Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide. COPD is characterized by poor treatment adherence, and patient medication preferences may contribute to adherence.
Methods
A discrete choice experiment with an internet panel drawn from the USA was used to evaluate preference and willingness to pay (WTP) of COPD patients for long-acting maintenance medications. Key attributes derived from earlier qualitative research (brief literature review and focus groups) with COPD patients on maintenance therapy included symptom relief, speed of feeling medication start to work, inhaler ease of use, rescue medication use, side effects, and monthly out-of-pocket co-pay. Patients were presented with hypothetical medications with different profiles and asked which they preferred. Utilities and marginal WTP in monthly co-pay dollars were estimated for all patients and by severity.
Results
Utilities for 515 participants were in the expected direction and highest for the most favorable attribute levels. Each attribute evaluated was important, and participants were willing to pay a premium to obtain each benefit. On average, WTP was as high as $US64 for complete symptom relief, $US59 for no side effects, $US32 to rarely use rescue medication, $US16 for a quick and easy to use inhaler, and $US13 for feeling medication work quickly (within 5 min; average WTP $US18/month for patients with severe/very severe COPD).
Conclusion
As expected, efficacy and safety were most valued by patients; however, this study showed that other COPD medication attributes, such as rescue medication, ease of use, and feeling medication work quickly, are also important in patient preferences.
Key Points for Decision Makers
| There are multiple attributes of a chronic obstructive pulmonary disease (COPD) maintenance therapy that patients may take into consideration in evaluating a medication. |
| In a US sample, COPD medication attributes of efficacy and safety were highly valued by patients, but other attributes, such as rescue medication, ease of use, and feeling medication work quickly, are also important in patient preferences. |
| Patients are willing to pay more for a medication with attractive characteristics. |
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality, with an estimated prevalence of 80 million people with moderate to severe COPD worldwide [1], and is predicted to become the third leading cause of death worldwide by 2030 [1, 2]. The three main symptoms of COPD are shortness of breath (dyspnea), cough, and excessive sputum production. COPD treatments focus on symptom alleviation, reduction of exacerbations, maintenance of good lung function, and improving health-related quality of life. Optimal management of COPD is complex, requiring behavioral and lifestyle changes from patients, including quitting smoking and adherence to prescribed medication. Pharmacological treatment for most patients includes prescription maintenance medication (taken on a regular daily schedule) and quick-acting ‘rescue’ medication (taken during acute symptoms) [3].
Despite the prevalence of this condition and the existence of clinical guidelines to manage COPD [3–5], treatment adherence remains poor, with recent estimates indicating that, on average, 60 % of patients with COPD are noncompliant with prescribed therapy [6]. COPD patients using multiple long-acting maintenance inhalers (two or more prescriptions) have also been shown to be less adherent and have higher discontinuation rates than single-inhaler users [7]. Improving compliance among this patient population is particularly important, as poor adherence to drug therapy has been identified as the major factor resulting in emergency hospitalization among COPD patients [8–10]. Conversely, better medication adherence has been associated with both a decrease in the number of hospitalizations and length of hospital stay among patients with chronic respiratory ailments [11]. Patient beliefs, experiences, and behaviors with regard to COPD and its treatment have been shown to be powerful predictors of medication adherence [12], which highlights the importance of including the patient perspective in COPD medication and management.
Although multiple factors may drive patient preference and subsequent medication adherence, a key step to improving compliance is to understand patients’ perspectives regarding favored attributes of their COPD medication. Attributes important to patients can include, but are not limited to, aspects such as efficacy, cost, ease of use, speed of onset, and side effects. To date, research on COPD patient preferences has focused on ease of use and convenience of different modes of administration (i.e., inhaler devices, nebulizers) [13–18]. Previous work among asthma patients has shown that they prefer a drug that they can feel working right away [19]. Patient preferences for medication attributes may play a role in the decision of COPD patients to take medications as directed [6], and understanding these preferences may help to improve patient adherence to treatment regimens.
Study Overview
The purpose of this study was twofold—first, to identify attributes that patients consider integral in a COPD maintenance medication and, second, to understand the relative value of each of these attributes as it relates to overall patient preference for a maintenance medication. The study was performed in two phases: initially, qualitative research was conducted to inform the types of COPD medication attributes that are important to patients and, subsequently, a discrete choice experiment (DCE) study was implemented to quantitatively evaluate patient preferences for each of the attributes. Each phase of this study was approved by the appropriate institutional review boards and all participants provided informed consent before the initiation of data collection procedures.
Methods
Focus Group Phase
In phase I, qualitative research was conducted to identify key medication attributes in COPD and the degrees (or levels) of each attribute that were important to patients. A brief, targeted review of recent literature was first conducted to identify potential attributes (or characteristics) of COPD medications that had salience for patients. Searches were conducted using the PubMed database, examining articles in English, published between 2000 and 2008. Appropriate articles were identified pertaining to treatment characteristics by using a search strategy that included descriptors related to COPD, patient satisfaction/preference, medication/treatment, and treatment effects or outcomes. The key concepts identified in the brief literature review informed potential areas of discussion in patient focus groups. The focus group discussions served to confirm treatment concepts reflected in the recent literature, identify any additional concepts that should be included, and further refine understanding of attributes relevant to treatment from the patient perspective.
Two focus group sessions of six to seven patients each were conducted in March 2009 at a pulmonary clinic in the USA. Participants were at least 40 years old, with a clinical diagnosis of moderate to very severe COPD and dyspnea, and receiving a long-acting COPD maintenance therapy at the time of the focus group. There were no exclusion criteria with regard to use of short-acting/rescue medications. A semi-structured focus group discussion guide was used to facilitate these conversations, and sessions were led by a trained moderator with research experience in COPD. The discussion guide was designed to elicit spontaneous feedback from patients about COPD symptoms experienced on a typical day, impact of symptoms, and their experiences with COPD long-acting or maintenance medications.
Discrete Choice Experiment (DCE) Study Phase
In phase II, a quantitative study using DCE methodology was designed based on the qualitative findings obtained in phase I. DCE is a type of conjoint analysis [20–24], and can be used to estimate willingness to pay (WTP) for healthcare interventions [25, 26]. The method allows respondents to choose their preferred option between hypothetical scenarios designed to reflect the different attributes that real world decisions would contain, and allow them to make ‘trade-offs’ between these attributes to reveal their preferences.
The DCE study was conducted in August 2009 among US patients with COPD on maintenance therapy (e.g., a long-acting β2-agonist [LABA]) via a web survey to measure patient preferences for attributes of COPD pharmaceutical treatment. A web-based platform for the survey was selected to maximize recruitment and study sample. Screening questions were used to determine eligibility of panelists who responded to the email invitation. Informed consent via YouGov (Palo Alto, CA, USA) was obtained prior to web survey completion. Participants completed the DCE questionnaire, which included screening, sociodemographic and clinical questions, a respiratory symptoms scale, instructions on how to complete DCE items, a description of the COPD attributes, and the choice tasks. The choice tasks contained 20 hypothetical scenarios composed of different levels of different attributes.
Study Population
The PollingPoint panel is a proprietary opt-in internet survey panel comprising 1.2 million US residents who participate in YouGov’s web surveys. The study goal was to recruit approximately 500 US participants with COPD (or chronic bronchitis and/or emphysema) through the panel database. The recruitment sample comprised US participants who self-reported a physician diagnosis of COPD, met the pre-screening criteria, and had previously completed YouGov surveys in English. In order to obtain approximately 500 completed surveys, YouGov issued email invitations to a pool of approximately 5,130 prospective panel participants in their database who (1) had previously reported having been diagnosed with COPD; (2) were 40 years or older, and (3) were current or former smokers.
A series of screening and eligibility questions determined eligibility once a potential participant responded to the email invitation. A flow diagram describing eligible participants is included in Fig. 1. Participants were eligible if they (1) were 40 years of age or older; (2) were a current or previous smoker with at least a 10-pack/year history (equivalent to one pack or 20 cigarettes per day for at least 10 years); (3) had been diagnosed with COPD (or chronic bronchitis and/or emphysema) by a clinician or healthcare provider; (4) were currently on a long-acting maintenance COPD therapy that included ADVAIR® (fluticasone and salmeterol) and/or SYMBICORT® (budesonide and formoterol); (5) were able to read and understand English; and (6) were willing and able to provide informed consent on the web link in the email invitation. There were no exclusion criteria with regard to use of short-acting/rescue medications. If confirmed eligible based on inclusion/exclusion criteria on the screening form, participants were directed to a YouGov HIPAA (Health Insurance Portability and Accountability Act)-compliant Internet portal. Respondents were provided with a consent statement, and those who agreed to participate were asked to complete the online survey.
Fig. 1.
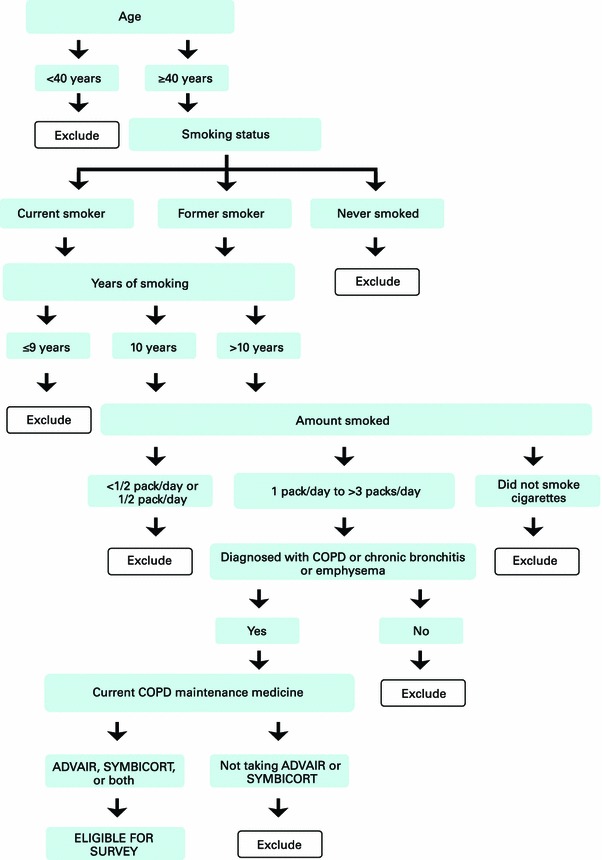
Flow diagram of participant eligibility. COPD chronic obstructive pulmonary disease
Patient Characteristics
Basic sociodemographic characteristics of interest collected included age, gender, race/ethnicity, employment status, education, living/domestic situation, and marital status. COPD and clinical characteristics collected included smoking history, diagnosis, disease duration, disease severity, use of maintenance and rescue medicines, overall health, and comorbid health conditions. The Breathlessness, Cough, and Sputum Scale (BCSS) was also administered; BCSS was developed as a three-item patient-reported instrument for tracking the severity of respiratory symptoms among patients with COPD [27]. Each COPD symptom (breathlessness, cough, sputum) is rated on a five-point Likert-type scale ranging from 0 to 4, where higher responses indicate more severity. Total BCSS scores range from 0 to 12, where higher scores indicate more overall severe symptoms for the current day.
DCE Questionnaire
The six study attributes assessed were symptom relief, speed of feeling the medication begin to work, side effects of medication, use of rescue (quick-relief) medication, ease of inhaler use, and monthly co-pay cost. The DCE questionnaire was composed of two medications with different levels for each of the six COPD medication attributes. Instructions were presented prior to the DCE questionnaire and described COPD and its usual symptoms, and explained the difference between maintenance and rescue (quick-relief) medicines that are used to treat COPD (or chronic bronchitis and/or emphysema). Each of the medication features was described, and the three levels for each attribute were detailed.
The complete set of COPD medication attributes and levels are presented in Table 1. Attribute levels ranged from best case to worst case. The symptom attribute mimics medication effectiveness, ranging from minimal to maximal relief. Levels for feeling the medication working were based on clinical reports and focus groups: some participants reported feeling their medication start to work quickly; others discussed adapting their morning routines to take medication well before they were ready to get up for the day so that the medication would be working when they needed it. Ease of use of an inhaler was based on literature noting that important features were ‘quick to use’ and ‘overall ease of use’ [15]. Levels for frequency of rescue medication use were set based on patients reporting hardly any use to daily need during the focus group discussions; this was also confirmed through clinical data gathered in this study. Rather than exploring specific side effects, the discussions focused on whether the side effects interfered with taking medication, and a continuum was provided from no side effects to side effects severe enough to affect whether to take a medication. Finally, the cost attribute reflects co-pay costs based on standard generic and formulary tiers used by prescription insurance plans.
Table 1.
Chronic obstructive pulmonary disease maintenance medicine attributes and levels
| Attribute 1: How well the maintenance medicine relieve symptoms |
| Level 1: Little or no relief of symptoms after taking the maintenance medicine |
| Level 2: Some relief of symptoms after taking the maintenance medicine |
| Level 3: Complete relief of symptoms after taking the maintenance medicine |
| Attribute 2: How quickly you feel the maintenance medicine start to work |
| Level 1: Feel maintenance medicine begin to work within 5 min of taking it |
| Level 2: Feel maintenance medicine begin to work within 20 min of taking it |
| Level 3: Feel maintenance medicine begin to work within 30 min or more of taking it |
| Attribute 3: How easy it is to use your inhaler device |
| Level 1: Inhaler is quick and easy to use |
| Level 2: Inhaler requires some practice and care to use |
| Level 3: Inhaler is more difficult and time-consuming to use |
| Attribute 4: Rescue (quick-relief) medicine use |
| Level 1: Rarely need to use rescue (quick-relief) inhaler, 1–2 times per week or less |
| Level 2: Need to use rescue (quick-relief) inhaler 3–5 times per week |
| Level 3: Need to use rescue (quick-relief) inhaler every day |
| Attribute 5: Side effects of maintenance medicine |
| Level 1: No side effects |
| Level 2: Mild side effects, but they do not interfere with taking the medicine |
| Level 3: Moderate to severe side effects that may stop you from taking the medicine |
| Attribute 6: Out-of-pocket cost of maintenance medicine |
| Level 1: $5 per month |
| Level 2: $25 per month |
| Level 3: $50 per month |
A balanced overlap design was used to create four versions of the DCE questionnaire, each of which included 20 choice tasks. Given that the attributes of symptom relief and the need to use rescue (quick-relief) medication are related, two prohibitions were built into the design used to randomly generate the medication choice tasks. The symptom relief attribute ‘Complete relief’ (level 3) was not allowed to appear in combination with either ‘3–5 times per week’ (level 2) or ‘every day’ (level 3) with the use of rescue (quick-relief) medicine attribute in any medication profile. Participants were randomly assigned to complete one of the four versions; the sample size of 500 was large enough to allow for over 100 participants to complete each separate version of the DCE questionnaire, thereby expanding the number and variety of scenarios that could be presented and reducing individual participant burden.
The DCE questionnaire included instructions, a description of the attributes, and the medication choice tasks. In each task, participants were asked to review the two medications in each scenario and indicate which medication they would prefer (Medicine A or Medicine B; a sample task is presented in Fig. 2 as an example). Each of the tasks was presented as a forced choice question, where the participant had to indicate which of the two medications they would prefer (e.g., one scenario might present a medication that costs $50 per month and have a 5-min onset, whereas another medication might cost $10 per month and have a 30-min onset). The participant would choose which scenario (or drug type) they preferred. The forced choice nature of the task provided help in understanding how participants make trade-offs between attributes when making their medication selections.
Fig. 2.
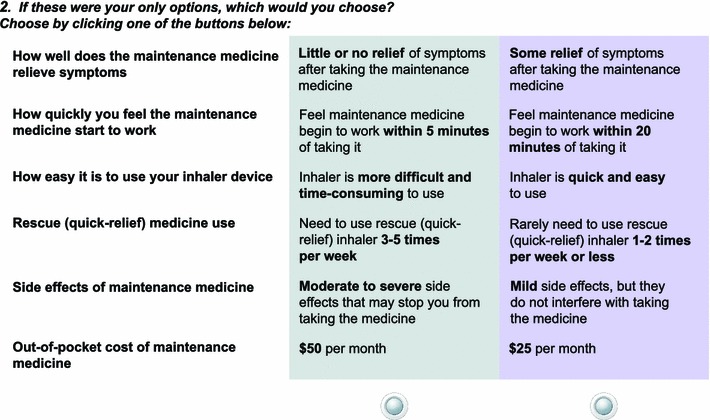
Example of discrete choice experiment choice task
Two fixed tasks with a clear preference were included in each questionnaire version as consistency checks to ensure that participants correctly understood how to complete the choice tasks. Each fixed task included a highly favorable medicine (complete relief, works within 5 min, quick and easy to use, rarely need to use rescue inhaler, no side effects/mild side effects, and $5 per month) that should have been chosen as the preferred medicine; failure to choose the preferred medicine could indicate that instructions on how to complete the choice tasks were not understood correctly.
Statistical Analyses
All data were collected in adherence with the study protocol and in accordance with good clinical research practice. Sawtooth Software version 6.6 (Sawtooth Software Inc., Sequim, WA, USA) and SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC, USA) were used to conduct the analyses. Missing data were handled per the instrument developer scoring instructions for the BCSS. No other imputation for missing data was performed; the DCE analysis does not allow for any data imputation.
Frequencies for participant disposition are reported for invitees (individuals issued an email invitation), responders (individuals who responded by opting in to the web link in the email invitations), eligible participants (individuals who responded and were eligible to complete the survey according to the inclusion/exclusion criteria), and completers (eligible participants who completed the web survey in its entirety with 10 % or less missing responses). Descriptive statistics for participant-reported sociodemographic and clinical questions, including the BCSS, are reported for the total sample. Categorical variables are summarized by frequency statistics, and continuous variables report mean, standard deviation (SD), and minimum/maximum.
Participant preference for COPD attributes was evaluated based on data from the DCE questionnaire. Comprehension of choice tasks was evaluated by examining response frequencies for consistency check questions and average utilities for participants with consistent and inconsistent response patterns. Utility scores were determined for each level of the COPD attributes as average zero-centered differences from a main-effects model. The utilities or preference weights reflect strength of participant preference for each of the COPD attributes. WTP was determined for each attribute level by assigning currency values to the utility (preference) scores. These values represent an estimate of the relative monetary value of obtaining improvements in the level of a COPD attribute. Multinomial logistic regression methods were used to estimate group-level preference values for each attribute level and the effect size of each attribute’s levels. The marginal effects of each attribute’s levels was divided by the marginal effect from a dollar change in co-pay amount to represent the participant’s marginal WTP for improvements within each COPD attribute, as measured in dollars of co-pay per month. Marginal effects and marginal WTP (to avoid the level) are presented for each attribute level. The reference level is the lowest (most favorable) level for each of the COPD attributes. WTP estimates of subgroups were also assessed for COPD severity level, age, and gender.
Results
Phase I: Qualitative Results
Slightly over one-half of the focus group participants (N = 13) were female (n = 7), and most were White (84.6 %), with a mean age of 70 years (SD 5.4). Patients had an overall disease severity of moderate (Global Initiative for COPD [GOLD]-2; 38.5 %) or severe COPD (GOLD-3; 61.5 %) and had been diagnosed with COPD for an average of 3.5 (SD 1.8) years. All were receiving a combination LABA + glucocorticosteroid inhaler, and the majority (92.3 %) also used an inhaled β2-agonist short acting/rescue medication.
Qualitative analysis of the focus group data involved the identification of themes based upon review of the transcripts. Important aspects of COPD and maintenance medications identified by patients included symptoms, medication efficacy/symptom relief, speed with which patients felt their medication working/onset of medication action, rescue inhaler/reliever use, comfort with delivery system/mode of administration, and side effects/adverse effects.
Study participants experienced typical COPD symptoms, including shortness of breath, chest tightness, cough, phlegm/mucus, fatigue, and wheezing, which tended to worsen upon exertion. The prevailing symptom experienced by patients was shortness of breath, described as “loss of breath,” “can’t get air,” and “takes your breath away.” Some patients described having more difficulty with symptoms at certain times of the day (e.g., morning) or times of the year (e.g., cold or humid weather, spring, certain months). Patients were very concerned with how well their maintenance medication worked. When describing their medication, comments such as “helping (them) to breathe,” “improving breathing,” “breathing without strain,” “feeling the medication open up/clear their lungs,” and “being able to go a longer time” were evidence to patients that the medication was working. How quickly their COPD maintenance medication worked was instrumental. Fast speed of action was described as allowing them “to start moving around” and “feeling better as soon as (they) took their medication.” Rescue medications were relied on by patients because they knew that they could take it immediately if their symptoms flared, and that it provided immediate relief that they could feel.
Ease of use in administering COPD maintenance medications was also important. For example, patients described difficulty with inserting a capsule into their delivery device and noted that medication with a metered dose was convenient and easy to use. Patients also cited preference for pill or aerosol spray form (similar to a rescue inhaler), as inhaling a dry powder medication could be difficult when they were experiencing shortness of breath. Patients were also concerned with side effects related to using COPD medication, and noted experiencing dizziness/lightheadedness, vision problems, leg cramping, and throat hoarseness, and discussed concerns about glaucoma and cataract risk. It should be noted that frequency of dosing was not a major issue for the patients, nor was cost, as study participants had prescription drug insurance coverage.
Results from a brief review of recent literature and qualitative research conducted with patients in phase I were used to develop the attributes that were evaluated in phase II of the study. The key concepts/themes related to COPD and its treatment identified in qualitative data analysis of focus group transcripts formed the basis of COPD maintenance medication attributes and levels used in the DCE study.
Phase II: DCE Results
Participant Disposition
YouGov issued email invitations to 5,130 enrollees who met the criteria for previous diagnosis of COPD. Of these, 57 % (n = 2,930) responded (Fig. 3). A total of 76 % (n = 2,234) of responders were ineligible for the survey; 24 % (n = 696) were eligible based on screening criteria, and the majority of these (n = 515; 74 %) completed the survey. The study sample is made up of 515 participants who completed the survey.
Fig. 3.
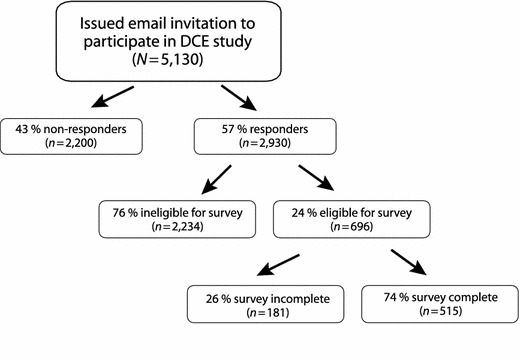
Survey participant disposition. DCE discrete choice experiment
Participant Characteristics
Slightly over one-half of survey participants were female, and the majority were White, with a mean age of 62 years (SD 9.99) (Table 2). Most were former smokers, and almost all had smoked for more than 10 years (Table 2). The time from diagnosis for COPD ranged from 2 to 10 years (Table 2). Almost one-half characterized themselves as having moderate COPD, with fewer reporting severe or very severe COPD. Most subjects reported using fluticasone/salmeterol as their maintenance medication and albuterol for rescue (Table 2). Almost one-quarter of respondents (24 %) reported using rescue medication less than once a week, while another one-quarter reported needing to use a rescue medication from one to five times a week. Nearly 15 % reported using rescue medications more than ten times per week.
Table 2.
Discrete choice experiment participant-reported sociodemographic and clinical characteristics (N = 515)
| Characteristics | Total (N = 515) |
|---|---|
| Age in yearsa, mean (SD) [range] | 62.3 (9.99) [40–88] |
| Men, n (%) | 230 (44.66 %) |
| Employment status, n (%) | |
| Employed, full-time, or part-time | 94 (18.25 %) |
| Retired | 220 (42.72 %) |
| Disabled | 154 (29.90 %) |
| Other | 47 (9.13 %) |
| Highest level of education, n (%) | |
| Elementary/primary school | 2 (0.39 %) |
| Secondary/high school | 136 (26.41 %) |
| Some college | 202 (39.22 %) |
| College degree or more | 162 (31.46 %) |
| Other | 13 (2.52 %) |
| Racial backgroundb, n (%) | |
| White | 490 (95.15 %) |
| Black or African American | 10 (1.94 %) |
| Asian | 1 (0.19 %) |
| American Indian or Alaska Native | 14 (2.72 %) |
| Other | 12 (2.33 %) |
| Hispanic or Latino, n (%) | 10 (1.94 %) |
| Marital status, n (%) | |
| Married | 280 (54.37 %) |
| Divorced | 112 (21.75 %) |
| Never married/single | 22 (4.27 %) |
| Widowed | 65 (12.62 %) |
| Other | 36 (6.99 %) |
| Smoking statusc, n (%) | |
| Current smoker | 178 (34.56 %) |
| Former smoker | 337 (65.44 %) |
| Typical amount smokedc, n (%) | |
| 1 pack/day | 185 (35.92 %) |
| 1½ packs/day | 140 (27.18 %) |
| 2 packs/day | 101 (19.61 %) |
| 2½ packs/day | 48 (9.32 %) |
| ≥3 packs/day | 41 (7.96 %) |
| COPD disease duration, n (%) | |
| <1 year | 23 (4.47 %) |
| 1 to <2 years | 54 (10.49 %) |
| 2 to <5 years | 182 (35.34 %) |
| 5–10 years | 161 (31.26 %) |
| >10 years | 94 (18.25 %) |
| Don’t know | 1 (0.19 %) |
| Participant-reported COPD disease severity, n (%) | |
| Mild | 108 (20.97 %) |
| Moderate | 238 (46.21 %) |
| Severe | 121 (23.50 %) |
| Very severe | 39 (7.57 %) |
| Don’t know | 9 (1.75 %) |
| Current maintenance medication for COPDc, n (%) | |
| Fluticasone propionate and salmeterol inhalation powder (ADVAIR®) | 421 (81.75 %) |
| Budesonide/formoterol fumarate dehydrate (SYMBICORT®) | 66 (12.82 %) |
| Both (ADVAIR and SYMBICORT) | 28 (5.44 %) |
| Rescue medicines for COPDd, n (%) | |
| Albuterol (e.g., VENTOLIN®, PROVENTIL®) | 334 (64.85 %) |
| Levalbuterol (e.g., XOPENEX®) | 30 (5.83 %) |
| Other | 64 (12.43 %) |
| Do not use rescue (quick-relief) medicine | 100 (19.42 %) |
COPD chronic obstructive pulmonary disease, SD standard deviation
aDate of birth was asked in participant eligibility screening. Age was computed using participated-reported date of birth
bParticipants may report more than one category; racial background may not be mutually exclusive
cQuestions about smoking history and current maintenance medicine were asked in eligibility screening
dParticipants may report more than one category; rescue medications may not be mutually exclusive
Over one-half (58 %) of participants felt that their overall health was either fair or poor. Most participants reported having at least one comorbid health condition. The mean total BCSS score was 4.5 (SD 2.42) on the day of survey completion; for individual COPD symptoms, patients on average reported moderate breathing difficulty (mean 2.1, SD 1.09), rare/occasional cough (mean 1.5, SD 1.03), and mild trouble with sputum (mean 1.0, SD 0.94).
Evaluation of DCE Task Comprehension
Task comprehension was evaluated using two consistency check questions (items 1 and 8). Out of the total sample of 515 participants, 441 (86 %) chose the preferred medicine for both consistency check questions, and 496 (96 %) chose the preferred medicine for at least one of the two consistency check questions. Utility (preference) scores were generated for the total sample, as well as for the two sub-samples based on participant responses to consistency check questions (data not shown). The same pattern of average utilities scores was generated using the total sample, as well as the two subsets of participants. These results suggested that unexpected responses to the consistency check questions did not produce substantially different results. In addition, no significant differences in sociodemographic characteristics were detected, with the exception that those with inconsistent responses tended to report having slightly more severe COPD than those with consistent responses. Therefore, subsequent analyses were conducted using all 515 participants.
Utility (Preference) Scores
The best or most favorable level was consistently preferred for each separate COPD medication attribute over the other less favorable levels. There was a logical ordering of utilities for the three levels presented for each COPD attribute (data not shown); for example, ‘complete relief’ had a higher preference score than ‘some relief,’ which in turn had a higher preference score than ‘little or no relief’ for the symptom relief attribute. Each COPD medication attribute (symptom relief, feeling maintenance medication begin to work, ease of use, rescue medicine use, side effects, and out-of-pocket cost) was independently important to participants.
Willingness to Pay (WTP)
WTP estimates were generated to assign monetary values (in monthly out-of-pocket co-pay dollars) to each COPD attribute level. This analysis evaluated whether participants would theoretically pay a premium to obtain each of the COPD maintenance medication attributes. The WTP analysis confirmed that participants found all of the attributes to be desirable elements in a COPD maintenance medication. Results indicated that participants were willing to pay a premium for better results from a maintenance medication (Table 3). Within each attribute, WTP estimates revealed that participants would pay to obtain the best (most favorable) level for each attribute, compared with the worst level. WTP estimates based on monthly co-pay dollars were $US64 for complete relief of symptoms, $US59 to have no side effects, $US32 to rarely use rescue medication, $US16 for an inhaler that is quick and easy to use, and $US13 for feeling medication work within 5 min, for the best (most favorable) level compared with the worst (least favorable) level for each attribute.
Table 3.
Willingness to pay for chronic obstructive pulmonary disease attributes (N = 515)
| Attributes and levels | Marginal effect (95 % CI) | Marginal WTP to avoid level (95 % CI) |
|---|---|---|
| Relieves symptoms (reference: complete relief) | ||
| Little or no relief | −1.23 (−1.33, −1.12) | $63.62 (58.16–69.08) |
| Some relief | −0.54 (−0.64, −0.43) | $27.74 (22.28–33.20) |
| Feel medicine start to work (reference: within 5 min) | ||
| Within 20 min | −0.19 (−0.24, −0.15) | $10.00 (7.74–12.27) |
| Within 30 min or more | −0.25 (−0.30, −0.21) | $13.02 (10.75–15.28) |
| Ease of use (reference: quick and easy) | ||
| Requires some practice and care | −0.09 (−0.14, −0.04) | $4.75 (2.25–7.25) |
| More difficult and time-consuming | −0.30 (−0.35, −0.25) | $15.53 (13.03–18.03) |
| Rescue medicine use (reference: rarely, 1–2 times per week or less) | ||
| 3–5 times per week | −0.38 (−0.48, −0.29) | $19.83 (14.99–24.67) |
| Every day | −0.61 (−0.70, −0.52) | $31.57 (26.73–36.41) |
| Side effects (reference: no side effects) | ||
| Mild side effects | −0.29 (−0.33, −0.24) | $14.81 (12.40–17.22) |
| Moderate to severe side effects | −1.13 (−1.18, −1.09) | $58.69 (56.28–61.11) |
Reference was the most favorable level for each COPD medication attribute. 95 % CIs that did not include zero were considered to be statistically significant
CI confidence interval, COPD chronic obstructive pulmonary disease, WTP willingness to pay
WTP varied with the level of COPD disease severity (Table 4). Participants who reported severe COPD (i.e., severe or very severe COPD) were willing to pay more per month to obtain favorable COPD medication attributes than were those classifying themselves as having mild or moderate COPD for some attributes, particularly for symptom relief and feeling medication start to work quickly. Those reporting mild COPD were willing to pay more for less frequent need to use rescue medicines. WTP estimates across COPD severity levels ranged from $US62 to 68 for complete relief of symptoms, $US58 to 61 to have no side effects, $US25 to 36 to rarely use rescue medication, $US12 to 19 for an inhaler that is quick and easy to use, and $US10 to 18 to feel medication work within 5 min, compared with the worst level for each attribute.
Table 4.
Willingness to pay estimates for chronic obstructive pulmonary disease attributes by disease severity (N = 506)
| Attributes and levels | Marginal WTP to avoid level by COPD disease severitya (95 % CI) | ||
|---|---|---|---|
| Mild (N = 108) | Moderate (N = 238) | Severe/very severe (N = 160) | |
| Relieves symptoms (reference: complete relief) | |||
| Little or no relief | $61.97 (51.21–72.74) | $65.54 (57.52–73.56) | $67.51 (56.31–78.71) |
| Some relief | $30.51 (19.74–41.27) | $29.36 (21.34–37.38) | $26.64 (15.44–37.83) |
| Feel medicine start to work (reference: within 5 min) | |||
| Within 20 min | $5.48 (0.92–10.04) | $10.42 (7.17–13.68) | $13.41 (8.72–18.10) |
| Within 30 min or more | $10.43 (5.87–15.00) | $10.04 (6.79–13.29) | $18.33 (13.64–23.02) |
| Ease of use (reference: quick and easy) | |||
| Requires some practice and care | $8.70 (3.55–13.86) | $6.08 (2.52–9.63) | −$0.19 (−$5.40–5.02) |
| More difficult and time-consuming | $11.88 (6.72–17.04) | $19.20 (15.65–22.76) | $12.81 (7.60–18.01) |
| Rescue medicine use (reference: rarely, 1–2 times per week or less) | |||
| 3–5 times per week | $20.50 (11.15–29.86) | $19.98 (12.88–27.07) | $18.13 (8.10–28.16) |
| Every day | $36.02 (26.67–45.38) | $33.68 (26.58–40.78) | $24.54 (14.51–34.56) |
| Side effects (reference: no side effects) | |||
| Mild side effects | $19.41 (14.45–24.38) | $13.46 (9.97–16.95) | $13.46 (8.57–18.35) |
| Moderate to severe side effects | $61.03 (56.07–66.00) | $57.77 (54.28–61.26) | $60.35 (55.46–65.24) |
Reference was the most favorable level for each COPD medication attribute. 95 % CIs that did not include zero were considered to be statistically significant. Negative marginal WTP value for ‘Ease of use’ was due to a relatively small difference in preference between attribute levels ‘Quick and easy’ and ‘Requires some practice and care’
CI confidence interval, COPD chronic obstructive pulmonary disease, WTP willingness to pay
aCOPD disease severity was participant-reported (Mild, Moderate, Severe, Very severe, Don’t know). Nine participants with response ‘Don’t know’ were excluded
Participants ranged from 40–88 years old, and WTP was evaluated by age groups (Table 5). WTP was estimated separately for two age groups; participants aged 40–62 years (n = 249) and participants aged 63–88 years (n = 266), based on a mean split. Older participants had higher WTP for virtually all COPD medication attributes than younger participants. WTP estimates in monthly co-pay dollars ranged from $US52 to 78 for complete relief of symptoms, $US48 to 71 to have no side effects, $US28 to 35 to rarely use rescue medication, $US13 to 18 for an inhaler that is quick and easy to use, and $US11 to 16 to feel medication work within 5 min, compared with the worst level for each attribute. WTP across attributes ranged from $US16 to 78 for participants aged 63–88 years and $US11 to 52 for participants aged 40–62 years.
Table 5.
Willingness to pay estimates for chronic obstructive pulmonary disease attributes by age group (N = 515)
| Attributes and levels | Marginal WTP to avoid level by age group (95 % CI) | |
|---|---|---|
| 40–62 years old (N = 249) | 63–88 years old (N = 266) | |
| Relieves symptoms (reference: complete relief) | ||
| Little or no relief | $51.59 (44.41–58.77) | $77.88 (69.51–86.24) |
| Some relief | $20.49 (13.31–27.67) | $36.41 (28.05–44.78) |
| Feel medicine start to work (reference: within 5 min) | ||
| Within 20 min | $8.28 (5.28–11.28) | $11.97 (8.53–15.41) |
| Within 30 min or more | $10.51 (7.51–13.51) | $15.95 (12.51–19.39) |
| Ease of use (reference: quick and easy) | ||
| Requires some practice and care | $4.46 (1.15–7.76) | $5.27 (1.46–9.07) |
| More difficult and time-consuming | $13.48 (10.17–16.78) | $18.07 (14.27–21.88) |
| Rescue medicine use (reference: rarely, 1–2 times per week or less) | ||
| 3–5 times per week | $19.71 (13.27–26.14) | $19.53 (12.21–26.85) |
| Every day | $28.41 (21.98–34.84) | $35.16 (27.84–42.48) |
| Side effects (reference: no side effects) | ||
| Mild side effects | $13.23 (10.11–16.35) | $17.00 (13.25–20.75) |
| Moderate to severe side effects | $48.29 (45.17–51.41) | $71.01 (67.26–74.76) |
Reference was the most favorable level for each COPD medication attribute. 95 % CIs that did not include zero were considered to be statistically significant
CI confidence interval, COPD chronic obstructive pulmonary disease, WTP willingness to pay
WTP was examined by gender of the participant with COPD (Table 6). COPD disease severity was similar by gender; almost half of men and women reported having moderate COPD. Men with COPD had higher WTP for complete symptom relief ($US70 vs. 59), quick and easy to use inhaler ($US19 vs. 13), less frequent need to use rescue medicine ($US35 vs. 29), and avoiding side effects ($US68 vs. 52) compared with women. However, women were willing to pay more monthly than men for a medicine that they can feel begin to work quickly ($US14 vs. 11). WTP estimates ranged from $US59 to 70 for complete relief of symptoms, $US52 to 68 to have no side effects, $US29 to 35 to rarely use rescue medication, $US13 to 19 for an inhaler that is quick and easy to use, and $US11 to 14 to feel medication work within 5 min, compared with the worst level for each attribute.
Table 6.
Willingness to pay estimates for chronic obstructive pulmonary disease attributes by gender (N = 515)
| Attributes and levels | Marginal WTP to avoid level by gender (95 % CI) | |
|---|---|---|
| Male (N = 230) | Female (N = 285) | |
| Relieves symptoms (reference: complete relief) | ||
| Little or no relief | $69.71 (60.62–78.80) | $59.10 (52.34–65.86) |
| Some relief | $27.30 (18.21–36.38) | $27.58 (20.83–34.34) |
| Feel medicine start to work (reference: within 5 min) | ||
| Within 20 min | $10.36 (6.63–14.09) | $9.72 (6.89–12.54) |
| Within 30 min or more | $11.45 (7.72–15.18) | $14.12 (11.29–16.94) |
| Ease of use (reference: quick and easy) | ||
| Requires some practice and care | $8.00 (3.78–12.22) | $2.58 (−0.48–5.64) |
| More difficult and time-consuming | $18.57 (14.35–22.79) | $13.32 (10.25–16.38) |
| Rescue medicine use (reference: rarely, 1–2 times per week or less) | ||
| 3–5 times per week | $23.29 (15.23–31.34) | $17.86 (11.87–23.85) |
| Every day | $35.23 (27.18–43.29) | $29.29 (23.30–35.27) |
| Side effects (reference: no side effects) | ||
| Mild side effects | $12.99 (8.97–17.01) | $15.92 (12.95–18.90) |
| Moderate to severe side effects | $67.57 (63.55–71.59) | $52.44 (49.47–55.42) |
Reference was the most favorable level for each COPD medication attribute. 95 % CIs that did not include zero were considered to be statistically significant
CI confidence interval, COPD chronic obstructive pulmonary disease, WTP willingness to pay
Discussion
This study identified and evaluated the importance of maintenance medication attributes to COPD patients. Based on findings from focus group discussions, attributes of COPD maintenance medication important to patients included symptom relief, speed with which patients felt their medication working, use of rescue medicine, ease of use for inhaler, presence of side effects, and out-of-pocket co-pay cost. Each attribute was associated with a value in co-pay dollars ranging from $US5 to 78, indicating that subjects would theoretically pay to obtain the benefit of a drug that had these attributes. These findings suggest that patients making individual health choices expressed WTP more out-of-pocket for a COPD maintenance medication with desirable features. Efficacy and side effects are often of primary concern for patients, and, as expected, symptom relief and avoiding side effects were highly valued, with higher WTP estimates for an efficacious medication with low risk. However, additional attributes, such as being able to feel the medicine begin to work quickly, inhaler ease of use, and the need to use rescue medicine were also associated with non-negligible WTP estimates, suggesting that these attributes are important considerations when evaluating a medication and are clearly meaningful to participants.
It should be noted that participants consistently preferred the best level for each individual COPD medication attribute on two consistency check questions embedded in each version of the DCE questionnaire, thus providing confidence in patient responses. Consistency in responses also helped to confirm that COPD medication attributes and levels derived from qualitative background research were appropriate.
Some differences were found in WTP when results were examined by COPD severity level. Patients with COPD classified as severe or very severe were willing to pay more for symptom relief and feeling the medication begin to work quickly. This may be due to a heightened awareness of symptoms and their impact among those with more severe disease. Severe patients were willing to pay almost $US8 more than less severe patients in order to obtain a medication they could feel working within 5 min. Interestingly, less frequent rescue medication use was not as important to patients with severe illness compared with those with less severe COPD. While older patients demonstrated higher WTP than younger patients, factors other than age may have contributed to this finding; confounding factors could include duration and severity of illness, in light of the progressive nature of COPD, and income and access to care for older patients. Additional research would be needed to further explore the relationship between age and WTP in COPD.
There are several limitations in this study. A limitation of the qualitative phase was that the background research conducted was solely focused on obtaining information about COPD medication delivery. A formal, systematic, and comprehensive review of all COPD literature was not conducted; rather, a brief, targeted review of recent literature (2000–2008) was performed that focused on COPD treatment/medication characteristics. Only two focus groups were conducted with COPD patients, and these groups also concentrated on COPD medication delivery as the main topic of discussion; there was limited inquiry into other aspects of the patient experience with COPD or other COPD treatments.
In the quantitative phase, medication scenarios posed to patients were hypothetical, and WTP in monthly co-pay dollars derived from preference weights; these stated preferences may differ from the actual choices that patients would make in a real-world setting. In addition, unmodeled heterogeneity in medication preferences across respondents may introduce bias and impact study findings. All survey data were based on respondent self-report; no physician records or diagnostic information were accessed. Participants self-reported both the diagnosis of COPD, as well as their severity level, and their perceptions may differ from information that would have been obtained from clinical sources. In addition, although minimum inclusion criteria were required, no exclusion criteria were specified for the study and participants were self-excluded by not selecting to participate in the survey. Based on the inclusion criteria that were used, patients could have had other comorbid health conditions present, which may have affected their responses. Data on household income, health plan coverage, and co-pay levels were not collected, so the impact of these factors as covariates for WTP could not be evaluated. Respondents were not asked after completing the choice tasks to rank-order the six medication attributes in their opinion from most to least important to solicit their direct preference; this additional information about their perceived ranking order, in addition to the indirect ranking generated by the preference utility analysis, would have been informative had it been collected.
Both phases of the study involved COPD patients in the USA who were largely treated with LABA or long-acting muscarinic antagonist (LAMA) therapies; this may limit the generalizability of these results to the larger population of COPD patients, as well as patients in other countries with different healthcare systems and approaches to therapy. However, the findings obtained in this study improve our understanding of factors that may contribute to patient preference for maintenance medications and potential areas for future research on patient beliefs and perceptions about medication that may subsequently help to improve adherence.
Conclusions
This study of US patients showed that patients take into consideration multiple attributes of a COPD maintenance therapy, and that, in hypothetical scenarios, patients expressed WTP more for a medication with attractive characteristics. Attributes such as decreased use of rescue medication, ease of use for inhaler, and being able to feel a medication begin to work quickly are aspects beyond safety and efficacy that patients consider in evaluating preference for a COPD maintenance medication. A medication that possesses an attribute that is particularly attractive or one that another medication lacks may also distinguish itself from other similar therapies and potentially provide added value from the patient perspective. Patients may also be more likely to adhere to a medication that they perceive to have attractive characteristics and valuable benefits. Findings in this study identified multiple concepts relevant to patient preference for maintenance medication in the context of hypothetical scenarios; additional research evaluating patient preference for specific medications with similar properties and in other countries/healthcare systems would be helpful to further evaluate this in a real-world setting.
Acknowledgments
Funding support for this research was provided by AstraZeneca LP, Wilmington, DE, USA. Dr. Kawata, Dr. Kleinman, and Ms. Harding are employees of Evidera, Bethesda, MD, USA. Dr. Ramachandran is an employee of AstraZeneca. The authors acknowledge Laurie Roberts, MPH, and Erin O’Rourke, BS, of Evidera for managing conduct of the focus groups; Anne Brooks, BS, of Evidera for assistance developing the web survey; Samantha Luks, PhD, and staff at YouGov (Palo Alto, CA, USA) for programming and implementation of the web survey; Fritz Hamme, BA, of Evidera for production support; Jeffrey Fletcher, PhD, Ubaldo Martin, MD, and Setareh Williams, PhD, and the global publications review team at AstraZeneca for review and commentary; and Mary Wiggin, BA, of AstraZeneca and Stella Chow, PhD, of Scientific Connexions (East Norriton, PA, USA) for editorial support in the preparation of this manuscript.
Conflict of interest
Funding support for this research was provided by AstraZeneca LP. Dr. Kawata, Dr. Kleinman, and Ms. Harding are employees of Evidera. Dr. Ramachandran is an employee of AstraZeneca.
The co-authors were involved in study design, statistical analysis, and interpretation of results. The authors had full access to data and were involved in critical review and editing of the manuscript. All authors provided approval prior to submission.
References
- 1.World Health Organization. Chronic obstructive pulmonary disease (COPD) burden; 2011. http://www.who.int/respiratory/copd/burden/en/index.html. Accessed March 2011.
- 2.Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of COPD. Revised 2011. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed 1 June 2012.
- 4.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence (NICE). Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (update); 2010. http://guidance.nice.org.uk/CG101. Accessed 1 June 2012.
- 6.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:371–384. doi: 10.2147/copd.s3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu AP, Guérin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14:486–496. doi: 10.3111/13696998.2011.576295. [DOI] [PubMed] [Google Scholar]
- 8.Col N, Fanale JE, Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch Intern Med. 1990;150:841–845. doi: 10.1001/archinte.1990.00390160093019. [DOI] [PubMed] [Google Scholar]
- 9.Fuso L, Incalzi RA, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98:272–277. doi: 10.1016/S0002-9343(99)80374-X. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Aymerich J, Barreiro E, Farrero E, et al. Patients hospitalized for COPD have a high prevalence of modifiable risk factors for exacerbation (EFRAM study) Eur Respir J. 2000;16:1037–1042. doi: 10.1034/j.1399-3003.2000.16f03.x. [DOI] [PubMed] [Google Scholar]
- 11.Balakrishnan R, Christensen DB. Inhaled corticosteroid use and associated outcomes in elderly patients with moderate to severe chronic pulmonary disease. Clin Ther. 2000;22:452–469. doi: 10.1016/S0149-2918(00)89013-X. [DOI] [PubMed] [Google Scholar]
- 12.George J, Kong DC, Thoman R, et al. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198–3204. doi: 10.1378/chest.128.5.3198. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DS, Gillion MS, Rees PJ. Use of dry powder inhalers in COPD. Int J Clin Pract. 2007;61:2005–2008. doi: 10.1111/j.1742-1241.2007.01593.x. [DOI] [PubMed] [Google Scholar]
- 14.Stoloff S, Samuels S, Kerney D, et al. Management of chronic obstructive pulmonary disease associated with chronic bronchitis with inhaled fluticasone propionate/salmeterol (ADVAIR DISKUS) 250/50: results of a patient experience trial. MedGenMed. 2006;8:86. [PMC free article] [PubMed] [Google Scholar]
- 15.Moore AC, Stone S. Meeting the needs of patients with COPD: patients’ preference for the Diskus inhaler compared with the Handihaler. Int J Clin Pract. 2004;58:444–450. doi: 10.1111/j.1368-5031.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Kozma CM, Slaton TL, Monz BU, et al. Development and validation of a patient satisfaction and preference questionnaire for inhalation devices. Treat Respir Med. 2005;4:41–52. doi: 10.2165/00151829-200504010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care. 2005;50:1313–21 (discussion 1321–2). [PubMed]
- 18.Barta SK, Crawford A, Roberts CM. Survey of patients’ views of domiciliary nebuliser treatment for chronic lung disease. Respir Med. 2002;96:375–381. doi: 10.1053/rmed.2001.1292. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser H, Parasuraman B, Boggs R, et al. Onset of effect of budesonide and formoterol administered via one pressurized metered-dose inhaler in patients with asthma previously treated with inhaled corticosteroids. Ann Allergy Asthma Immunol. 2008;101:295–303. doi: 10.1016/S1081-1206(10)60495-4. [DOI] [PubMed] [Google Scholar]
- 20.Johansson G, Ställberg B, Tornling G, et al. Asthma treatment preference study: a conjoint analysis of preferred drug treatments. Chest. 2004;125:916–923. doi: 10.1378/chest.125.3.916. [DOI] [PubMed] [Google Scholar]
- 21.Maas A, Stalpers L. Assessing utilities by means of conjoint measurement: an application in medical decision analysis. Med Decis Making. 1992;12:288–297. doi: 10.1177/0272989X9201200408. [DOI] [PubMed] [Google Scholar]
- 22.Verhoef LC, Stalpers LJ, Verbeek AL, et al. Breast-conserving treatment or mastectomy in early breast cancer: a clinical decision analysis with special reference to the risk of local recurrence. Eur J Cancer. 1991;27:1132–1137. doi: 10.1016/0277-5379(91)90310-A. [DOI] [PubMed] [Google Scholar]
- 23.Harwood RH, Rogers A, Dickinson E, et al. Measuring handicap: the London Handicap Scale, a new outcome measure for chronic disease. Qual Health Care. 1994;3:11–16. doi: 10.1136/qshc.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakim Z, Pathak DS. Modelling the EuroQol data: a comparison of discrete choice conjoint and conditional preference modelling. Health Econ. 1999;8:103–116. doi: 10.1002/(SICI)1099-1050(199903)8:2<103::AID-HEC393>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Kleinman L, McIntosh E, Ryan M, et al. Willingness to pay for complete symptom relief of gastroesophageal reflux disease. Arch Intern Med. 2002;162:1361–1366. doi: 10.1001/archinte.162.12.1361. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd A, Penson D, Dewilde S, et al. Eliciting patient preferences for hormonal therapy options in the treatment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:153–159. doi: 10.1038/sj.pcan.4500992. [DOI] [PubMed] [Google Scholar]
- 27.Leidy NK, Schmier JK, Jones MK, et al. Evaluating symptoms in chronic obstructive pulmonary disease: validation of the Breathlessness, Cough, and Sputum Scale. Respir Med. 2003;97(suppl A):S58–S70. [PubMed] [Google Scholar]


