Abstract
Purpose
The purpose of this work was to evaluate a prospectively initiated two-center protocol of risk-adapted single-fraction (SRS) or fractionated radiotherapy (SRT) in patients with nonsecretory pituitary adenomas (NSA).
Patients and methods
A total of 73 NSA patients (39 men/34 women) with a median age of 62 years were prospectively included in a treatment protocol of SRS [planning target volume (PTV) < 4 ccm, > 2 mm to optic pathways = low risk] or SRT (PTV ≥ 4 ccm, ≤ 2 mm to optic pathways = high risk) in two Novalis® centers. Mean tumor volume was 7.02 ccm (range 0.58–57.29 ccm). Based on the protocol guidelines, 5 patients were treated with SRS and 68 patients with SRT.
Results
Median follow-up (FU) reached 5 years with 5-year overall survival (OS) of 90.4 % (CI 80.2–95 %) and 5-year local control and progression-free survival rates of 100 % (CI 93.3–100 %) and 90.4 % (CI 80.2–95 %), respectively. A post-SRS/SRT new visual disorder occurred in 2 patients (2.7 %), a new oculomotor nerve palsy in one pre-irradiated patient, in 3 patients (4.1 %) a pre-existing visual disorder improved. New complete hypopituitarism occurred in 4 patients (13.8 %) and in 3 patients (25 %) with pre-existing partial hypopituitarism. Pituitary function in 26 % of patients retained normal. Patients with tumor shrinkage (65.75 %) had a significantly longer FU (p = 0.0093). Multivariate analysis confirmed correlation of new hypopituitarism with duration of FU (p = 0.008) and correlation of new hypopituitarism and tumor volume (p = 0.023). No significant influence factors for occurrence of visual disorders were found.
Conclusion
Our SRS/SRT protocol proved to be safe and successful in terms of tumor control and protection of the visual system, especially for large tumors located close to optic pathways.
Keywords: Nonsecretory pituitary adenoma, Radiosurgery, Stereotactic radiotherapy, Hypopituitarism
Abstract
Ziel
Evaluation eines prospektiv angelegten Behandlungsprotokolls einer risikoadaptierten Radiochirurgie (SRS) oder stereotaktischen Radiotherapie (SRT) von Patienten mit hormoninaktiven Hypophysenadenomen (NSA) aus zwei Zentren.
Patienten und Methoden
73 NSA-Patienten (39 Männer, 34 Frauen) mit einem medianen Alter von 62 Jahren wurden nach einem prospektiven Protokoll entweder mit SRS (PTV < 4 ccm, > 2 mm zum optischen System = geringes Risiko) oder fraktionierter SRT (PTV ≥ 4 ccm, ≤ 2 mm zum optischen System = hohes Risiko) in zwei Novalis®-Zentren behandelt. Das mittlere Tumorvolumen betrug 7,02 ccm (Spanne 0,58–57,29 ccm). Nach diesem Protokoll wurden 5 Patienten mit SRS und 68 mit SRT behandelt.
Ergebnisse
Ein medianes Follow-up von 5 Jahren wurde mit einer 5-Jahres-Überlebensrate von 90,4 % (CI 80,2–95 %) und einer lokalen 5-Jahres-Kontrollrate und progressionsfreier Überlebensrate von 100 % (CI 93,3–100 %) bzw. 90,4 % (CI 80,2–95 %) erreicht. Bei 2 Patienten (2,7 %) trat eine post-SRS/SRT-Störung des optischen Systems und bei einem auswärtig vorbestrahlten Patienten eine neue Okulomotoriusparese auf; bei 3 Patienten (4,1 %) wurde eine Sehverbesserung beobachtet. Eine neue komplette Hypophyseninsuffizienz post-SRS/SRT wurde in 4 (13,8 %) und in 3 Patienten (25 %) mit vorbestehender partieller Hypophyseninsuffizienz beobachtet. Bei 26 % der Patienten blieb die Hypophysenfunktion normal. Patienten mit Tumorschrumpfung (65,75 %) hatten ein deutlich längeres Follow-up (p = 0,0086). Die multivariate Analyse bestätigte eine Korrelation von neuer Hypophyseninsuffizienz mit der Follow-up-Dauer (p = 0,007) und eine Korrelation einer neuen Hypophyseninsuffizienz mit dem Tumorvolumen (p = 0,019). Signifikante Einflussfaktoren für das Auftreten einer Sehstörung wurden nicht identifiziert.
Schlussfolgerung
Unser SRS/SRT-Behandlungsprotokoll erwies sich vor allem bei großen Tumoren in der Nähe des optischen Systems als erfolgreich und sicher hinsichtlich Tumorkontrolle und Schutz des optischen Systems.
Schlüsselwörter: Hormoninaktive Hypophysenadenome, Radiochirurgie, Stereotaktische Radiotherapie, Hypophyseninsuffizienz
Abbreviations
- PA
Pituitary adenoma
- NSA
Nonsecretory pituitary adenoma
- SRS
Stereotactic radiosurgery
- SRT
Stereotactic radiotherapy
- OS
Overall survival
- PFS
Progression free survival
- PTV
Planning target volume
- CTV
Clinical target volume
- RTOG
Radiation therapy oncology group
- OARs
Organs at risk
- LINAC
Linear accelerator
- MRI
Magnetic resonance imaging
- RT
Radiotherapy
- Gy
Gray
- CTC
Common Terminology Criteria for adverse events
- RECIST
Response Evaluation Criteria in Solid Tumors
- TSH
Thyroid-stimulating hormone
- fT4
Free thyroxin
- ACTH
Adrenocorticotropic hormone
- LH
Luteinizing hormone
- FSH
Follicle-stimulating hormone
- IGF-1
Insulin-like growth factor 1
- (hf) SRT
hypofractionated stereotactic radiotherapy
- N.D.
not done
Pituitary adenomas (PA) are divided into secretory and nonsecretory varieties and the intention of treatment differ for the two entities. Treatment for secretory PA mainly aims to prevent excessive secretion of hormones, whereas treatment of nonsecretory PA (NSA) is intended to control tumor growth and prevent or reverse visual disorders and endocrinopathies [8, 12, 27]. So far, most articles have been summarizing both entities together; however we consider these two varieties in terms of clinic as well as in terms of treatment completely differently, which underlines the need to investigate these two varieties entirely separately. Thus, we excluded the hormone secreting tumors from the study presented herein.
Approximately 30 % of PA are nonsecretory and especially when causing visual symptoms are treated primarily with transsphenoidal surgery or craniotomy. Yet frequently patients have residual postoperative tumors and several studies have reported recurrences in about 20–50 % of cases treated with surgery alone [3, 6, 13, 28].
There are still no clear guidelines with regard to radiotherapy (RT) because of the lack of randomized controlled studies. RT is commonly considered in cases in which a large amount of tumor is left behind or if the residual tumor is located close to the optic nerves/chiasm and regrowth may lead to visual compromise. RT is also considered if residual or recurrent tumors invade in the cavernous sinus or in cases in which repeated surgeries have resulted in fibrosis and inoperability [1, 10, 31, 33, 36, 39]. A review on conventional radiotherapy for NSAs demonstrated an overall progression-free survival of 80–90 % at 10 years and 75–90 % at 20 years [25]. Considering the proximity of organs at risk (OARs) such as the optic nerves, chiasm and brain stem, the use of stereotactic irradiation has been increasingly considered [29, 36]. A review on stereotactic radiosurgery (SRS) for NSA reported a tumor growth control rate of 87–100 % with a follow-up of 6–60 months [26]. However, a single high-dose treatment may not be appropriate for large tumors or tumors adjacent to optic pathways because of the limited dose tolerance for these structures [37]. Thus, protection of optic pathways and the brain stem may be more efficiently achieved by using lower daily doses with a fractionated regime rather than SRS [17]. More recently, some reports have indicated promising outcomes with stereotactic radiotherapy (SRT) using conventional fractionation or moderate hypofractionation [5, 15, 16, 23]. However, these data are still preliminary with a relatively short follow-up.
In the study presented herein, however a high number of NSA has been prospectively followed after fractionated SRT and SRS with complete radiological, endocrinological, and ophthalmological data. All patients had been treated with harmonized protocols at two institutions with the same irradiation system either as SRS or SRT in a risk-adapted manner.
Methods and patients
Patients and treatment protocol
We included 73 patients with NSA from July 2000 to March 2010 fulfilling following eligibility criteria: (1) histologically confirmed or image diagnosed PA with endocrinological findings indicating NSA; (2) recurrent cases, patients receiving postoperative adjuvant SRS/SRT, inoperable patients, and patients who refused surgical resection; (3) no prior RT or chemotherapy for other cranial disease; and (4) willingness to provide written informed consent. The patient and tumor characteristics are summarized in Table 1.
Table 1.
Overview of clinical data before and after irradiation and irradiation parameters
| Variable | Overall | Average | |
|---|---|---|---|
| N | 73 | ||
| Age (range) | (30–82) | 60 (mean) | |
| No. of surgeries (range) | (0–4) | 1.41 (mean) | |
| Fractionation | |||
| SRS | 5 | 7 % | |
| SRT | 67 | 92 % | |
| (hf) SRT | 1 | 1 % | |
| RT | Adjuvant | 63 | 86 % |
| primary | 10 | 14 % | |
| CTV (range), ccm | (0.58–57.29) | 7.02 (mean) | |
| SRS | (1.04–1.94) | 1.69 (median) | |
| SRT | (0.58–57.29) | 4.05 (median) | |
| (hf) SRT | 2.21 | ||
| Fx (range) | (1.00–31.00) | ||
| SRS | 1 | ||
| SRT | 25–31 | 26 (mean) | |
| (hf) SRT | 7 | ||
| Daily dose, Gy | |||
| SRS | 15, 18, 20 | ||
| SRT | 1.8–2.0 | ||
| (hf) SRT | 5 | ||
| Total dose (range), Gy | (15.00–56.00) | ||
| SRS | 18, 20, 30 | ||
| SRT | 45–62 | 52 (median) | |
| (hf) SRT | 35 | ||
| Follow up (range) years | (0.5–11.0) | 5.16 (mean) | |
| Tumor size (at last follow-up) | |||
| Smaller | 48 | 66 % | |
| Stable | 25 | 34 % | |
| Larger | 0 | 0 % | |
| N.D. | 0 | ||
| Hypopituitarism (before RT) | |||
| Full | 32 | 43.8 % | |
| Partial | 12 | 16.4 % | |
| None | 29 | 39.7 % | |
| N.D. | 0 | ||
| Hypopituitarism (after RT) | |||
| Full | 39 | 53.4 % | |
| Partial | 15 | 20.5 % | |
| None | 19 | 26.0 % | |
| N.D. | 0 | ||
| Dysfunction of optical system (before RT) | |||
| Yes | 36 | 49.3 % | |
| None | 37 | 50.7 % | |
| Dysfunction of optical system (after RT) | |||
| None | 36 | 49.3 % | |
| Idem | 31 | 42.5 % | |
| Improvement | 3 | 4.1 % | |
| Aggravation | 3 | 4.1 % |
RT radiotherapy, SRS stereotactic radiosurgery, SRT stereotactic radiotherapy, (hf) SRT hypofractionated stereotactic radiotherapy, CTV clinical target volume, Gy Gray, N.D. not done
The treatment algorithm followed at the two institutions was as follows: SRS was considered the preferred treatment, if the target volume was smaller than 4 ccm and the closest distance to the optic pathways was above 2 mm. The single dose given was 15–20 Gy prescribed to the 80 % isodose line. In all other cases, SRT was preferred, consisting of 25–31 fractions in 1.8–2.0 Gy daily doses. Based on the protocol guidelines we used, only 5 patients were treated with SRS and the majority of 68 patients with SRT (see Table 1). Patient immobilization, treatment planning, geometrical accuracy of and clinical experiences with the Novalis® system used here have been reported by several investigators [9, 11, 14].
Follow-up evaluation and statistical analysis
After SRS/SRT, the patients were followed at 6 and 12 months during the first year, and at intervals of 12 months thereafter. Regular follow-up studies included clinical examination, brain MRI, visual perception tests, and examinations of hormonal levels.
The following hormones were routinely tested: thyroid-stimulating hormone (TSH), free tyroxin (fT4), adrenocorticotropic hormone (ACTH), cortisol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, prolactin, and insulin-like growth factor 1 (IGF-1). A 24-h cortisol urine test was performed only in some cases. Any deviation from the age and gender adjusted hormone levels of the corticotrope, thyrotrope, and gonadotrope hormone axes was counted as hypopituitarism, a hypofunction of all hormone axes represents a complete insufficiency, an isolated somatotrope dysfunction before or after irradiation was not counted as a partial hypopituitarism. New deficits 3 months after radiotherapy were regarded as being associated with radiotherapy.
The local responses to RT were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST). If there were no computer-generated tumor volumes available on follow-up MRI, tumor progression was defined as an increase in mean tumor dimension of more than 2 mm persisting on 2 or more consecutive studies. Tumor response was defined as decrease in mean tumor dimension of more than 2 mm persisting on 2 or more consecutive studies. Stable tumor was defined as no change in size or change of 2 mm or less. In most cases the DICOM data of the follow-up MRIs were available, so we could perform an image fusion with the planning MRI, generate and compare the tumor volumes, where response was defined as minus > 20 % volume and progression as plus 20 %.
The rates of overall survival, local control, and progression-free survival were calculated using the Kaplan–Meier method. Stepwise multiple linear regression tests were used in the analysis of following potential influence factors for tumor shrinkage, new hypopituitarism, or new visual deficit: age at treatment, SRS vs. SRT, primary vs. adjuvant SRS/SRT, CTV, total dose, number of fractions, follow-up in years, number of surgeries. Statistical analysis was performed using SPSS® (SPSS Inc., Chicago, IL, USA) software.
Results
Of the 73 patients, 10 patients were irradiated without primary surgery (all of them received SRT), the remaining 63 patients received irradiation to a progressive and/or residual tumor after one or more surgeries. Most patients had one or two surgeries before irradiation, namely 36 and 17, respectively. There were 5 patients who had 3 and even 5 patients who had 4 prior surgeries.
SRS received 5 patients with small sized residual tumors (all CTVs < 2 ccm) with additional sufficient distance from the optical system (> 2 mm). Only one patient received a hypofractionated (hf) SRT with 7 fractions of 5 Gy, because the CTV was too large for SRS, but the distance from the optical system was large enough for the higher daily dose. SRT was prescribed in the 67 remaining patients (see Table 1).
The figures show examples of treatment planning’s with dose–volume histograms, isodose lines and OARs including the chiasm, optic nerves, and brain stem as well as MRI follow-ups in a typical SRS (Fig. 1a and b) and a typical SRT case (Fig. 2a and b).
Fig. 1.
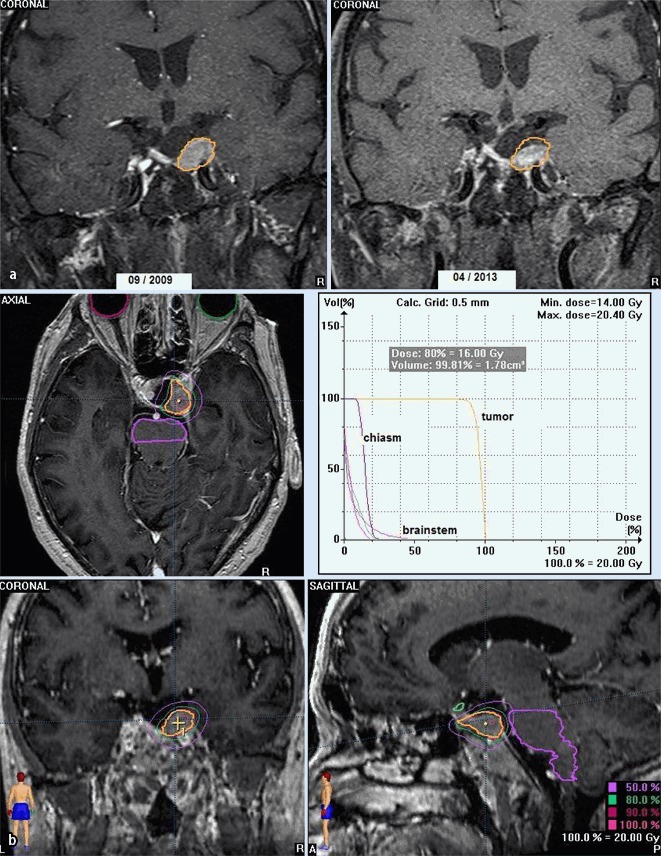
a, b MRI of patient (male, 72 years) with tumor progression after 2 transsphenoidal surgeries (the last in 2005), SRS with 20 Gy in March 2009 (CTV 1.88 ccm). At 4-year follow-up, tumor regression, no visual disorder, no hypopituitarism
Fig. 2.
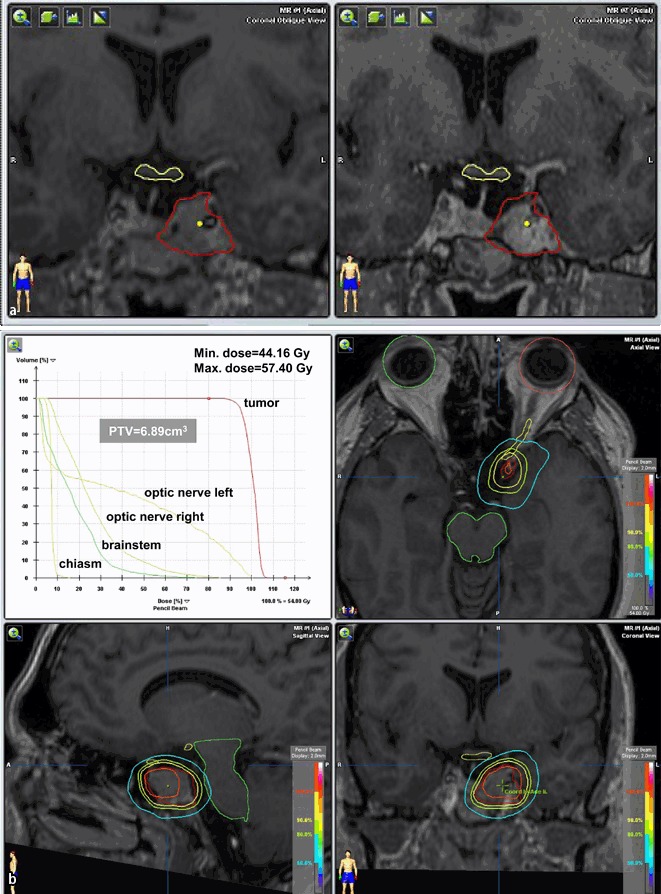
a, b MRI of patient (male, 41 years) with tumor progression after 2 transsphenoidal surgeries (the last in 2007), SRT with 5 × 1.8 ad 54 Gy in 2009 (CTV 5.13 ccm). At 3-year follow-up, tumor regression, no visual disorder, hypopituitarism (partial) idem
Survival and local control
Follow-up varied from 0.5–11 years (median, 5 years). A total of 69 patients were followed at least 1 year. There were 48 patients (65.75 %) who showed a response with tumor shrinkage, 25 patients (34.25 %) with stable disease, and no patient showed progressive disease at the latest follow-up (Table 1).
The 5-year overall survival rate reached 90.4 % (95 % confidence interval [CI] 80.2–95 %). We have seen no local recurrences in our series. The 5-year local control and progression-free survival rates were 100 % (CI 93.3–100 %) and 90.4 % (CI 80.2–95 %), respectively. The difference between the values is due to disease-unrelated death of some patients.
Side effects and complications
A post-SRS/SRT visual disorder was observed in 2 patients (2.7 %; one worsening of a visual field disturbance and one vision acuity decrease in a previously elsewhere irradiated patient). However, there was also an improvement of a visual disorder in 3 patients (4.1 %; visual field disturbance in all and one additional vision acuity improvement in one patient with a primary SRT). For overview see Table 2.
Table 2.
Overview of the patients with improvement or aggravation of dysfunction of the optical system after RT
| Patients | CTV (ccm) | RT | Surgeries (n) | Follow-up (years) | Dysfunction before RT | Outcome |
|---|---|---|---|---|---|---|
| RH, m, 69 years | 2.46 | SRT | 1 | 6 | None | New oculomotor nerve palsy; serveral other causes possible: dilatative angiopathy, ischaemic neuropathy, neurofibromatosis type 2 |
| TH, m, 61 years | 2.49 | SRT | 2 | 9 | Visual field | (mild) Aggravation |
| BM, f, 72 years | 7.73 | RT and SRT | 2 | 1 | None | New visual acuity impairment left (improvement after cortisone); preexisting amaurosis right after surgery; conventional pre-irradiation in 1996 and 04–05/2005 SRT; cardiac death 18 months after SRT |
| GR, f, 72 years | 3.47 | SRT | 1 | 11 | Visual field | Improvement |
| TU, f, 62 years | 19.81 | (primary) SRT | 0 | 4 | Visual acuity/visual field | Improvement (visual field >visual acuity) |
| ER, m, 64 years | 7.66 | SRT | 2 | 7 | Visual acuity/visual field | Improvement (visual field) |
f female, m male, RT radiotherapy, SRT stereotactic radiotherapy, CTV clinical target volume
Only 29 of 73 patients (39.7 %) had normal pituitary function before SRS/SRT. Twelve of 73 patients (16.4 %) had partial dysfunction before SRS/SRT.
Post-SRS/SRT new complete hypopituitarism was observed in 4 patients who received no hormone replacement therapy after surgery (13.79 %) and in 3 patients with partial hypopituitarism after surgery (25 %). Post-SRS/SRT new partial hypopituitarism was observed in 4 patients (13.79 %). In one patient partial hypopitutarism had normalized after SRT. A total of 19 of 29 (66 %) of the patients with normal function before SRS/SRT remain with a normal pituitary function after SRS/SRT. No radiation-induced brain necrosis and only one new paralysis of an oculomotor nerve in a patient with additional neurofibromatosis was observed, whereby the causal relationship of the oculomotor nerve palsy with the irradiation could not be proven (see Table 2).
Univariate and multivariate analysis
Patients with tumor shrinkage (n = 48) had compared to patients without tumor shrinkage (n = 25) a significantly longer follow-up (6 vs. 4 years, t-test, p = 0.0093). The positive correlation between tumor shrinkage and duration of follow-up remains significant in the stepwise (backward and forward) multivariate analysis of the data (p = 0.020).
Patients with new endocrine deficits had a significantly longer post-radiation follow-up-time compared to patients without new endocrine deficits (7 vs. 4 years, t-test, p = 0.0001). The stepwise multivariate analysis (backward and forward) of the data confirmed a correlation of new endocrine deficits with the duration of follow-up (p = 0.008 and p = 0.006, respectively) meaning that the longer the follow-up period goes, the more likely a pituitary hypofunction appears. Additionally a weak positive correlation of new hypopituitarism with the CTV was found (only stepwise backward, p = 0.023).
No correlation between a new post-SRS/SRT visual damage and any of the predictors in the multivariate analysis was detected.
Discussion
In this two center study, we prospectively treated a relatively high number of NSA with a comparably long follow-up and complete radiological, endocrinological and ophthalmological data at the last follow-up. In addition all patients were irradiated with the same irradiation system either as SRS or SRT in a harmonized risk-adapted protocol achieving a high 5-year local control (100 %) and progression-free survival rate (90.4 %) with low toxicity. A post-SRS/SRT visual disorder occurred only in 2 patients (2.7 %), the only other neurological deficit was a new oculomotor nerve palsy which occurred in a pre-irradiated patient. The rate of 39.7 % patients with normal pituitary function before radiotherapy decreased to 26 % after radiotherapy.
While concerning conventional irradiation [3, 7, 21, 25, 32, 33] and also classical radiosurgery with gamma knife or LINAC [2, 22, 31, 34–36], case series with long-term follow-up were already published, these have been lacking so far for fractionated stereotactic radiotherapy. This is understandable, since this technique has only been used regularly since the end of the 1990s [4, 16]. In the last 5 years, the first of such studies have been published [18–20, 24, 30, 38]. First, we want to put our data in respect of three in our view relevant series published in recent years.
In 2007 Kong et al. [18] published a retrospective cohort study of 125 patients including 54 hormone secreting PA. Of the 71 NSA, 42 were treated with LINAC SRT (48–54 Gy) and 29 tumors were treated with Gamma knife SRS (20–28 Gy). The mean follow-up was 36.7 months. They presented indeed a large case number, but also included patients with secretory adenomas. We have excluded all those cases to avoid the bias of different therapeutic approaches (higher radiation dose and antihormonal adjuvant therapy in secretory adenomas) in these two types of pituitary adenomas. Nevertheless, our case number of 73 patients with NSA and a median follow-up of 5 years compares favorably. Kong et al. found an overall actuarial progression-free survival rate of 97 % at 4 years. We can confirm the long-term high tumor control and progression-free rate of more than 90 %. A differentiated comparison with our data regarding the long-term effects on damage of the optical system or the pituitary does not appear reasonable since in the study of Kong et al. no objective visual testing was done in the majority of patients and no differentiation between complete and partial insufficiency was undertaken.
In 2013 Kopp et al. [20] reported their experience with 37 PA including 29 NSA. In their series all patients had surgery before SRT; the median follow-up was 57 months. The tumor control rate was 91.9 % with tumor size unchanged in 59.5 % and only 3 (8.1 %) progressions in two hormone secreting tumors. Visual acuity improved in 7 cases (19 %) and deteriorated in 2 cases (5 %). Visual field improved in 1 and worsened in 1 patient (both 2.7 %). The pituitary function of 22 % was normal after SRT (24 % before SRT), all other patients had a partial (43 %) or complete dysfunction (35 %). This is largely in line with our results. A total of 19 of 73 patients (26 %) remain with a normal pituitary function after (mostly one or more surgeries plus) SRS/SRT in our series. Whereas post-SRS/SRT new complete hypopituitarism was observed in 4 patients who received no hormone replacement therapy after surgery (13.79 %), the rate of complete hypopituitarism in patients with partial hypopituitarism before SRS/SRT was even higher (25 %). We can confirm a very low rate of new damage of the optical system. In our series there were only 2 patients (2.7 %; one worsening of a visual field disturbance and one vision acuity decrease in a previously elsewhere irradiated patient). But we also observed an improvement of a visual disorder in 3 patients (4.1 %). These results are also in line with the data of Kocher et al. [17] reporting about SRT of perioptic tumors. Regarding risk factors for occurrence of a damage of the optical system, we found both in the univariate and in the multivariate analysis, no statistically significant influence factors.
Rieken et al. [30] reported in 2013 about their experience with 92 patients with pituitary adenomas including 55 NSA. RT was conducted using either 3D conformal or fractionated stereotactic techniques (76 patients). Median follow-up was 152.5 months. Before treatment, 2 % of all patients were diagnosed with adenoma-related hypopituitarism. Following surgery, 68 % suffered from new pituitary deficits. RT was associated with 5.4 % new visual deficits and 10.9 % new hypopituitarism in their series. PFS following RT was 90.4 and 75.5 % at 120 and 240 months. Despite their long follow-up the new hypopituitarism rates are low, but again in the long term only 20–25 % of the patients retain a normal pituitary function. In our series we could demonstrate a significant correlation between length of follow-up and occurrence of new hypopituitarism.
Possible differences of the incidence of hypopituitarism after (S)RT or SRS [40] may reflect different patient selection and length of follow-ups, also our data provide no conclusive information about this aspect and large comparative prospective studies are needed to clarify this issue. If there is a trend, then it is a correlation with tumor volume and length of follow-up. That adenomas with a large volume are supplied mostly to fractionated (stereotactic) irradiation would then have to be taken into account.
In our series no secondary cancer was observed. Since our follow-up times are fairly long, we are able to confirm that secondary malignancies are no primary concern in the treatment of pituitary adenoma patients.
In the absence of comparative studies, the choice of the radiation technique should be based on tumor characteristics. SRS is usually offered to patients with relatively small adenomas with a distance of at least 2 mm from the optic system. SRT should be preferred in patients with large tumors in close proximity of the optic apparatus, since the treatment is delivered within the radiation tolerance limits of cranial nerves, including the optic apparatus.
Our experience demonstrated that the Novalis® system is very versatile in delivering SRS or SRT so that both modalities can be offered and one can search for the best individual solution.
Limitations
Because this study is a two center study, the data presented are not representative, and the results cannot be generalized. However, the study can still be of value as a sample study submitted by two major departments of radiosurgery and stereotactic radiotherapy.
Conclusion
After 10 years of experience, we consider our risk-adapted protocol of radiosurgery or fractionated SRT depending on tumor volume and distance between tumor and the optical system as safe and successful in terms of tumor control and protection of the visual system, especially for large tumors and tumors located near to the optic pathways. However, one has to accept an increased rate of new hypopituitarism.
Compliance with ethical guidelines
Conflict of interest
J.P. Boström, A. Meyer, B. Pintea, R. Gerlach, G. Surber, G. Lammering, and K. Hamm state that there are no conflicts of interest.
All studies on humans in the present manuscript were carried out with the approval of the responsible ethics committee with national law and the Helsinki Declaration of 1975 (in its current, revised form). Informed consent was obtained from all patients included in studies.
Acknowledgements
Open access was sponsored by IPSEN Pharma GmbH, Ettlingen, Germany.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Boelaert K, Gittoes NJ. Radiotherapy for non-functioning pituitary adenomas. Eur J Endocrinol. 2001;144:569–575. doi: 10.1530/eje.0.1440569. [DOI] [PubMed] [Google Scholar]
- 2.Brada M, Ajithkumar TV, Minniti G. Radiosurgery for pituitary adenomas. Clin Endocrinol (Oxf) 2004;61:531–543. doi: 10.1111/j.1365-2265.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 3.Brada M, Rajan B, Traish D, Ashley S, Holmes-Sellors PJ, Nussey S, Uttley D. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol (Oxf) 1993;38:571–578. doi: 10.1111/j.1365-2265.1993.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 4.Coke C, Andrews DW, Corn BW, Werner-Wasik M, Downes B, McCune C, Curran WJ., Jr Multiple fractionated stereotactic radiotherapy of residual pituitary macroadenomas: initial experience. Stereotact Funct Neurosurg. 1997;69:183–190. doi: 10.1159/000099872. [DOI] [PubMed] [Google Scholar]
- 5.Colin P, Jovenin N, Delemer B, Caron J, Grulet H, Hecart AC, Lukas C, Bazin A, Bernard MH, Scherpereel B, Peruzzi P, Nakib I, Redon C, Rousseaux P. Treatment of pituitary adenomas by fractionated stereotactic radiotherapy: a prospective study of 110 patients. Int J Radiat Oncol Biol Phys. 2005;62:333–341. doi: 10.1016/j.ijrobp.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 6.Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer. 1991;68:860–866. doi: 10.1002/1097-0142(19910815)68:4<860::AID-CNCR2820680431>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Cornett MS, Paris KJ, Spanos WJ, Jr, Lindberg RD, Jose B. Radiation therapy for pituitary adenomas. A retrospective study of the University of Louisville experience. Am J Clin Oncol. 1996;19:292–295. doi: 10.1097/00000421-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2008;93:3717–3726. doi: 10.1210/jc.2008-0643. [DOI] [PubMed] [Google Scholar]
- 9.Ernst-Stecken A, Lambrecht U, Ganslandt O, Mueller R, Fahlbusch R, Sauer R, Grabenbauer G. Radiosurgery of small skull-base lesions. No advantage for intensity-modulated stereotactic radiosurgery versus conformal arc technique. Strahlenther Onkol. 2005;181:336–344. doi: 10.1007/s00066-005-1371-1. [DOI] [PubMed] [Google Scholar]
- 10.Erridge SC, Conkey DS, Stockton D, Strachan MW, Statham PF, Whittle IR, Grant R, Kerr GR, Gregor A. Radiotherapy for pituitary adenomas: long-term efficacy and toxicity. Radiother Oncol. 2009;93:597–601. doi: 10.1016/j.radonc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Fahrig A, Ganslandt O, Lambrecht U, Grabenbauer G, Kleinert G, Sauer R, Hamm K. Hypofractionated stereotactic radiotherapy for brain metastases–results from three different dose concepts. Strahlenther Onkol. 2007;183:625–630. doi: 10.1007/s00066-007-1714-1. [DOI] [PubMed] [Google Scholar]
- 12.Greenman Y, Stern N. Non-functioning pituitary adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:625–638. doi: 10.1016/j.beem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Greenman Y, Ouaknine G, Veshchev I, Reider G, II, Segev Y, Stern N. Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin Endocrinol (Oxf) 2003;58:763–769. doi: 10.1046/j.1365-2265.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 14.Henzel M, Hamm K, Gross MW, Surber G, Kleinert G, Failing T, Sitter H, Strassmann G, Engenhart-Cabillic R. Fractionated stereotactic radiotherapy of glomus jugulare tumors. Local control, toxicity, symptomatology, and quality of life. Strahlenther Onkol. 2007;183:557–562. doi: 10.1007/s00066-007-1701-6. [DOI] [PubMed] [Google Scholar]
- 15.Iwata H, Sato K, Tatewaki K, Yokota N, Inoue M, Baba Y, Shibamoto Y. Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro Oncol. 2011;13:916–922. doi: 10.1093/neuonc/nor055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalali R, Brada M, Perks JR, Warrington AP, Traish D, Burchell L, McNair H, Thomas DG, Robinson S, Johnston DG. Stereotactic conformal radiotherapy for pituitary adenomas: technique and preliminary experience. Clin Endocrinol (Oxf) 2000;52:695–702. doi: 10.1046/j.1365-2265.2000.00967.x. [DOI] [PubMed] [Google Scholar]
- 17.Kocher M, Treuer H, Hoevels M, Semrau R, Sturm V, Mueller RP. Endocrine and visual function after fractionated stereotactic radiotherapy of perioptic tumors. Strahlenther Onkol. 2013;189:137–141. doi: 10.1007/s00066-012-0269-y. [DOI] [PubMed] [Google Scholar]
- 18.Kong DS, Lee JI, Lim do H, Kim KW, Shin HJ, Nam DH, Park K, Kim JH. The efficacy of fractionated radiotherapy and stereotactic radiosurgery for pituitary adenomas: long-term results of 125 consecutive patients treated in a single institution. Cancer. 2007;110:854–860. doi: 10.1002/cncr.22860. [DOI] [PubMed] [Google Scholar]
- 19.Kopp C, Theodorou M, Poullos N, Jacob V, Astner ST, Molls M, Grosu AL. Tumor shrinkage assessed by volumetric MRI in long-term follow-up after fractionated stereotactic radiotherapy of nonfunctioning pituitary adenoma. Int J Radiat Oncol Biol Phys. 2012;82:1262–1267. doi: 10.1016/j.ijrobp.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 20.Kopp C, Theodorou M, Poullos N, Astner ST, Geinitz H, Stalla GK, Meyer B, Molls M, Nieder C, Grosu AL. Fractionated stereotactic radiotherapy in the treatment of pituitary adenomas. Strahlenther Onkol. 2013;189:932–937. doi: 10.1007/s00066-013-0433-z. [DOI] [PubMed] [Google Scholar]
- 21.Langsenlehner T, Stiegler C, Quehenberger F, Feigl GC, Jakse G, Mokry M, Langsenlehner U, Kapp KS, Mayer R. Long-term follow-up of patients with pituitary macroadenomas after postoperative radiation therapy: analysis of tumor control and functional outcome. Strahlenther Onkol. 2007;183:241–247. doi: 10.1007/s00066-007-1706-1. [DOI] [PubMed] [Google Scholar]
- 22.Laws ER, Sheehan JP, Sheehan JM, Jagnathan J, Jane JA, Jr, Oskouian R. Stereotactic radiosurgery for pituitary adenomas: a review of the literature. J Neurooncol. 2004;69:257–272. doi: 10.1023/B:NEON.0000041887.51906.b7. [DOI] [PubMed] [Google Scholar]
- 23.Milker-Zabel S, Debus J, Thilmann C, Schlegel W, Wannenmacher M. Fractionated stereotactically guided radiotherapy and radiosurgery in the treatment of functional and nonfunctional adenomas of the pituitary gland. Int J Radiat Oncol Biol Phys. 2001;50:1279–1286. doi: 10.1016/S0360-3016(01)01535-8. [DOI] [PubMed] [Google Scholar]
- 24.Minniti G, Traish D, Ashley S, Gonsalves A, Brada M. Fractionated stereotactic conformal radiotherapy for secreting and nonsecreting pituitary adenomas. Clin Endocrinol (Oxf) 2006;64:542–548. doi: 10.1111/j.1365-2265.2006.02506.x. [DOI] [PubMed] [Google Scholar]
- 25.Minniti G, Jaffrain-Rea ML, Osti M, Cantore G, Enrici RM. Radiotherapy for nonfunctioning pituitary adenomas: from conventional to modern stereotactic radiation techniques. Neurosurg Rev. 2007;30:167–175. doi: 10.1007/s10143-007-0072-x. [DOI] [PubMed] [Google Scholar]
- 26.Minniti G, Gilbert DC, Brada M. Modern techniques for pituitary radiotherapy. Rev Endocr Metab Disord. 2009;10:135–144. doi: 10.1007/s11154-008-9106-0. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson B, Gustavasson-Kadaka E, Bengtsson BA, Jonsson B. Pituitary adenomas in Sweden between 1958 and 1991: incidence, survival, and mortality. J Clin Endocrinol Metab. 2000;85:1420–1425. doi: 10.1210/jcem.85.4.6498. [DOI] [PubMed] [Google Scholar]
- 28.Oruckaptan HH, Senmevsim O, Ozcan OE, Ozgen T. Pituitary adenomas: results of 684 surgically treated patients and review of the literature. Surg Neurol. 2000;53:211–219. doi: 10.1016/S0090-3019(00)00171-3. [DOI] [PubMed] [Google Scholar]
- 29.Pollock BE. Radiosurgery for pituitary adenomas. Prog Neurol Surg. 2007;20:164–171. doi: 10.1159/000100108. [DOI] [PubMed] [Google Scholar]
- 30.Rieken S, Habermehl D, Welzel T, Mohr A, Lindel K, Debus J, Combs SE. Long term toxicity and prognostic factors of radiation therapy for secreting and non-secreting pituitary adenomas. Radiat Oncol. 2013;8:18. doi: 10.1186/1748-717X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Runge MJ, Maarouf M, Hunsche S, Kocher M, Ruge MI, El Majdoub F, Treuer H, Mueller RP, Voges J, Sturm V. LINAC-radiosurgery for nonsecreting pituitary adenomas. Long-term results. Strahlenther Onkol. 2012;188:319–325. doi: 10.1007/s00066-011-0052-5. [DOI] [PubMed] [Google Scholar]
- 32.Rush S, Cooper PR. Symptom resolution, tumor control, and side effects following postoperative radiotherapy for pituitary macroadenomas. Int J Radiat Oncol Biol Phys. 1997;37:1031–1034. doi: 10.1016/S0360-3016(96)00586-X. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki R, Murakami M, Okamoto Y, Kono K, Yoden E, Nakajima T, Nabeshima S, Kuroda Y. The efficacy of conventional radiation therapy in the management of pituitary adenoma. Int J Radiat Oncol Biol Phys. 2000;47:1337–1345. doi: 10.1016/S0360-3016(00)00503-4. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan JP, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97:408–414. doi: 10.3171/jns.2002.97.2.0408. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan JP, Jagannathan J, Pouratian N, Steiner L. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res. 2006;34:185–205. doi: 10.1159/000091581. [DOI] [PubMed] [Google Scholar]
- 36.Sheehan JP, Starke RM, Mathieu D, Young B, Sneed PK, Chiang VL, Lee JY, Kano H, Park KJ, Niranjan A, Kondziolka D, Barnett GH, Rush S, Golfinos JG, Lunsford LD. Gamma Knife radiosurgery for the management of nonfunctioning pituitary adenomas: a multicenter study. J Neurosurg. 2013;119:446–456. doi: 10.3171/2013.3.JNS12766. [DOI] [PubMed] [Google Scholar]
- 37.Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, Gorman DA, Schomberg PJ. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177–1181. doi: 10.1016/S0360-3016(02)04380-8. [DOI] [PubMed] [Google Scholar]
- 38.Sun DQ, Cheng JJ, Frazier JL, Batra S, Wand G, Kleinberg LR, Rigamonti D, Quinones-Hinojosa A, Salvatori R, Lim M. Treatment of pituitary adenomas using radiosurgery and radiotherapy: a single center experience and review of literature. Neurosurg Rev. 2010;34:181–189. doi: 10.1007/s10143-010-0285-2. [DOI] [PubMed] [Google Scholar]
- 39.Woollons AC, Hunn MK, Rajapakse YR, Toomath R, Hamilton DA, Conaglen JV, Balakrishnan V. Non-functioning pituitary adenomas: indications for postoperative radiotherapy. Clin Endocrinol (Oxf) 2000;53:713–717. doi: 10.1046/j.1365-2265.2000.01153.x. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z, Lee Vance M, Schlesinger D, Sheehan JP. Hypopituitarism after stereotactic radiosurgery for pituitary adenomas. Neurosurgery. 2013;72:630rosu–637. doi: 10.1227/NEU.0b013e3182846e44. [DOI] [PubMed] [Google Scholar]


