Abstract
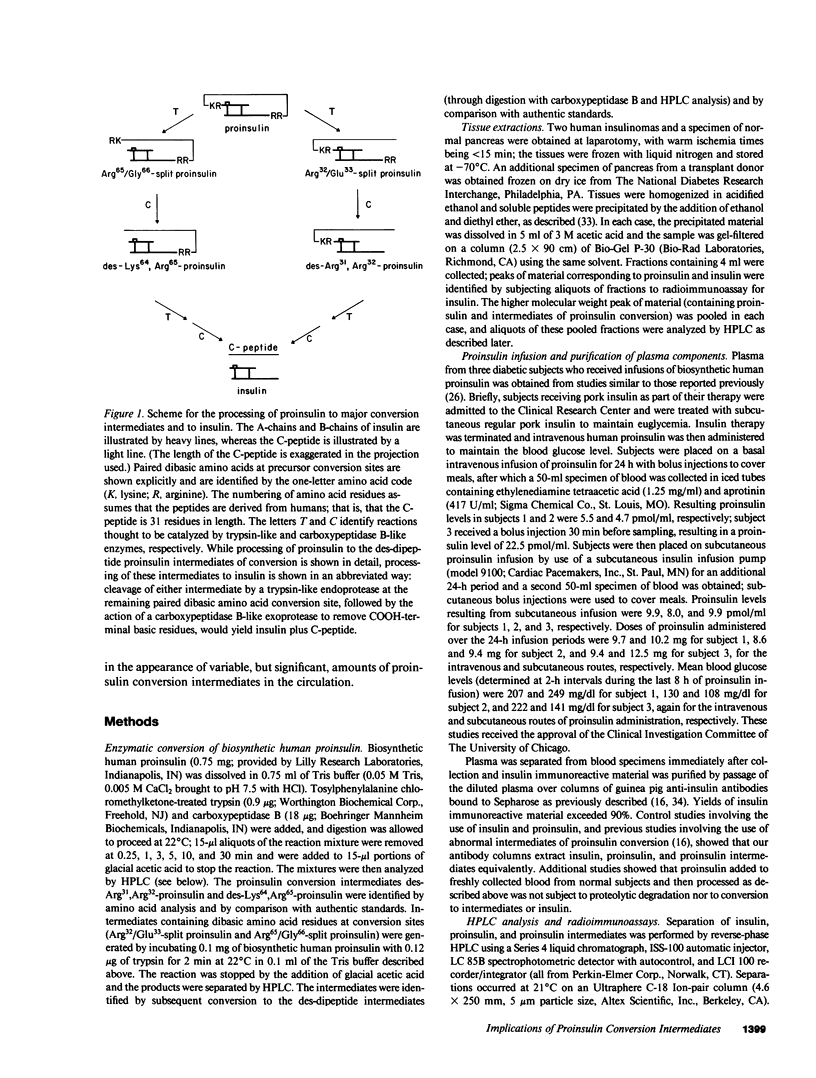
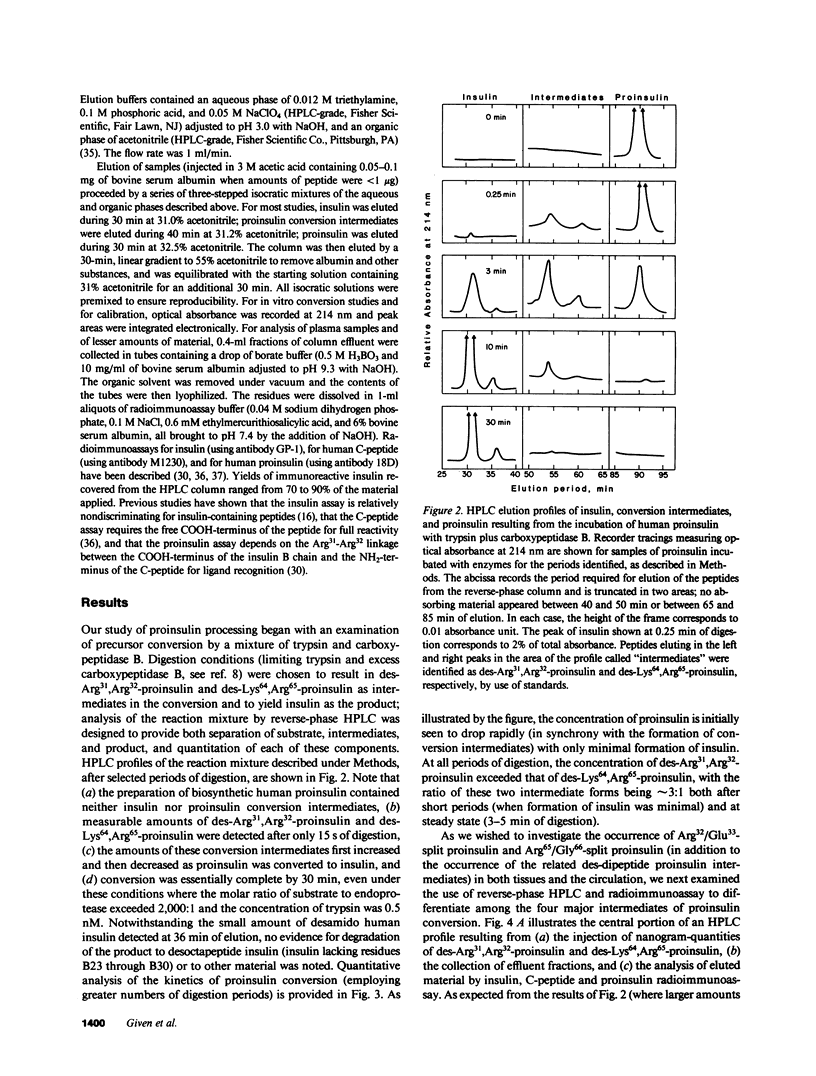
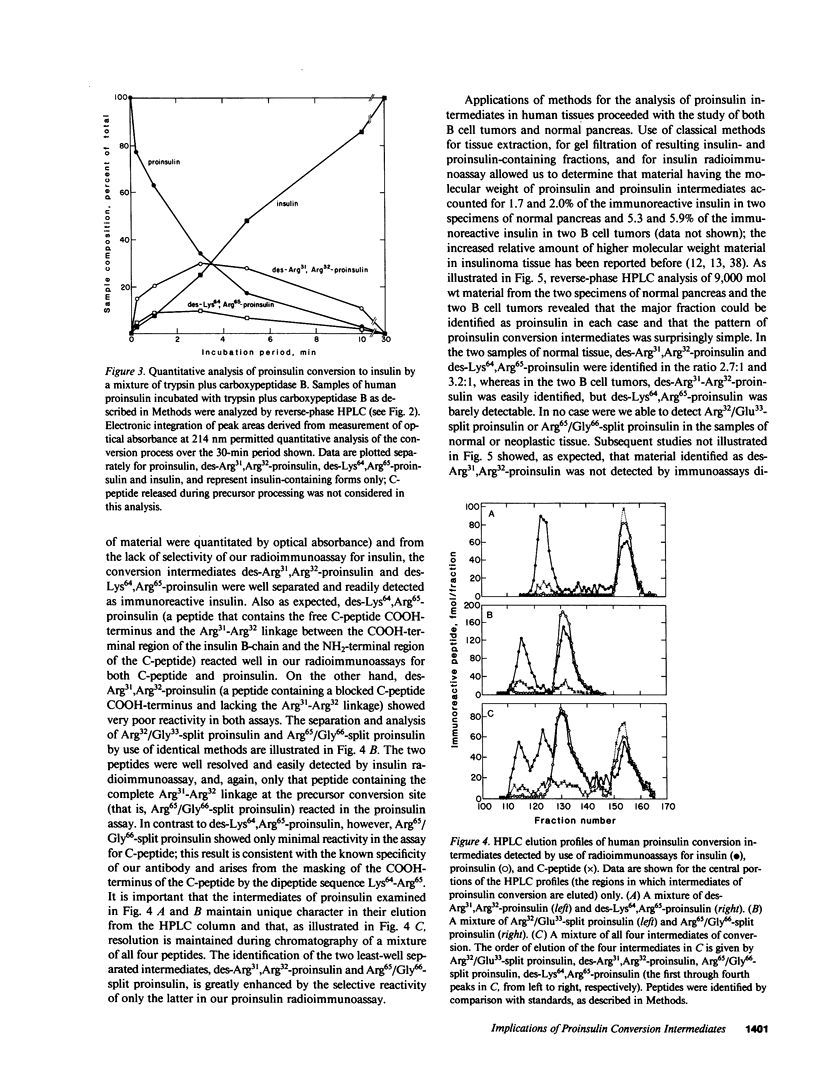
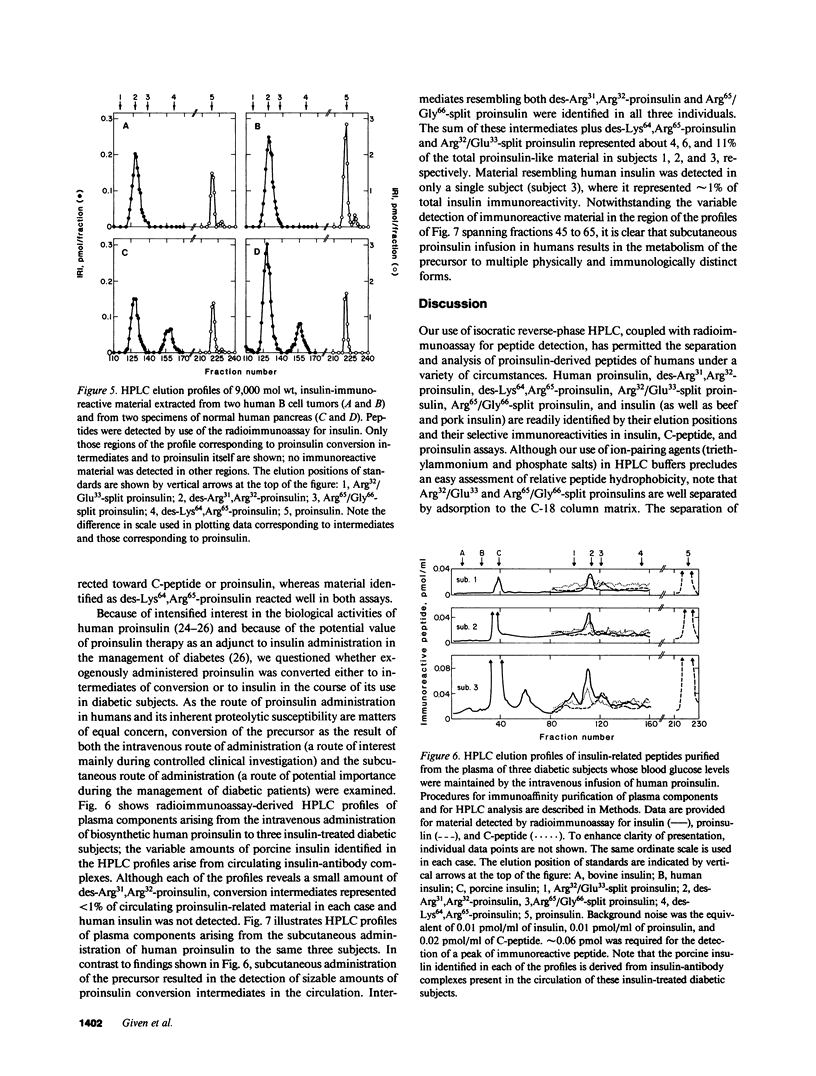
Since a complete map of insulin-related peptides in humans requires consideration of proinsulin, Arg32/Glu33-split proinsulin, Arg65/Gly66-split proinsulin, des-Arg31,Arg32-proinsulin, des-Lys64, Arg65-proinsulin, and insulin, we applied high performance liquid chromatography coupled with radioimmunoassay to investigate the formation of proinsulin conversion intermediates in vitro and in vivo. Kinetic analysis of proinsulin processing by a mixture of trypsin and carboxypeptidase B (to stimulate in vivo processes) revealed (a) a rapid decline in proinsulin concommitant with formation of conversion intermediates, (b) formation of des-Arg31, Arg32-proinsulin and des-Lys64,Arg65-proinsulin in the ratio 3.3:1 at steady state, and (c) complete conversion of the precursor to insulin during extended incubation. Studies on normal human pancreas identified a similar ratio of des-Arg31,Arg32-proinsulin to des-Lys64,Arg65-proinsulin (approximately 3:1), whereas two insulinomas contained sizable amounts of des-Arg31,Arg32-proinsulin, but barely detectable amounts of des-Lys64,Arg65-proinsulin. None of the tissues contained measurable quantities of Arg32/Glu33- or Arg65/Gly66-split proinsulin. Analysis of plasma from three diabetic subjects managed by the intravenous infusion of human proinsulin revealed less than 1% processing of the circulating precursor to conversion intermediates and no processing of the precursor to human insulin. Nevertheless, analysis of plasma from the same subjects managed by the subcutaneous infusion of proinsulin revealed 4-11% processing of the precursor to intermediates that had the properties of des-Arg31,Arg32-proinsulin and Arg65/Gly66-split proinsulin. We conclude that (a) processing of proinsulin to insulin in vivo as in vitro likely occurs by preferential cleavage at the Arg32-Glu33 peptide bond in proinsulin, (b) proinsulin is inefficiently processed in the vascular compartment, and (c) subcutaneous administration of the precursor can result in the formation of conversion intermediates with the potential for contributing to biological activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsever R. N., Roberts J. P., Gerber J. G., Mako M. E., Rubenstein A. H. Insulinoma with low circulating insulin levels: the diagnostic value of proinsulin measurements. Ann Intern Med. 1975 Mar;82(3):347–350. doi: 10.7326/0003-4819-82-3-347. [DOI] [PubMed] [Google Scholar]
- Bergenstal R. M., Cohen R. M., Lever E., Polonsky K., Jaspan J., Blix P. M., Revers R., Olefsky J. M., Kolterman O., Steiner K. The metabolic effects of biosynthetic human proinsulin in individuals with type I diabetes. J Clin Endocrinol Metab. 1984 Jun;58(6):973–979. doi: 10.1210/jcem-58-6-973. [DOI] [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. M., Nakabayashi T., Blix P. M., Rue P. A., Shoelson S. E., Root M. A., Frank B. H., Revers R. R., Rubenstein A. H. A radioimmunoassay for circulating human proinsulin. Diabetes. 1985 Jan;34(1):84–91. doi: 10.2337/diab.34.1.84. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt C., Track N. S., Creutzfeldt W. In vitro studies of the rate of proinsulin and insulin turnover in seven human insulinomas. Eur J Clin Invest. 1973 Sep;3(5):371–384. doi: 10.1111/j.1365-2362.1973.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C., Markussen J., Heding L. G., Naithani V. K., Kuzuya H., Blix P., Horwitz D. L., Rubenstein A. H. Characterization of seven C-peptide antisera. Diabetes. 1978;27 (Suppl 1):170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., DeLuca K., Fisher J. N., Jr, Mako M. E., Rubenstein A. H. Familial hyperproinsulinemia. An autosomal dominant defect. N Engl J Med. 1976 Apr 22;294(17):911–915. doi: 10.1056/NEJM197604222941701. [DOI] [PubMed] [Google Scholar]
- Given B. D., Mako M. E., Tager H. S., Baldwin D., Markese J., Rubenstein A. H., Olefsky J., Kobayashi M., Kolterman O., Poucher R. Diabetes due to secretion of an abnormal insulin. N Engl J Med. 1980 Jan 17;302(3):129–135. doi: 10.1056/NEJM198001173020301. [DOI] [PubMed] [Google Scholar]
- Gutman R. A., Lazarus N. R., Penhos J. C., Fajans S., Recant L. Circulating proinsulin-like material in patients with functioning insulinomas. N Engl J Med. 1971 May 6;284(18):1003–1008. doi: 10.1056/NEJM197105062841803. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Specific and direct radioimmunoassay for human proinsulin in serum. Diabetologia. 1977 Sep;13(5):467–474. doi: 10.1007/BF01234498. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen E. P., Courtney J. W., 3rd, Duckworth W. C. Insulin resistance caused by massive degradation of subcutaneous insulin. Diabetes. 1979 Jul;28(7):640–645. doi: 10.2337/diab.28.7.640. [DOI] [PubMed] [Google Scholar]
- Peavy D. E., Abram J. D., Frank B. H., Duckworth W. C. In vitro activity of biosynthetic human proinsulin. Receptor binding and biologic potency of proinsulin and insulin in isolated rat adipocytes. Diabetes. 1984 Nov;33(11):1062–1067. doi: 10.2337/diab.33.11.1062. [DOI] [PubMed] [Google Scholar]
- Podlecki D. A., Frank B. H., Olefsky J. M. In vitro characterization of biosynthetic human proinsulin. Diabetes. 1984 Feb;33(2):111–118. doi: 10.2337/diab.33.2.111. [DOI] [PubMed] [Google Scholar]
- Powers C. A., Nasjletti A. A novel kinin-generating protease (kininogenase) in the porcine anterior pituitary. J Biol Chem. 1982 May 25;257(10):5594–5600. [PubMed] [Google Scholar]
- Rainbow S. J., Woodhead J. S., Yue D. K., Luzio S. D., Hales C. N. Measurement of human proinsulin by an indirect two-site immunoradiometric assay. Diabetologia. 1979 Oct;17(4):229–234. doi: 10.1007/BF01235859. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Henry R., Schmeiser L., Kolterman O., Cohen R., Bergenstal R., Polonsky K., Jaspan J., Rubenstein A., Frank B. The effects of biosynthetic human proinsulin on carbohydrate metabolism. Diabetes. 1984 Aug;33(8):762–770. doi: 10.2337/diab.33.8.762. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Henry R., Schmeiser L., Kolterman O., Cohen R., Rubenstein A., Frank B., Galloway J., Olefsky J. M. Biosynthetic human insulin and proinsulin have additive but not synergistic effects on total body glucose disposal. J Clin Endocrinol Metab. 1984 Jun;58(6):1094–1098. doi: 10.1210/jcem-58-6-1094. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Blix P. M., Rubenstein A. H., Kanazawa Y., Kosaka K., Tager H. S. A human proinsulin variant at arginine 65. Nature. 1981 Jun 25;291(5817):679–681. doi: 10.1038/291679a0. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Rubenstein A. H., Tager H. S. Familial hyperproinsulinemia. Two cohorts secreting indistinguishable type II intermediates of proinsulin conversion. J Clin Invest. 1984 Mar;73(3):714–719. doi: 10.1172/JCI111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. C., Tager H. S., Rubenstein A. H. Biologic and clinical importance of proinsulin. N Engl J Med. 1984 May 3;310(18):1165–1175. doi: 10.1056/NEJM198405033101807. [DOI] [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Pottenger L. A., Mako M., Getz G. S., Steiner D. F. The metabolism of proinsulin and insulin by the liver. J Clin Invest. 1972 Apr;51(4):912–921. doi: 10.1172/JCI106886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman B. M., Pek S., Fajans S. S., Floyd J. C., Jr, Conn J. W. Plasma proinsulin in patients with functioning pancreatic islet cell tumors. J Clin Endocrinol Metab. 1972 Aug;35(2):271–280. doi: 10.1210/jcem-35-2-271. [DOI] [PubMed] [Google Scholar]
- Shoelson S., Fickova M., Haneda M., Nahum A., Musso G., Kaiser E. T., Rubenstein A. H., Tager H. Identification of a mutant human insulin predicted to contain a serine-for-phenylalanine substitution. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7390–7394. doi: 10.1073/pnas.80.24.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S., Haneda M., Blix P., Nanjo A., Sanke T., Inouye K., Steiner D., Rubenstein A., Tager H. Three mutant insulins in man. Nature. 1983 Apr 7;302(5908):540–543. doi: 10.1038/302540a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L., Nolan C., Rubenstein A. H., Margoliash E., Aten B., Oyer P. E. Proinsulin and the biosynthesis of insulin. Recent Prog Horm Res. 1969;25:207–282. doi: 10.1016/b978-0-12-571125-8.50008-9. [DOI] [PubMed] [Google Scholar]
- Steiner D. F. Cocrystallization of proinsulin and insulin. Nature. 1973 Jun 29;243(5409):528–530. doi: 10.1038/243528a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager H. S., Patzelt C., Assoian R. K., Chan S. J., Duguid J. R., Steiner D. F. Biosynthesis of islet cell hormones. Ann N Y Acad Sci. 1980;343:133–147. doi: 10.1111/j.1749-6632.1980.tb47247.x. [DOI] [PubMed] [Google Scholar]
- Tager H., Given B., Baldwin D., Mako M., Markese J., Rubenstein A., Olefsky J., Kobayashi M., Kolterman O., Poucher R. A structurally abnormal insulin causing human diabetes. Nature. 1979 Sep 13;281(5727):122–125. doi: 10.1038/281122a0. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M. A., Vassalli J. D., Estensen R. D., Reich E. Plasminogen activator of islets of Langerhans: modulation by glucose and correlation with insulin production. Proc Natl Acad Sci U S A. 1980 Feb;77(2):875–879. doi: 10.1073/pnas.77.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. S., Kitbachi A. E. Biological activity of proinsulin and related polypeptides in the fat tissue. J Biol Chem. 1973 Jun 10;248(11):3753–3761. [PubMed] [Google Scholar]
- de Haën C., Little S. A., May J. M., Williams R. H. Characterization of proinsulin-insulin intermediates in human plasma. J Clin Invest. 1978 Oct;62(4):727–737. doi: 10.1172/JCI109183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ole-MoiYoi O. K., Pinkus G. S., Spragg J., Austen K. F. Identification of human glandular kallikrein in the beta cell of the pancreas. N Engl J Med. 1979 Jun 7;300(23):1289–1294. doi: 10.1056/NEJM197906073002301. [DOI] [PubMed] [Google Scholar]



