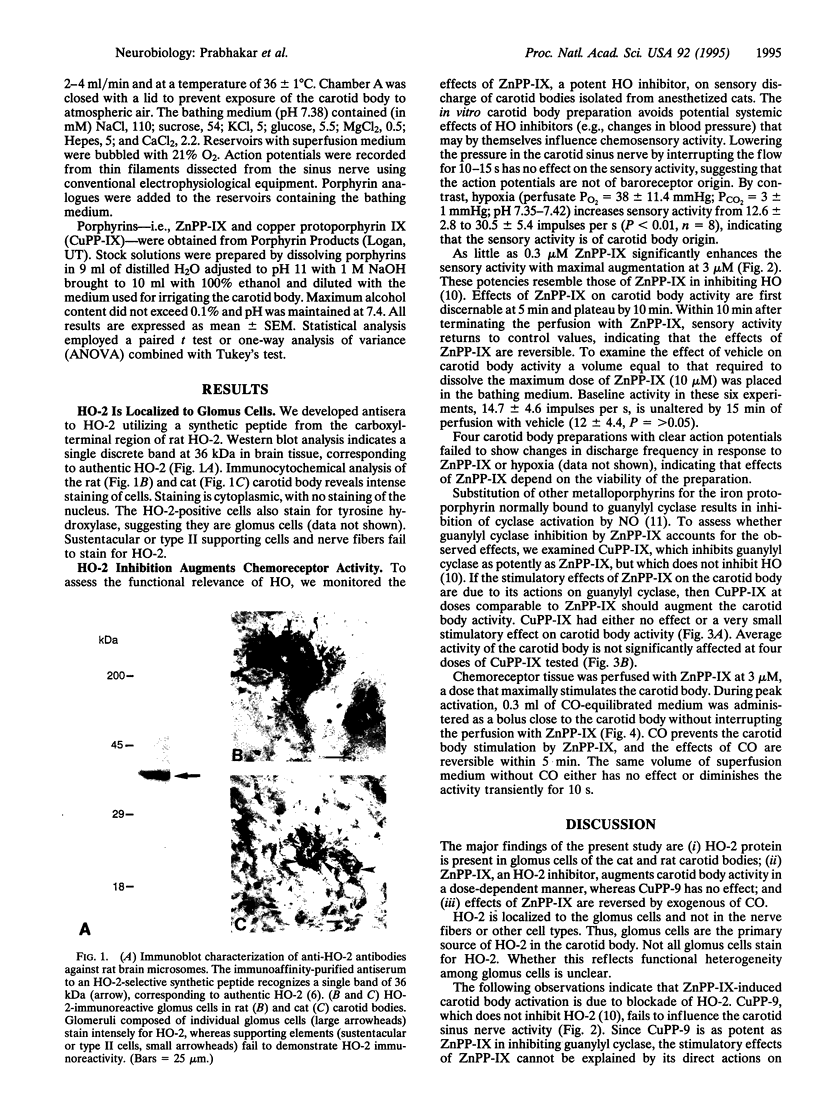
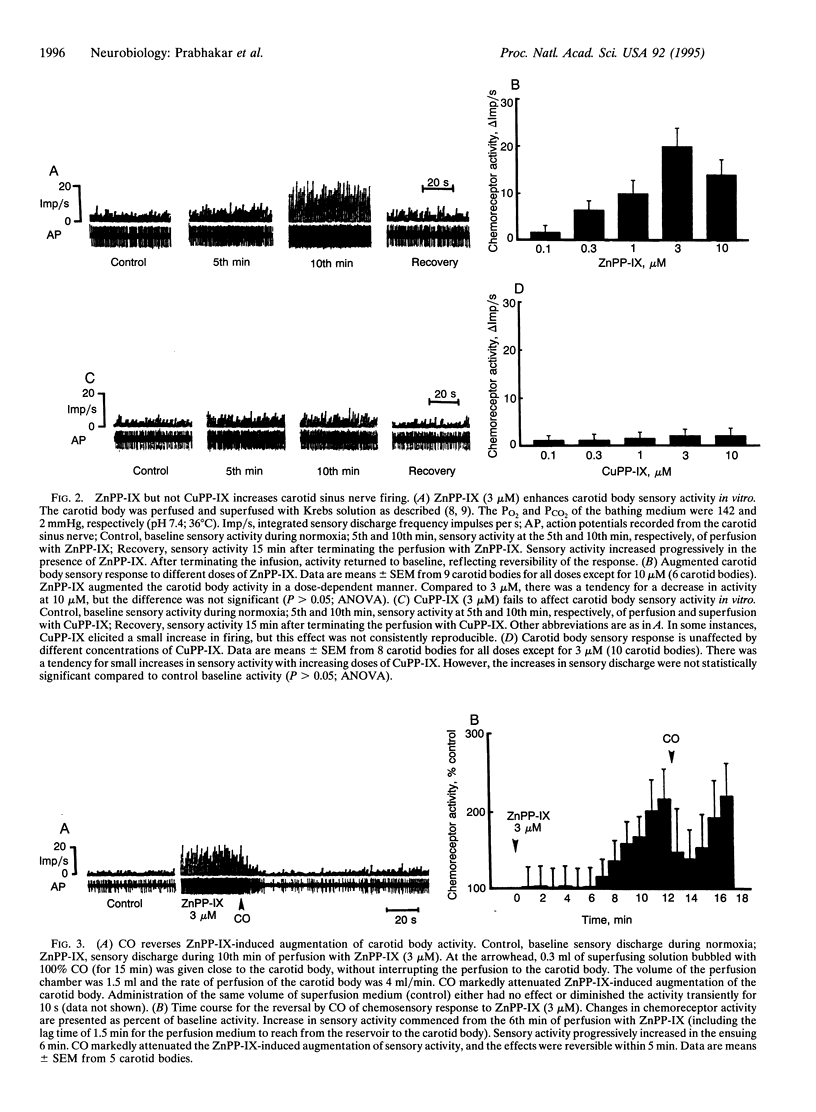
Abstract
Carbon monoxide (CO), produced endogenously by heme oxygenase, has been implicated as a neuronal messenger. Carotid bodies are sensory organs that regulate ventilation by responding to alterations of blood oxygen, CO2, and pH. Changes in blood gases are sensed by glomus cells in the carotid body that synapse on afferent terminals of the carotid sinus nerve that projects to respiratory-related neurons in the brainstem. Using immunocytochemistry, we demonstrate that heme oxygenase 2 is localized to glomus cells in the cat and rat carotid bodies. Physiological studies show that zinc protoporphyrin IX, a potent heme oxygenase inhibitor, markedly increases carotid body sensory activity, while copper protoporphyrin IX, which does not inhibit the enzyme, is inactive. Exogenous CO reverses the stimulatory effects of zinc protoporphyrin IX. These results suggest that glomus cells are capable of synthesizing CO and endogenous CO appears to be a physiologic regulator of carotid body sensory activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H. PO2 chemoreception in arterial chemoreceptors. Annu Rev Physiol. 1989;51:835–844. doi: 10.1146/annurev.ph.51.030189.004155. [DOI] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987 Oct;32(4):497–504. [PubMed] [Google Scholar]
- Docherty J. C., Schacter B. A., Firneisz G. D., Brown S. B. Mechanism of action of heme oxygenase. A study of heme degradation to bile pigment by 18O labeling. J Biol Chem. 1984 Nov 10;259(21):13066–13069. [PubMed] [Google Scholar]
- Fotuhi M., Sharp A. H., Glatt C. E., Hwang P. M., von Krosigk M., Snyder S. H., Dawson T. M. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993 May;13(5):2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28(1-3):52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Ballot B., Wood K. S. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins. J Biol Chem. 1984 May 25;259(10):6201–6207. [PubMed] [Google Scholar]
- Itano H. A., Hirota T. A two-molecule mechanism of haem degradation. Biochem J. 1985 Mar 15;226(3):767–771. doi: 10.1042/bj2260767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S., Iturriaga R., Mokashi A., Ray D. K., Chugh D. CO reveals dual mechanisms of O2 chemoreception in the cat carotid body. Respir Physiol. 1993 Nov;94(2):227–240. doi: 10.1016/0034-5687(93)90050-k. [DOI] [PubMed] [Google Scholar]
- López-López J. R., González C. Time course of K+ current inhibition by low oxygen in chemoreceptor cells of adult rabbit carotid body. Effects of carbon monoxide. FEBS Lett. 1992 Mar 16;299(3):251–254. doi: 10.1016/0014-5793(92)80126-2. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. R., Kumar G. K., Chang C. H., Agani F. H., Haxhiu M. A. Nitric oxide in the sensory function of the carotid body. Brain Res. 1993 Oct 15;625(1):16–22. doi: 10.1016/0006-8993(93)90132-7. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. Nature. 1993 Jul 8;364(6433):147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- Sun Y., Rotenberg M. O., Maines M. D. Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem. 1990 May 15;265(14):8212–8217. [PubMed] [Google Scholar]
- Vedernikov Y. P., Gräser T., Vanin A. F. Similar endothelium-independent arterial relaxation by carbon monoxide and nitric oxide. Biomed Biochim Acta. 1989;48(8):601–603. [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Wang Z. Z., Bredt D. S., Fidone S. J., Stensaas L. J. Neurons synthesizing nitric oxide innervate the mammalian carotid body. J Comp Neurol. 1993 Oct 15;336(3):419–432. doi: 10.1002/cne.903360308. [DOI] [PubMed] [Google Scholar]
- Wang Z. Z., Stensaas L. J., de Vente J., Dinger B., Fidone S. J. Immunocytochemical localization of cAMP and cGMP in cells of the rat carotid body following natural and pharmacological stimulation. Histochemistry. 1991;96(6):523–530. doi: 10.1007/BF00267078. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982 Jul 10;257(13):7778–7785. [PubMed] [Google Scholar]
- Zhuo M., Small S. A., Kandel E. R., Hawkins R. D. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993 Jun 25;260(5116):1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]