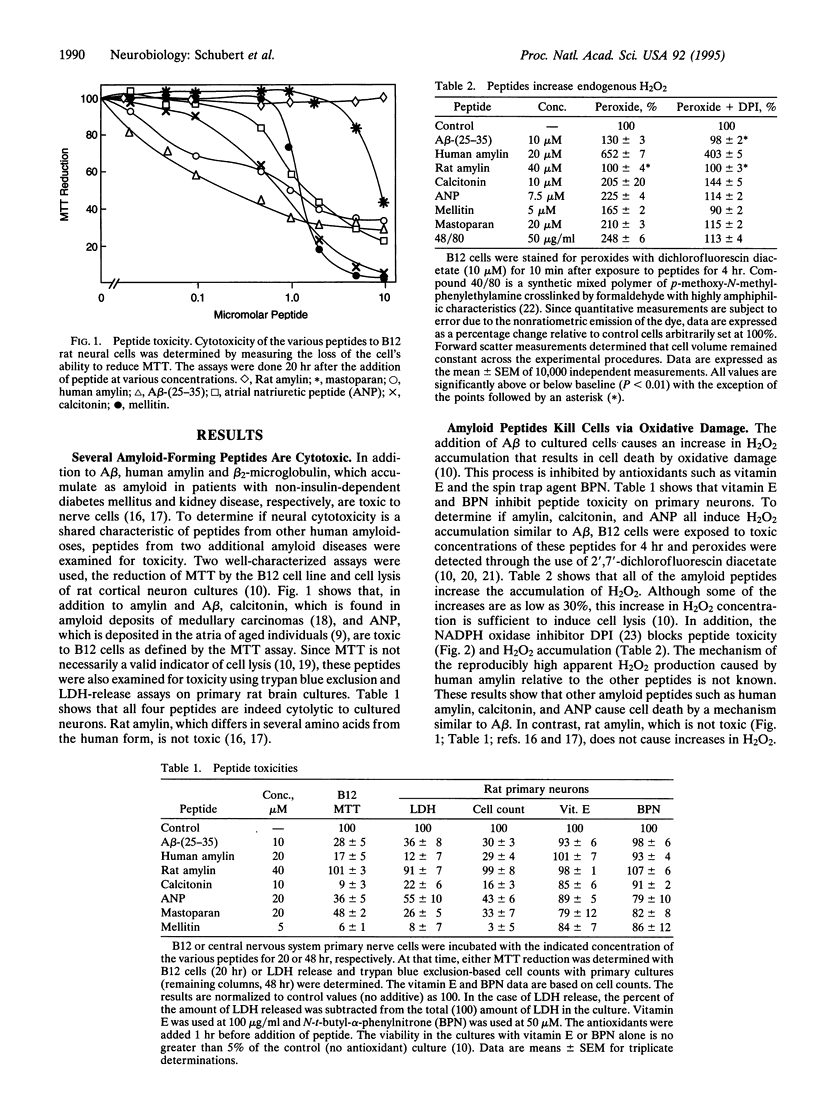
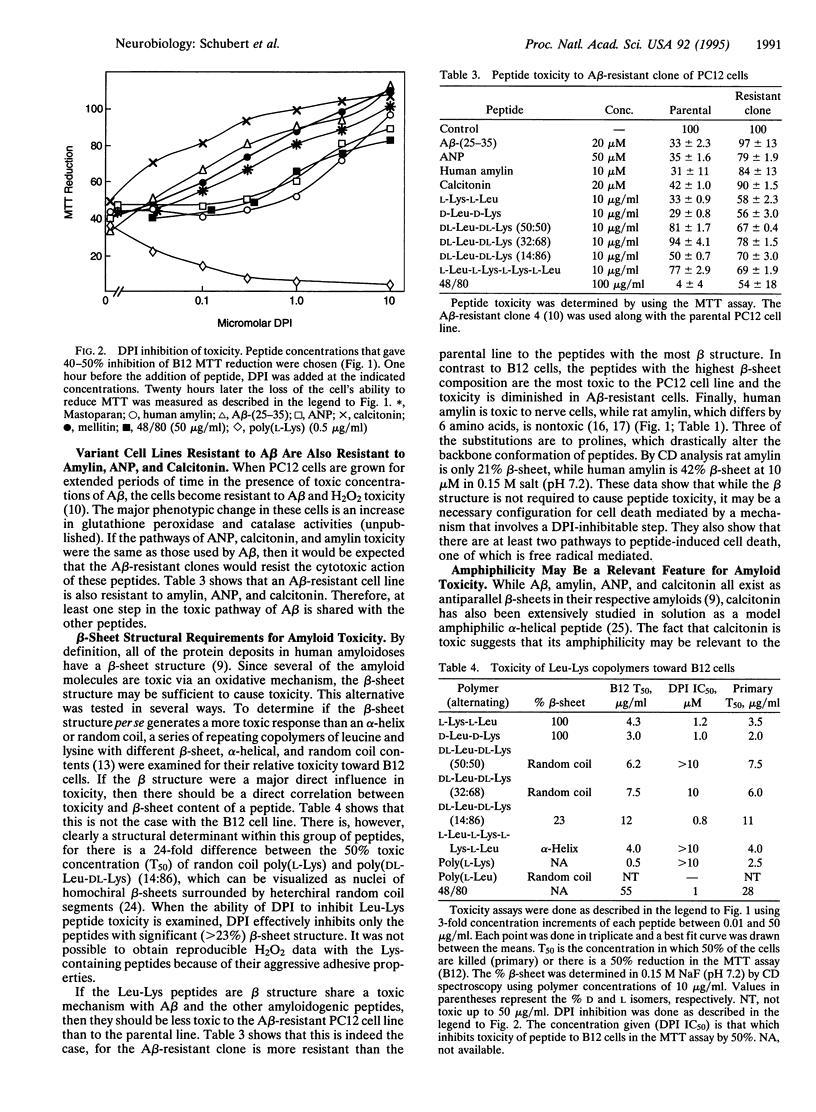
Abstract
beta-Amyloid protein (A beta) is a member of a small group of proteins that accumulate as amyloid deposits in various tissues. It has recently been demonstrated that the toxicity of A beta toward some neural cells is caused by oxidative damage. Since all of the amyloid diseases are characterized by protein deposited in the antiparallel beta-sheet conformation, it was asked whether there is a common toxic mechanism. It is shown here that the protein components of other human amyloidoses, including amylin, calcitonin, and atrial natriuretic peptide, are all toxic to clonal and primary cells. The toxicity is mediated via a free radical pathway indistinguishable from that of A beta. Experiments with synthetic peptides suggest that it is the amphiphilic nature of the peptides generated by their beta structure rather than their beta structure per se that causes toxicity. These results tend to rule out the alternative that amyloid toxicity is exclusively mediated via specific cell surface receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Takayanagi M., Saito H. Effects of recombinant human basic fibroblast growth factor and its modified protein CS23 on survival of primary cultured neurons from various regions of fetal rat brain. Jpn J Pharmacol. 1990 Jun;53(2):221–227. doi: 10.1254/jjp.53.221. [DOI] [PubMed] [Google Scholar]
- Asai J., Nakazato M., Kangawa K., Matsukura S., Matsuo H. Isolation and sequence determination of rat islet amyloid polypeptide. Biochem Biophys Res Commun. 1989 Oct 16;164(1):400–405. doi: 10.1016/0006-291x(89)91733-6. [DOI] [PubMed] [Google Scholar]
- Ballermann B. J., Brenner B. M. George E. Brown memorial lecture. Role of atrial peptides in body fluid homeostasis. Circ Res. 1986 May;58(5):619–630. doi: 10.1161/01.res.58.5.619. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J., Cole G. M., Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992 Jul 31;186(2):944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- Brack A., Caille A. Synthesis and beta-conformation of copolypeptides with alternating hydrophilic and hydrophobic residues. Int J Pept Protein Res. 1978 Feb;11(2):128–139. doi: 10.1111/j.1399-3011.1978.tb02831.x. [DOI] [PubMed] [Google Scholar]
- Brack A., Spach G. Beta-structures of polypeptides with L-and D-residues. Part I. Synthesis and conformational studies. J Mol Evol. 1979 Jun 8;13(1):35–46. doi: 10.1007/BF01732752. [DOI] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M. C., Crawford F., Houlden H., Warren A., Hughes D., Fidani L., Goate A., Rossor M., Roques P., Hardy J. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991 Oct 31;353(6347):844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Murdoch W. L., Kyle R. A., Westermark P., Pitkänen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med. 1983 Oct;75(4):618–623. doi: 10.1016/0002-9343(83)90443-6. [DOI] [PubMed] [Google Scholar]
- Cross A. R. Inhibitors of the leukocyte superoxide generating oxidase: mechanisms of action and methods for their elucidation. Free Radic Biol Med. 1990;8(1):71–93. doi: 10.1016/0891-5849(90)90147-b. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Burnier J., Ross E. M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J Biol Chem. 1990 Aug 25;265(24):14176–14186. [PubMed] [Google Scholar]
- KESTON A. S., BRANDT R. THE FLUOROMETRIC ANALYSIS OF ULTRAMICRO QUANTITIES OF HYDROGEN PEROXIDE. Anal Biochem. 1965 Apr;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Secondary structures of proteins and peptides in amphiphilic environments. (A review). Proc Natl Acad Sci U S A. 1983 Feb;80(4):1137–1143. doi: 10.1073/pnas.80.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf C., Klausner R. D., Weinstein J. N., Van Renswoude J., Pincus M., Blumenthal R. Voltage-dependent trans-bilayer orientation of melittin. J Biol Chem. 1982 Mar 10;257(5):2469–2476. [PubMed] [Google Scholar]
- Krejchi M. T., Atkins E. D., Waddon A. J., Fournier M. J., Mason T. L., Tirrell D. A. Chemical sequence control of beta-sheet assembly in macromolecular crystals of periodic polypeptides. Science. 1994 Sep 2;265(5177):1427–1432. doi: 10.1126/science.8073284. [DOI] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. C., Boggs L. N., Fuson K. S. Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer's disease amyloid-beta neurotoxicity. J Neurochem. 1993 Dec;61(6):2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Balasubramaniam A. Promotion of beta-structure by interaction of diabetes associated polypeptide (amylin) with phosphatidylcholine. Biochim Biophys Acta. 1992 Aug 21;1122(3):317–320. doi: 10.1016/0167-4838(92)90411-6. [DOI] [PubMed] [Google Scholar]
- Moe G. R., Kaiser E. T. Design, synthesis, and characterization of a model peptide having potent calcitonin-like biological activity: implications for calcitonin structure/activity. Biochemistry. 1985 Apr 9;24(8):1971–1976. doi: 10.1021/bi00329a026. [DOI] [PubMed] [Google Scholar]
- Mousli M., Bueb J. L., Bronner C., Rouot B., Landry Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol Sci. 1990 Sep;11(9):358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- Murrell J., Farlow M., Ghetti B., Benson M. D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall J. A., Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993 May;302(2):348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Ragan C. I., Iversen L. L. Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of beta-amyloid-mediated cell death. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1470–1474. doi: 10.1073/pnas.91.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe J. D. Amyloidosis. Annu Rev Biochem. 1992;61:947–975. doi: 10.1146/annurev.bi.61.070192.004503. [DOI] [PubMed] [Google Scholar]
- Song D. L., Chang G. D., Ho C. L., Chang C. H. Structural requirements of mastoparan for activation of membrane-bound guanylate cyclase. Eur J Pharmacol. 1993 Nov 15;247(3):283–288. doi: 10.1016/0922-4106(93)90196-g. [DOI] [PubMed] [Google Scholar]
- Soreghan B., Kosmoski J., Glabe C. Surfactant properties of Alzheimer's A beta peptides and the mechanism of amyloid aggregation. J Biol Chem. 1994 Nov 18;269(46):28551–28554. [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Shih I. L., Lees A. M., Lees R. S. Surface-induced conformational switching in amphiphilic peptide segments of apolipoproteins B and E and model peptides. Int J Pept Protein Res. 1993 Jun;41(6):536–547. doi: 10.1111/j.1399-3011.1993.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]



