toward natural products application.
In our experiment, to discover that whether methanol extract of S. cucumber as one of chief source of curing bone injury in traditional medicine could stimulate osteoblastic differentiation, we employed methanol S. cucumber extract (exterior part of body) as an assay system for osteoblast differentiation on mesenchymal stem cells isolated from male wistar rat in vitro. We measured the activity of alkaline phosphatase and mineralization of BMSCs pretreated with S. cucumber extract (with or without osteogenic medium) on day 14th. In the presence of alkaline phosphatase p-nitro-phenyl phosphate (p NPP) converts to the p-nitro-phenol (pNP) which produces yellow color. Our results revealed that alkaline phosphatase activity was considerably higher in all sea cucumber extract treated groups rather than control group. S. cucumber alcoholic extract (alone in a dose of 12.5 and 25 µg/ml) and (in the presence of osteogenic medium) at a dose of 12.5 µg/ml created 3-fold improvement compared to BMSCs control group. Our findings from Alizarin red staining were consistent with alkaline phosphatase activity results indicating that this extract also promoted extracellular matrix mineralization in the presence of osteogenic medium and showed that S. cucumber methanol extract have osteogenic differentiation potency. According to another research study performed by Ran et al Phorbaketal isolated from sponge can be mentioned as an example to show that marine ecosystem had efficient role in osteoblastic differentiation. In 2012 Ran and his coworker were revealed that Phorbaketal isolated from sponge species Phorbas via activation of ERK pathway induced osteoblastic differentiation on C3H10T1/2 cells (11).
In order to understand the action of S. cucumber alcoholic extract on osteogenic differentiation of BMSCs, we evaluated the effects of the extract on the expression of the osteopontin via RT-PCR. Pretreatment of BMSCs alone or along with osteogenic medium with sea cucumber extract for twenty one days increased osteopontin expression. The treated cells with S. cucumber alcoholic extract 14 days after induction of differentiation evidenced increases in alkaline phosphatase activity. RT-PCR analysis also showed that osteopontin expression increased significantly during osteogenic differentiation in the cells exposed to S. cucumber extract (with or without osteogenic medium) compared with control BMSCs supporting the existence of valuable compounds in the body wall of S. cucumber such as Fucan sulfates which have capacity of challenging against osteoclastogenesis.
Zhang in 2008 studied the role of flavonoids on osteoblastic proliferation and differentiation and he concluded that flavonoids could enhance osteoblast development with improvement in alkaline phosphatase activity (12). His finding is consistent with our results indicating that S. cucumber methanol extract as natural adjuvant can be considered valuable due to the presence of mineral compounds in treatment of bone disease in future. Ryu and his colleagues in 2010 were isolated and identified peptide sequence as known as LEDPFDKDDWDNWK (1821Da) from sea horse and indicated this peptide stimulate osteogenic differentiation in MG-63 and chondrogenic differentiation in SW-1353 via increasing alkaline phosphatase activity, mineralization and collagen synthesize (13). Similarly in our experiments we showed that, S. cucumber extract promoted osteoblastic differentiation via alkaline phosphatase activity.
In 2011, Park and coworkers were reported that Fucoidan a type of algae Sulfated Polysaccharide induced osteogenic differentiation on human adipose derived stem cells (14). Shahrulazua et al in 2013 examined the effects of S. cucumber extract of Stichopus sp1 Malaysia (gamat) on osteoblast cell survival and functional activity and concluded that special concentration of S. cucumber extract enhanced cell viability and osteoblast cell function (15). Our finding also demonstrated that synergistic effect of sea cucumber extract with osteogenic medium enhanced differentiation to osteoblasts but S. cucumber extract alone can potentially induced osteoblast differentiation.
Conclusion
In conclusion, the results of our experiments indicated that pretreatment by Persian Gulf S. cucumber alcoholic extract alone (without osteogenic medium) can be used in induction of osteogenic differentiation which was documented by morphological assessment, ALP activity, mineralization and RT-PCR. Therefore, it has been suggested that alcoholic crude extract of S. cucumber due to possessing bioactive components in the exterior part of the body can direct the fate of mesenchymal stem cells toward osteogenic lineage and can be promising as a novel therapeutic potential of natural source for the treatment of skeletal disorders.
Acknowledgment
This work was performed in Research Center for Animal Development Applied Biology and was supported by Vice Chancellor Scientific Research of Mashhad Branch, Islamic Azad University. The authors have special thanks to the central lab of Mashhad University of medical sciences.
References
- 1.Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, et al. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontal. 2004;75:281–287. doi: 10.1902/jop.2004.75.9.1281. [DOI] [PubMed] [Google Scholar]
- 2.Cragg GM, Newman DJ. Natural products : A continuing source of novel drug leads. Biochim Biophys Acta. 2013;830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venugopal V. Marine products for healthcare: functional and bioactive nutraceutical compounds from the ocean. J Aquat Food ProductTechnol. 2010;19:48–54. [Google Scholar]
- 4.Shaifuzain AR, Amran AS, Muzaffar TMS, Tengku AR, Shaifulizan Effect of oral sea cucumber (Stichopus sp1) extract on fracture healing. Malays J Med Sci. 2007;14:172. [Google Scholar]
- 5.Kariya Y, Mulloy B, Imai K, Tominaga A, Kaneko T, Asari A, Suzuki K, et al. Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopusjaponicus and their ability to inhibit osteoclastogenesis. Carbohydr Res. 2004;339:1339–1346. doi: 10.1016/j.carres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr Drug DiscovTechnol. 2008;5:289–301. doi: 10.2174/157016308786733564. [DOI] [PubMed] [Google Scholar]
- 7.Chen SG, Li GY, Yin LA, Huang WC, Dong P, Xu J, et al. Identification of eight species of sea cucumber chondroitin sulfates by high temperature ~1H NMR. J Instrumental Analysis. 2010. pp. 29–38.
- 8.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061–5067. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740–747. [PubMed] [Google Scholar]
- 10.Eijken M, Koedam M, van Driel M, Buurman CJ, Pols HA, van Leeuwen JPV. The essential role of glucocorticoids for proper human osteoblast differentiation and matrix mineralization. Mol Cell Endocrinol. 2006;248:87–93. doi: 10.1016/j.mce.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Byuna MR, Kim AR, Hwang JH, Sung MK, Lee YK, Hwang BS, et al. Phorbaketal A stimulates osteoblast differentiation through TAZ mediated Runx2 activation. FEBS Lett. 2012;586:1086–1092. doi: 10.1016/j.febslet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhang DW, Cheng Y, Wang NL, Zhang JC, Yang MS, Yao XS. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine. 2008;15:55–61. doi: 10.1016/j.phymed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Ryu B, Qian ZJ, Kim SK. Purification of a peptide from seahorse , that inhibits TPA-induced MMP , iNOS and COX-2 expression through MAPK and NF- B activation, and induces human osteoblastic and chondrocytic differentiation. ChemBiol Interact. 2010;184:413–422. doi: 10.1016/j.cbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Park SJ, Lee KW, Lim DS, Lee S. The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 2011;21:2204–2211. doi: 10.1089/scd.2011.0521. [DOI] [PubMed] [Google Scholar]
- 15.Shahrulazua A, Samsudin AR, Iskandar MA, Amran AS. The in vitro effects of sea cucumber (Stichopus sp1) extract on human osteoblast cell line. J Malay Ortho. 2013;7:41–48. doi: 10.5704/MOJ.1303.015. [DOI] [PMC free article] [PubMed] [Google Scholar]