Abstract
INTRODUCTION: Incisional biopsies, including the diagnostic core needle biopsy (CNB), routinely performed before surgical excision of breast cancer tumors are hypothesized to increase the risk of metastatic disease. In this study, we experimentally determined whether CNB of breast cancer tumors results in increased distant metastases and examine important resultant changes in the primary tumor and tumor microenvironment associated with this outcome. METHOD: To evaluate the effect of CNB on metastasis development, we implanted murine mammary 4T1 tumor cells in BALB/c mice and performed CNB on palpable tumors in half the mice. Subsequently, emulating the human scenario, all mice underwent complete tumor excision and were allowed to recover, with attendant metastasis development. Tumor growth, lung metastasis, circulating tumor cell (CTC) levels, variation in gene expression, composition of the tumor microenvironment, and changes in immunologic markers were compared in biopsied and non-biopsied mice. RESULTS: Mice with biopsied tumors developed significantly more lung metastases compared to non-biopsied mice. Tumors from biopsied mice contained a higher frequency of myeloid-derived suppressor cells (MDSCs) accompanied by reduced CD4 + T cells, CD8 + T cells, and macrophages, suggesting biopsy-mediated development of an increasingly immunosuppressive tumor microenvironment. We also observed a CNB-dependent up-regulation in the expression of SOX4, Ezh2, and other key epithelial-mesenchymal transition (EMT) genes, as well as increased CTC levels among the biopsy group. CONCLUSION: CNB creates an immunosuppressive tumor microenvironment, increases EMT, and facilitates release of CTCs, all of which likely contribute to the observed increase in development of distant metastases.
Abbreviations: CCAC, Canadian Council on Animal Care; CNB, core needle biopsy; CTCs, circulating tumor cells; EMT, epithelial-mesenchymal transition; H&E, hematoxylin and eosin; MDSCs, myeloid-derived suppressor cells; PGE2, prostaglandin E2
Keywords: breast cancer, core needle biopsy, metastasis immunosuppression, epithelial-mesenchymal transition
Introduction
Breast cancer is the most common cancer affecting women and accounts for the second highest incidence of cancer-related death, after lung cancer [1]. There are numerous factors known to influence the metastatic potential of any given breast cancer (tumor size, receptor status, lymph node involvement at the time of diagnosis, age of the patient, menopausal status, and family history) [2], [3], [4], [5]. However, the most consistent predictors of metastasis continue to be tumor size and lymph node involvement at the time of diagnosis [6], [7]. Because of this, breast cancer screening and early detection are critically important in improving outcomes for women with breast cancer.
Suspicious lesions detected on screening mammograms are generally biopsied to confirm or rule out a diagnosis of cancer. The most common form of biopsy administered today is a core needle biopsy (CNB) [8], [9], [10], [11], [12], [13], [14]. A CNB is a form of incisional biopsy whereby a portion of a tumor is removed for histologic evaluation leaving the remainder in vivo to be removed at a later date following a definitive diagnosis. Typically with breast cancer, tissue samples are collected by administering three to nine passes with a 14G biopsy needle [15], [16]. Biopsy needle sizes can range from 9G to 18G depending on the particular form of image guidance and system of sample acquisition used for the core biopsy [17], [18], [19].
Other breast cancer biopsy techniques include complete excisional biopsy, open incisional biopsy, and fine needle aspiration. CNB and fine needle aspiration are favored over open incisional or excisional biopsies because they are less invasive, produce a smaller cosmetic post-operative footprint, and result in faster patient recovery. This is particularly appealing given that the majority of biopsied breast lesions are ultimately ruled benign [20], [21]. Furthermore, in addition to distinguishing invasive from non-invasive cancer, tissue obtained from CNB can be used to perform nucleic acid analysis, immunohistochemistry, or analysis of prognostic biomarkers [22], [23], [24]. As a consequence, stereotactic or ultrasonographically guided CNB is currently the predominant biopsy method employed in breast cancer management [16].
Incisional surgical procedures, including incisional biopsies, on cancers have historically been associated with higher local recurrence rates and elevated incidence of lymph node metastasis [25], [26], [27], [28], [29]. There is also a growing body of evidence suggesting that surgical trauma in the presence of an established neoplasm can potentiate its growth and metastatic proliferation [30], [31], [32], [33].
Current literature also increasingly notes the occurrence of post-surgical immunosuppressive changes and their relevance to metastatic spread and disease recurrence [33]. These observations suggest that surgically instigated changes in the tumor and subsequent host interaction with residual disease can influence the tumor’s metastatic potential.
The clinical impact and potential risk associated with performing a CNB has long been debated. There is compelling evidence that CNB increases the risk of needle track seeding and local tumor recurrence in patients with breast cancer [28], [34], [35]. There is also little question that cancer cells from both invasive and non-invasive breast cancers enter lymphatic channels and migrate to lymph nodes following a biopsy procedure [27], [36]. However, whether or not cancer cells displaced into lymphatic and vascular channels are capable of effectively establishing distant metastases remains unproven [34], [37], [38], [39].
To test the hypothesis that surgically initiated changes in the tumor microenvironment due to CNB results in increased metastatic spread, we used the 4T1-BALB/c mouse model, a well-established, immune-competent, cancer animal model considered to closely mimic metastatic breast cancer in humans. Murine mammary 4T1 tumor cells were orthotopically implanted in BALB/c mice and tumors large enough to biopsy developed within 2 to 3 weeks. Biopsies were then performed in a manner designed to experimentally replicate the human clinical experience of using CNB for the diagnostic workup of breast cancer as well as study the impact of the CNB on metastatic outcomes. In this model, tumors spontaneously metastasize from the mammary fat pad to lymph nodes, lung, and bone in a similar pattern to that observed in human breast cancers [40]. This immunologically intact model also enabled study of the immunologic changes associated with CNB within the local tumor microenvironment, in distant organs, and peripheral circulation. These changes were assessed to detect those events that might be associated with tumor progression and metastasis [41]. Gene expression profiles were evaluated to detect changes in known key epithelial-mesenchymal transition (EMT) genes [42]. Recent studies have drawn associations between surgical procedures and increased levels of tumor cells in circulation [43]. In consideration of this finding, and the documented link between circulating tumor cell (CTC) levels and metastasis [44], [45], the impact of CNB on CTC levels was also measured.
Methods
Cells
Metastatic murine breast cancer cell line 4T1 (ATCC CRL-2539) cells were cultured in RPMI-1640, supplemented with 10% FBS and antibiotic-antimycotic, at 37°C at 90% humidity and 5% CO2. All media, sera, and supplements used for the murine metastasis model were purchased from Invitrogen, Life Technologies (Burlington, Ontario).
Animals
Female BALB/c mice were obtained from Charles River Laboratory (St-Constant, Canada) and housed in the Carleton Animal Care Facility at Dalhousie University. After a 1 week acclimatization period, the mice were used in the study. All protocols (No. 09-044 and No. 13-088) were approved by the Dalhousie University Committee on Laboratory Animals in keeping with guidelines established by the Canadian Council on Animal Care (CCAC).
Metastasis Model
Mice received a subcutaneous orthotopic injection of 7 × 103 4T1 tumor cells into the third thoracic mammary fat pad. Primary tumors were palpable in 10 to 16 days and reached the biopsy size of 6 to 8 mm by 15 to 20 days after implantation. Mice with slow-growing tumors that failed to achieve the required size for experimentation were excluded from the study (this was typically no more than 3 mice from a starting set of 23 mice). Tumor growth was determined by measuring two axes of the tumor and determining the mean tumor diameter. At the CNB time point, mice were alternately assigned to biopsy and non-biopsy groups to eliminate bias. Mice assigned to the biopsy group were fully anesthetized using inhaled isoflurane (2% body weight) and their biopsy sites were shaved and subjected to aseptic surgical preparation; they then received CNB with an 18-gauge needle attached to a 5-ml syringe. Six to eight cutting passes were administered in a palmate-radiating pattern through a single cutaneous insertion point so as to reduce injury to the skin. On occasion, when a tumor was multilobed or irregularly shaped, an additional skin puncture was required to achieve six to eight passes distributed through the tumor with the biopsy needle. The non-biopsy (control) groups received no biopsy but were subjected to an equal duration of complete anesthesia in a modified induction chamber. Mice were maintained in a recovery area for 24 hours post procedure and observed to assure return to normal grooming and feeding behavior. All mice were monitored regularly throughout the experiment for any signs of illness or distress and were killed if and when they became moribund, according to CCAC guidelines.
In the clinical setting, breast cancers are generally biopsied using image-guided CNB technology to confirm the diagnosis with definitive (excisional) surgical treatment typically performed 2 or more weeks following performance of a diagnostic CNB. To reproduce this process, tumors were excised in both biopsy and non-biopsy control mice groups 7 days post biopsy/non-biopsy assignment (Figure 1A). Mice were anesthetized and tumors were surgically (and aseptically) excised, removing all tumor tissue to prevent regrowth. Incisions were sutured shut using Novaril 4. Mice were given analgesic Ketoprofen (5 mg/kg), monitored regularly for any signs of illness, and were killed if judged moribund according to CCAC guidelines.
Figure 1.
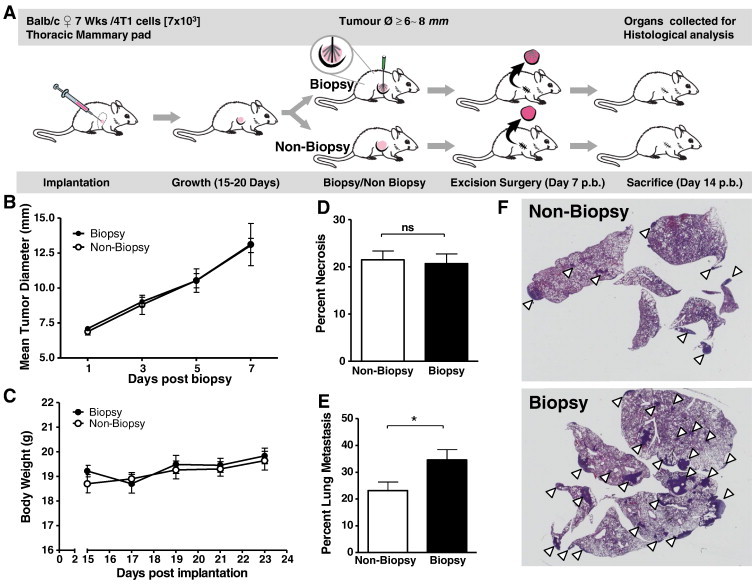
CNB significantly increases lung metastasis. (A) Experiment schematic mimicking the format of progression in the human scenario Detection > Biopsy > Excision > Outcome. Mice were implanted with 4T1 cells, and after 6- to 8-mm diameter tumors developed, the mice are alternately assigned to two groups, a biopsied group (n = 40), and a non-biopsy group (n = 44). This was followed by surgical excision of the tumors 7 days post biopsy and sacrifice of the mice at 14 days post biopsy (circular inset: details radiating a palamate biopsy pattern). (B) Tumor growth and (C) body weight were monitored post biopsy. (D) Percent necrosis of excised tumors and (E) percent metastasis of the lungs were determined by H&E staining of fixed tissue sections. (F) Representative section of lungs from non-biopsy and biopsy mice groups. White pointers indicate lung metastases. Significance determined by an unpaired t test, *P ≤ .05; error bars represent the SEM.
Excised tumors were divided with a 2 mm × 2 mm × 6 mm sliver of tissue being snap frozen in TRIzol and RNAlater (Qiagen GmbH, Hilden, Germany) for later RNA extraction and quantitative polymerase chain reaction (qPCR) gene expression analysis, as described in the Quantification of Gene Expression by qPCR section. The remaining tumor tissue was placed in 10% formalin buffered with sodium acetate for paraffin embedding and histologic analysis.
Histologic Quantification of Pulmonary Metastasis and Tumor Necrosis
To compare the level of metastasis and associated necrosis between biopsied and non-biopsied mice, a total of 84 mice in four cohorts underwent the metastasis model described above. Cohort size was designed to allow manageable numbers of mice and enable consistent treatment and surgical handling. Seven days after the tumor excision, each cohort of mice consisting, on average, of biopsy (n = 10) and non-biopsy (n = 11) mice was sacrificed. The draining lymph nodes and lungs were harvested, formalin fixed, and paraffin embedded with each block assigned an anonymizing numerical identifier to allow unbiased evaluation. The anonymized lungs, lymph nodes, and tumors were serial sectioned into 5-μm thin tissue sections and stained with hematoxylin and eosin (H&E) for histologic evaluation. Imaging was performed using a Zeiss Axiocam HRC Color, mounted on a Zeiss Stemi. The percentage of metastatic (lung and lymph node) and necrotic (tumor) tissue areas relative to total section area was established using an overlay grid imposed on images captured from H&E-stained tissue sections.
Quantification of CTCs in Peripheral Circulation post Biopsy Using 4T1neo Cells
To quantify CTCs in circulation, we generated neomycin cassette-bearing 4T1 cells using the Clontech retroviral expression system (Clontech Laboratories, Mountain View, CA; Cat. No. 634401). The cells were selected for stable expression of the neomycin resistance gene by multiple passages under RPMI-1640 with 100 μg/ml G418 sulfate (Fisher Scientific Co., Fair Lawn, NJ), and the resulting cells were designated 4T1neo cells.
To assess changes in the level of CTCs, the 4T1neo cells were implanted in the mammary fat pad of a single group of 12 BALB/c mice using a procedure identical to that used for unmodified 4T1 cells. The mice developed similar sized tumors in the same time frame as unmodified 4T1 implanted mice. The 12 mice implanted with 4T1neo cells were biopsied when the 6- to 8-mm tumor diameter threshold was achieved. Groups of mice (n = 4) were processed for each post-biopsy time point (6 hours, 24 hours, and 10 days). Mice were bled through facial vein with 200 μl of blood collected and immediately mixed with 1 ml of TRIzol reagent. This was snap frozen in liquid nitrogen and stored at − 80°C until RNA was extracted and qPCR was performed for neomycin gene expression. Mice were sacrificed, and their tumors harvested and processed in RNAlater for subsequent RNA extraction and gene expression analysis by qPCR.
Quantification of Gene Expression by qPCR
Immediately after blood collection for analysis, mice from biopsy (n = 5) and non-biopsy (n = 5) groups were sacrificed for the 0-, 3-, 12-, and 24-hour post biopsy time points (n = 40). Sections of tumor and lung were harvested and snap frozen in TRIzol and RNAlater (Qiagen Inc). Frozen tissue samples were later thawed, the tissue was crushed, and the RNA extracted using Purelink RNA Kit (Invitrogen Inc). Generation of cDNA was performed using Superscript II (Invitrogen Inc). For qPCR analysis of relative expression of genes of interest, specific primers for neomycin resistance gene (Neo), S100A8, Ly-6G (Gr-1), CXCL2 (MIP-2), CCL3 (MIP-1a), FOXP3, transforming growth factor–β (TGF-β), SOX-4, Ezh2, SNAI2, ZEB2, CDH2 (N cadherin), IL-1β, IL-10, TNF-α, matrix metalloproteinase 9, and urokinase plasminogen activator were designed on the basis of RefSeq data for Mus musculus using the NCBI Primer-BLAST online primer designing tool (Table S1).
Using the Bio-Rad CFX-96 system, qPCR assay was performed and analysis of results was done using the CFX manager software applying ΔΔCt analysis with relative normalization calculations based on the murine glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin housekeeping genes.
Flow Cytometry
To study variation in immune cellular populations in the tumor and lung microenvironments and systemic immune changes related to performance of a CNB, a single cohort of 60 mice was used. Twenty mice from the biopsy (n = 10) and non-biopsy (n = 10) groups were sacrificed on each assigned post-biopsy time point (days 3, 7, and 10). Sections of tissue from tumor, lung, spleen, and lymph node were harvested and immediately processed and labeled with CD4 − fluorescein isothiocyanate, CD3 − Phycoerythrin (PE), CD8 − PerCP, Dx5 (Pan-Natural Killer [NK])− APC, CD45 − fluorescein isothiocyanate, Ly6G (Gr-1)–PE, CD11b-PerCP, and F4/80-APC antibodies (eBioscience Inc., San Diego, CA) allowing quantification of CD4 + T cells, CD8 + T cells, NK cells, macrophages, and myeloid-derived suppressor cells (MDSCs). Using a FACScalibur flow cytometer (BD Biosciences, CA), data were collected and analyzed using CellQuest Pro (BD Biosciences), FCS express ver. 4 (DeNovo Software, CA), and Flowing Software ver. 2.5 (University of Turku, Turku, Finland).
Statistical Analysis
Statistical significance was established relative to baseline values at 0 hour post biopsy or values recorded for non-biopsied samples and determined using non-parametric two-tailed t test performed on GraphPad Prism software. For all analyses, a P value greater than .05 was considered not significant, while significant P values were demarcated using the standard increasing asterisk scale to indicate level of significance, *P ≤ .05, **P ≤ .01, and ***P ≤ .001.
Results
CNB Is Associated with Significantly Increased Pulmonary Metastases
To investigate whether CNB has an effect on the development of pulmonary metastasis, we first implanted the mammary fat pads of BALB/c mice with 4T1 murine breast cancer cells. Tumors were allowed to grow to 6- to 8-mm diameter at which point CNB was performed in half the mice and the tumor growth rate was quantified in all mice for the following week (Figure 1A). Our results illustrate that tumor growth rates were not significantly affected by CNB (Figure 1B). There was also no significant difference between the body weights of the biopsied and non-biopsied groups (Figure 1C).
Next, to reproduce the human experience, we surgically removed the tumors from all the mice (biopsied and non-biopsied) and the mice were allowed to recover. Histologic comparison of excised tumors from biopsied mice versus those from non-biopsied mice showed similar levels of necrosis (Figure 1D). One week following surgical excision of their tumors, all mice were sacrificed and the lungs were analyzed for metastasis. Mice that had undergone CNB demonstrated significantly greater lung metastasis (Figure 1, E and F). Importantly, this suggests that CNB performed before surgical excision of a tumor may unintentionally promote metastatic dissemination.
CNBs Create an Immunosuppressive Tumor Microenvironment
It is well established that an immunosuppressive tumor microenvironment facilitates malignant progression by promoting tumor immune evasion, angiogenesis, and metastasis [46], [47], [48]. Furthermore, surgical procedures are known to induce suppressive immune conditions including infiltration by immune cells with suppressive capacities such as MDSCs [46], [49]. Therefore, following the regimen illustrated in Figure 2A, we investigated whether the increased metastasis observed in mice after CNB is also associated with the development of a pro-tumor immunosuppressive microenvironment. Flow cytometry was used to quantify macrophages (CD45 +, CD11blow, F4/80 +), MDSCs (CD45 +, CD11b +, Gr-1 +), CD4 + T cells, CD8 + T cells, and NK cells (Pan-NK, CD3 +) (Figure S1A). Data were collected on the frequencies of these cells in the primary tumor, spleen, lymph node, and lungs. It was found that on day 3 post biopsy there was a significant increase in the frequencies of MDSCs with a concurrent decrease in macrophage, CD4 + T cell, and CD8 + T cell frequencies in the tumors of mice from the biopsy group compared to non-biopsied controls. On day 7, CD4 + and CD8 + T cell and macrophage levels were significantly higher in lung tissue from the biopsy group compared to non-biopsy controls (Figure 2B). Additionally, during the post-biopsy period, there was a gradual decrease in the frequencies of MDSCs, CD4 +, and CD8 + T cells and an increase in macrophages in the spleen in both groups. Furthermore, monitoring the frequencies of the NK cells during the first 7 days post biopsy revealed that there were higher frequencies of these innate immune cells in the lung tissue from the biopsy group on day 7 post biopsy compared to non-biopsied controls (Figure 2B). Although trends were observed in the spleen (Figure 2B) and lymph nodes (Figure S1B), both showed no significant difference in recorded immune cell frequencies when comparing biopsy and non-biopsy groups over 10 days post CNB.
Figure 2.
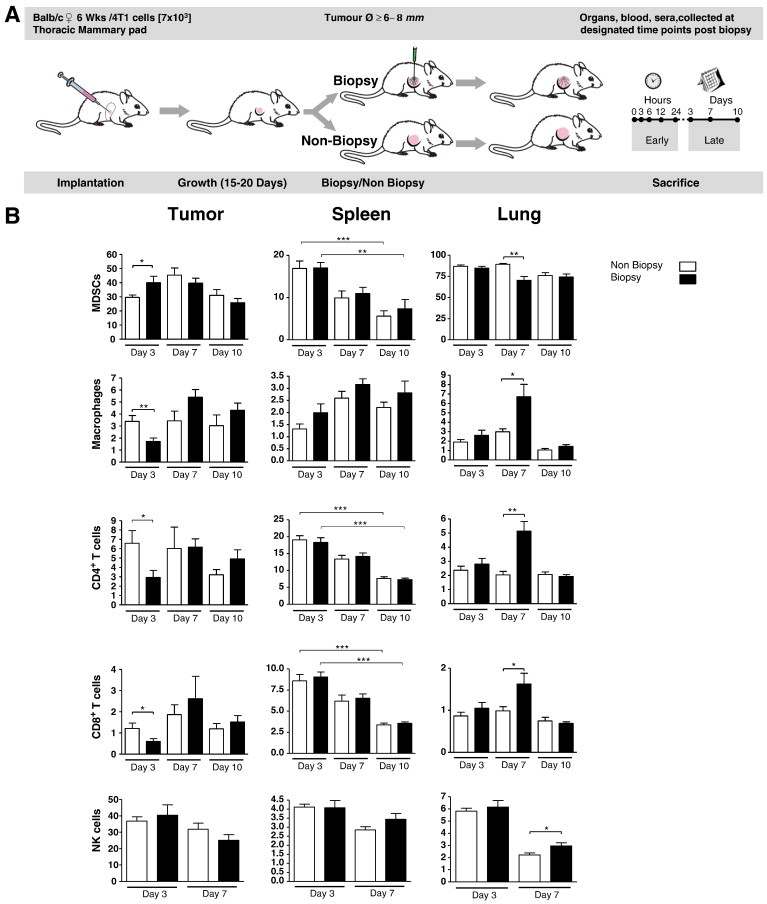
CNBs induce an immunosuppressive tumor microenvironment. (A) Mice were implanted with 4T1 cells, and after 6- to 8-mm diameter tumors developed, half the mice were biopsied (n = 30), while the other half were not biopsied (n = 30). The tumor, spleen, and lungs were collected from 10 mice from each group for flow cytometry analysis of the indicated immune cells at 3, 7, and 10 days after the biopsy date. NK cells were only monitored on days 3 and 7 post-biopsy date. Significance determined by unpaired t test, *P ≤ .05; error bars represent SEM.
Together, our data demonstrate that the CNB procedure impacts the level of immune cells at both local and systemic sites and promotes the development of an immunosuppressive immune milieu in the local tumor microenvironment immediately following CNB.
CNB Alters the Expression of Cytokines in the Local Tumor Microenvironment
The recruitment, pathophysiology, and functionality of immune cells are dictated by various soluble mediators (cytokines and chemokines) [50]. Hence, we next investigated whether CNB altered the expression of various pro-inflammatory factors such as S100A8, CXCL1, CXCL2, IL-1β, TNF-α, and COX2 that can influence the recruitment and survival of MDSCs, macrophages, and other immune cells at the tumor site [51], [52], [53]. To this end, tumor samples collected at various time points from biopsied or non-biopsied groups were processed and analyzed for the expression of a panel of cytokines. The qPCR analysis of these samples revealed a significant elevation in the expression of S100A8, CXCL2 (MIP-2α), CCL3(MIP-1α), and COX2 in tumor tissues as early as 3 hours post biopsy, with this elevation persisting up to 24 hours post biopsy (Figure 3, A–F).
Figure 3.
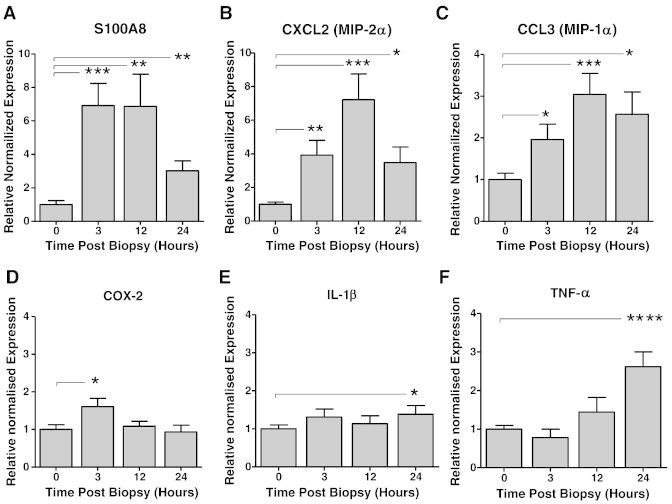
CNB modulates the expression of pro-inflammatory factors within the local tumor microenvironment. Tumor samples collected from biopsied and non-biopsied mice were processed, and RNA was extracted, purified, and reversed transcribed using random primers. Real-time qPCR was conducted on the following gene-specific primers: (A) S100A8, (B) CXCL2 (MIP-2α), (C) CCL3(MIP-1α), (D) COX2, (E) IL-1β, and (F) TNF-α. Gene expression at 0, 3, 12, and 24 hours post biopsy was analyzed using ΔΔCt analysis with relative normalization with murine GAPDH and β-actin (n = 5; significance is determined relative to levels at 0 hour post biopsy by unpaired t test, *P ≤ .05; error bars represent SEM).
EMT-Related Changes in Gene Expression Occur within Hours of a CNB
Activation of the processes of EMT promotes tumor-cell metastasis through development of dysfunctional cell-cell adhesive interactions, loss of cell-cell junctions, and restructuring of the cytoskeleton. These changes, along with the loss of apical polarity, signal the development of an invasion-permissive, and hence pro-metastatic, microenvironment [54]. To determine if EMT contributes to the changes that occur post biopsy, we assessed the change in gene expression levels of known EMT regulators. Our analysis showed that tumor samples from the biopsied group had significantly increased expression of TGF-β and SOX-4 compared to that of tumors from the non-biopsied control group, as early as 3 hours post biopsy (Figure 4A). This was followed by a significant increase in expression of Ezh2 (a member of the transcription repressive Polycomb group family) by 24 hours post biopsy (Figure 4A). Expression of downstream EMT genes SNAI2 (Slug), ZEB2, and CDH2 (N-cadherin) (Figure 4B) was also statistically significant at 24 hours post biopsy. The timing of TGF-β/SOX-4/Ezh2 expression changes is consistent with involvement of a TGF-β–initiated, Sox4/Ezh2, EMT pathway. These results suggest that the CNB enhances the expression of genes involved in the development of EMT within the tumor.
Figure 4.
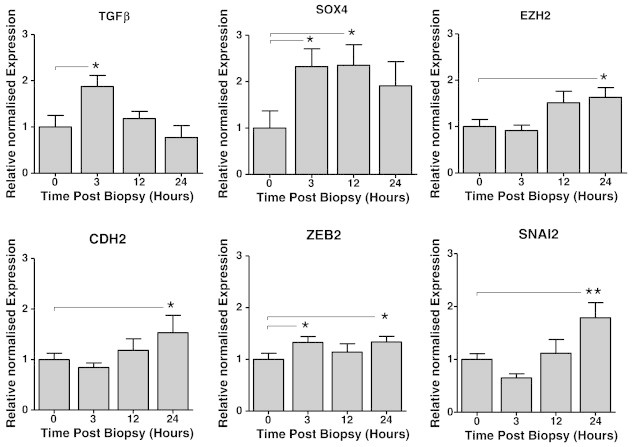
Increase in SOX-4/TGF-β and EMT marker expression post biopsy. qPCR was conducted on tumor-derived cDNA samples from biopsied or non-biopsied mice, and the relative gene expression of TGF-β, SOX-4, EZH2, CDH2, ZEB2, and SNAI2 was normalized to GAPDH and β-actin and analyzed at 0, 3, 12, and 24 hours post biopsy (n = 5; significance is determined relative to levels at 0 hour post biopsy by unpaired t test, *P ≤ .05; error bars represent SEM).
CNB Increases the Number of CTCs
Recent studies have highlighted the prognostic relevance of CTCs for metastatic breast cancer [55], [56]. Reports of generating increased CTCs as a consequence of incisional biopsies and surgical manipulation raise concern about increased metastasis as a consequence of biopsy-generated CTCs [43]. We therefore sought to determine whether CNB affects the level of CTCs in our experimental model. To accomplish this, we first modified the 4T1 cells to express a neomycin resistance gene (4T1neo cells) that could be detected sensitively by qPCR. The 4T1neo cells were implanted and processed as in previous experiments (Figure 2A). At 6 hours, 24 hours, and 10 days post biopsy, blood was collected and the relative expression of the neomycin resistance gene was quantified as a measure of the number of 4T1neo cells in peripheral circulation. A significant elevation in expression of the neomycin resistance gene was seen in the biopsied group at the 6-hour (P = .008) and 24-hour (P = .034) time points (Figure 5). These data indicate that the performance of a biopsy is associated with significantly higher number of CTCs as early as 6 hours and persisting to beyond 24 hours post biopsy.
Figure 5.
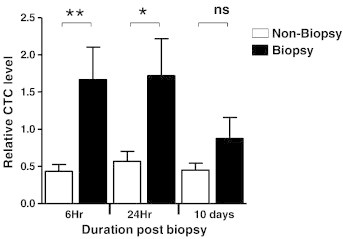
CNB promotes increased frequency of CTCs in peripheral blood. Blood collected from biopsied and non-biopsied mice at 6 hours, 24 hours, and 10 days post biopsy was analyzed using real-time qPCR for the relative expression of neomycin (neo) resistance gene as a measure of the levels of 4T1neo cells in the peripheral circulation (n = 15; significance determined by unpaired t test, *P ≤ .05, **P ≤ .01; error bars represent SEM).
Discussion
CNB, a type of incisional biopsy procedure, is commonly performed in the diagnostic workup of breast cancer. We tested the hypothesis that surgically initiated changes in the tumor microenvironment resulting from a CNB and the ensuing host’s interaction with the residual cancer influence the metastatic potential of that tumor resulting in increased metastatic spread. Using a 4T1:BALB/c murine metastatic breast cancer model, we evaluated the impact of performing a CNB on the following: 1) the development of distant metastases that occur over and above those expected in this metastatic model, 2) the status of immune mediators (cells and cytokines) within the tumor microenvironment, 3) the expression of molecules involved in EMT, and 4) the number of CTCs.
Our findings demonstrate that CNB performed on malignant breast cancers significantly increased the incidence of distant metastases above that which would be expected without biopsy. The observed increase in metastases is associated with early immunosuppressive changes within the tumor microenvironment that facilitate metastatic progression. These changes include recruitment of MDSCs and a concomitant down-regulation of CD4 + T cells, CD8 + T cells, and NK cells. Additional findings that support the hypothesis of a CNB-facilitated metastatic process include the up-regulation early post biopsy of genes responsible for EMT including involvement of a TGF-β–initiated, SOX-4/Ezh2, EMT pathway.
Significant decreases in splenic CD4 + T cells and CD8 + T cells, with associated increase in macrophages, have previously been recorded in tumor-bearing mice with no biopsy performed demonstrating that these changes would occur independent of any biopsy-associated effects [57], [58].While these changes in spleen cellular population are presumed to be a consequence of dynamic interaction between the tumor microenvironment and the immune system, the specific mechanism or exact implications of the changes remain unknown.
In keeping with the cellular changes demonstrated in the post-biopsy tumor microenvironment, we observed an up-regulation of many key inflammatory cytokines and mediators including S100A8, CXCL1, CXCL2, IL-1β, TNF-α, and COX2, which are responsible for the recruitment of multiple inflammatory and immune cell populations including neutrophils, macrophages, MDSCs, and Tregs. Increased S100A8 expression is reportedly associated with chemotactic recruitment of MDSCs as well as the promotion of increased vascular permeability [49], [59]. Increased expression of CCL3 and CXCL2 also indicate enhanced chemoattraction of granulocytes and MDSCs. COX2 stimulates production of prostaglandin E2 (PGE2) and has been reported to increase recruitment of EP2-expressing FoxP3 + Tregs to the tumor through a PGE2/EP2 interaction–dependent pathway [53]. Collectively, this post-biopsy cytokine profile favors a pro-metastatic tumor microenvironment.
We established that TGF-β expression was significantly increased post biopsy as was the SOX-4 gene, a master EMT regulator and a member of the C subgroup of SRY-related HMG box (SOX) transcription factor family [60]. Further investigation showed accompanying increases in expression of the epigenetic modifier Ezh2 as well as increases in the expression of other attendant downstream EMT genes (ZEB2, SNAI2, and CDH2). These findings suggest involvement of the TGF-β/SOX-4 EMT pathway, which would contribute to the increased metastasis we observed through increased migration and extravasation. Previous studies have shown that SOX-4 expression is associated with poor metastasis-related outcomes among early-stage lymph node–negative patients [42], [61], while a separate study correlates SOX-4 expression with triple-negative breast cancer patients and consequently poor prognosis [60]. In conjunction with these studies, our findings suggest a mechanistic role for EMT and related molecules in CNB-induced breast cancer metastasis.
The observed cytokine and EMT-associated gene expression changes also correlated with significantly elevated CTCs from biopsied tumor-bearing mice that persisted up to 24 hours post biopsy. Importantly, other studies have demonstrated that CTC persistence post-surgery extending to, and beyond, 24 hours is considered a robust predictor for metastatic recurrence [62]. The relevance of the CTC/EMT relationship and its role in determining metastatic outcomes in breast cancer is explored in a recent study by Yu et al., which highlights the dynamic relationship between EMT and CTC level and shows the association between cancer subtype, treatment response, and stage of disease. They reveal that EMT, and consequently mesenchymal CTCs, occur more prevalently in aggressive disease subtypes typically characterized as being highly pro-metastatic [44], [63]. The persistent increase in the total CTC numbers we observed in biopsied mice likely has a cumulative impact that favors the possibility of successful metastatic colonization (Figure 6).
Figure 6.
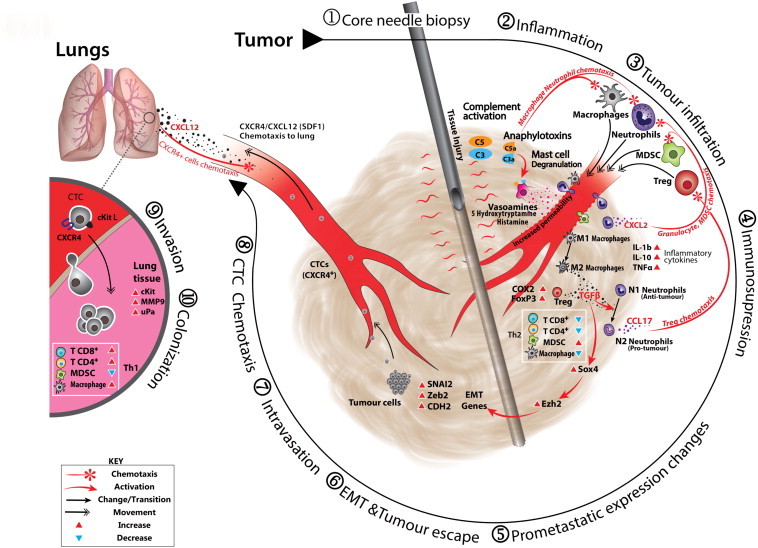
Hypothetical schematic for biopsy-associated metastasis. Clockwise: (1) CNB, with attendant tissue injury, is rapidly followed by (2) inflammation, acute inflammatory reaction with complement-mediated anaphylatoxin release, macrophage and neutrophil chemoattraction accompanied by associated mast cell degranulation, vasoamine release, and corresponding increase in vascular permeability [64], [65]. (3) Tumor infiltration, this adds to the population of leucocytes already present in the tumor and initially involves anaphylatoxin and leukotriene chemotaxis drawing neutrophils, other granulocytes (including MDSCs), more mast cells and monocytes, and macrophages; T lymphocytes would typically arrive later, but these are already well represented in the tumor’s Cell Mediated Immunity (CMI) environment [66], [67]. (4) Immunosuppression, events occur that drive the tumor milieu toward an immunosuppressive phenotype. They include conversion of tumor-associated macrophages from an M1 to an M2 phenotype that increases TGF-β expression [68], [69]. This COX2 expression in the tumor stimulates conversion of nascent naïve CD4 + T cells to FoxP3 + Tregs as well as their recruitment through interaction between COX2-stimulated PGE2 and Treg-expressed EP2. Elevated MDSC and Treg populations drive the immunosuppression process. The increase in TGF-β expression allows conversion of tumor-associated neutrophils from N1 to N2; N2 tumor-associated neutrophils express CCL17, a Treg chemotactic accelerating Treg infiltration [53], [70]. (5) Pro-metastatic expression changes. Increase in TGF-β stimulates up-regulation of master EMT regulator, SOX-4. SOX-4 directly regulates expression of the Ezh2 gene that encodes the Polycomb group histone methyltransferase and epigenetically modifies expression of several EMT genes [42], [60]. (6) EMT and tumor escape, EMT allows the conversion of tumor cells toward an invasive mesenchymal phenotype allowing (7) intravasation into the circulatory system and (8) CTC chemotactic migration to the lung through the CXCR4/SDF1 axis [71], [72], [73], (9) invasion (extravasation), once in the lung the cells invade the lung tissue at the metastatic niche expressing Extra-cellular Matrix (ECM) degrading matrix metalloproteinase 9 and urokinase plasminogen activator completing the process of [74], [75], [76] (10) metastasis by successful colonization and formation of metastases.
Conclusion
Our experimental findings demonstrate for the first time that in the setting of malignant breast cancer, performance of a CNB is associated with a significantly increased incidence of pulmonary metastases. We also show that an additional impact of CNB includes creation of a distinctly immunosuppressive and pro-metastatic tumor microenvironment with elevated TGF-β/SOX-4–associated EMT and significantly higher CTC levels.
In this era of digital mammography when smaller breast cancers are being detected, presumably in a pre-metastatic state, biologic knowledge of the potential harms associated with the traditional workup of breast cancer through the application of a CNB needs to be considered. Further research to better understand the biologic pathways associated with surgically induced metastases needs to be conducted.
The following are the supplementary data related to this article.
Gene-Specific qPCR Primer List: Sequence Information for Primers Used for Real-Time qPCR Gene Expression Analysis
(A) Flow cytometry analysis was used to quantify frequencies of macrophages (CD45 +, CD11blow, F4/80 +), MDSCs (CD45 +, CD11b +, Gr-1 +), CD4 + T cells, CD8 + T cells, and NK cells (Pan-NK, CD3 +). (B) Immune cellular population in the axillary lymph node showed no significant difference between biopsied and non-biopsied mice frequencies for CD4 + T cells, CD8 + T cells, MDSCs, macrophages, or NK cells over the 10-day period (n = 10, t test, *P ≤ .05; error bars represent SEM).
Acknowledgements
The authors thank Patricia Colp of the HRS Laboratory in the Department of Pathology for her invaluable assistance in the histologic processing and analysis of tissues evaluated. Author contributions: E.G.M. participated in the design of the study, performed animal experiments, surgical procedures, sample and data collection, sample archiving and processing, histologic and molecular assays, interpretation and analysis of data, statistical analysis and reporting, manuscript writing, coordination of review and editing, and preparation for submission. C.A.D. participated in the design of the study and performed animal experiments, surgical procedures, sample and data collection, histologic assays, the statistical analysis interpretation and reporting, and manuscript review and editing. D.C. performed sample collection and processing, performed FACS data collection and supervision of analysis, data interpretation and reporting, and manuscript drafting, review, and editing. A.V.-K. performed sample processing, qPCR assay and analysis of resulting data, and manuscript review and editing. S.P. performed animal surgical procedures, generation of modified 4T1neo cells, sample and data collection and collation, and manuscript review and editing. K.M.C. performed sample and data collection and manuscript review and editing. M.G. performed sample and data collection and sample processing and carried out qPCR assay and analysis of resulting data. B.M. performed animal surgical procedures, sample and data collection, and data interpretation. A.N. performed sample collection and processing and data collection and manuscript review. J.J. participated in the design of animal experiments, development of data collection and analysis planning, and manuscript review and editing. J.D.L. participated in development of the study, preliminary data acquisition and model determination, and manuscript writing, review, and editing. S.A.G. participated in the design of the study and performed FACS data collection and supervision of analysis and interpretation, and manuscript writing, review, and editing. P.M. participated in the design of the study, generation of modified 4T1neo cells, and manuscript writing, review, and editing. P.W.K.L. participated in the design of the study and manuscript review and editing. C.A.G. participated in the design and development of the study, acquisition of funding and coordination of the research group, supervisory oversight, and manuscript writing, review, and editing. All authors read and approved the final manuscript.
Footnotes
This article refers to supplementary materials, which are designated by Table S1 and Figure S1 and are available online at www.neoplasia.com.
The authors thank the Canadian Breast Cancer Foundation, the Beatrice Hunter Cancer Research Institute, the Dalhousie University Senior Clinical Scholar Award, and Dalhousie Medical Research Foundation Adopt-a-Researcher Program for their financial support that made this study possible.
Contributor Information
Edward Gitau Mathenge, Email: edward.mathenge@gmail.com.
Cheryl Ann Dean, Email: deanc@dal.ca.
Derek Clements, Email: derek.clements@dal.ca.
Ahmad Vaghar-Kashani, Email: ahmadvk@gmail.com.
Steffany Photopoulos, Email: steffany.photopoulos@dal.ca.
Krysta Mila Coyle, Email: krysta.coyle@dal.ca.
Michael Giacomantonio, Email: mgiacomantonio1@gmail.com.
Benjamin Malueth, Email: benjamin.a.malueth@dal.ca.
Anna Nunokawa, Email: an539251@dal.ca.
Julie Jordan, Email: julie.jordan@dal.ca.
John D. Lewis, Email: jdlewis@ualberta.ca.
Shashi Ashok Gujar, Email: shashi.gujar@dal.ca.
Paola Marcato, Email: paola.marcato@dal.ca.
Patrick W.K. Lee, Email: patrick.lee@dal.ca.
Carman Anthony Giacomantonio, Email: carman.giacomantonio@cdha.nshealth.ca.
References
- 1.Koka R, Ioffe OB. Breast carcinoma: is molecular evaluation a necessary part of current pathological analysis? Semin Diagn Pathol. 2013;30:321–328. doi: 10.1053/j.semdp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 3.Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, Easton D, Forbes JF, Key T, Hankinson SE. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- 4.Jacobi CE, de Bock GH, Siegerink B, van Asperen CJ. Differences and similarities in breast cancer risk assessment models in clinical practice: which model to choose? Breast Cancer Res Treat. 2009;115:381–390. doi: 10.1007/s10549-008-0070-x. [DOI] [PubMed] [Google Scholar]
- 5.Wolf I, Ben-Baruch N, Shapira-Frommer R, Rizel S, Goldberg H, Yaal-Hahoshen N, Klein B, Geffen DB, Kaufman B. Association between standard clinical and pathologic characteristics and the 21-gene recurrence score in breast cancer patients: a population-based study. Cancer. 2008;112:731–736. doi: 10.1002/cncr.23225. [DOI] [PubMed] [Google Scholar]
- 6.Gipponi M, Bassetti C, Canavese G, Catturich A, Di Somma C, Vecchio C, Nicolò G, Schenone F, Tomei D, Cafiero F. Sentinel lymph node as a new marker for therapeutic planning in breast cancer patients. J Surg Oncol. 2004;85:102–111. doi: 10.1002/jso.20022. [DOI] [PubMed] [Google Scholar]
- 7.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Breast Cancer Statistics & Survival Rates: Breast Cancer Cases/Deaths per Year (U.S. and World) http://www.imaginis.com/breast-health/breast-cancer-statistics-on-incidence-survival-and-screening-2
- 9.Doberneck RC. Breast biopsy: a study of cost-effectiveness. Ann Surg. 1980;192:152–156. doi: 10.1097/00000658-198008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duijm LE, Groenewoud JH, Roumen RM, de Koning HJ, Plaisier ML, Fracheboud J. A decade of breast cancer screening in The Netherlands: trends in the preoperative diagnosis of breast cancer. Breast Cancer Res Treat. 2007;106:113–119. doi: 10.1007/s10549-006-9468-5. [DOI] [PubMed] [Google Scholar]
- 11.Holloway CM, Gagliardi AR. Percutaneous needle biopsy for breast diagnosis: how do surgeons decide? Ann Surg Oncol. 2009;16:1629–1636. doi: 10.1245/s10434-009-0451-3. [DOI] [PubMed] [Google Scholar]
- 12.Holloway CM, Saskin R, Brackstone M, Paszat L. Variation in the use of percutaneous biopsy for diagnosis of breast abnormalities in Ontario. Ann Surg Oncol. 2007;14:2932–2939. doi: 10.1245/s10434-007-9362-3. [DOI] [PubMed] [Google Scholar]
- 13.Holloway CM, Saskin R, Paszat L. Geographic variation and physician specialization in the use of percutaneous biopsy for breast cancer diagnosis. Can J Surg. 2008;51:453–463. [PMC free article] [PubMed] [Google Scholar]
- 14.Breast Cancer Statistics at a Glance. http://www.cancer.ca/en/cancer-information/cancer-101/cancer-statistics-at-a-glance/?region=ns
- 15.Londero V, Zuiani C, Furlan A, Nori J, Bazzocchi M. Role of ultrasound and sonographically guided core biopsy in the diagnostic evaluation of ductal carcinoma in situ (DCIS) of the breast. Radiol Med. 2007;112:863–876. doi: 10.1007/s11547-007-0183-z. [DOI] [PubMed] [Google Scholar]
- 16.Hemmer JM, Kelder JC, van Heesewijk HP. Stereotactic large-core needle breast biopsy: analysis of pain and discomfort related to the biopsy procedure. Eur Radiol. 2008;18:351–354. doi: 10.1007/s00330-007-0762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataraman S, Dialani V, Gilmore HL, Mehta TS. Stereotactic core biopsy: comparison of 11 gauge with 8 gauge vacuum assisted breast biopsy. Eur J Radiol. 2012;81:2613–2619. doi: 10.1016/j.ejrad.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Philpotts LE. Controversies in core-needle breast biopsy. Semin Roentgenol. 2001;36:270–283. doi: 10.1053/sroe.2001.25121. [DOI] [PubMed] [Google Scholar]
- 19.Ames V, Britton PD. Stereotactically guided breast biopsy: a review. Insights Imaging. 2011;2:171–176. doi: 10.1007/s13244-010-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ioffe OB, Berg WA, Silverberg SG, Kumar D. Mammographic-histopathologic correlation of large-core needle biopsies of the breast. Mod Pathol. 1998;11:721–727. [PubMed] [Google Scholar]
- 21.Boerner S, Sneige N. Specimen adequacy and false-negative diagnosis rate in fine-needle aspirates of palpable breast masses. Cancer. 1998;84:344–348. [pii] [PubMed] [Google Scholar]
- 22.Ellis MJ, Dixon M, Dowsett M, Nagarajan R, Mardis E. A luminal breast cancer genome atlas: progress and barriers. J Steroid Biochem Mol Biol. 2007;106:125–129. doi: 10.1016/j.jsbmb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Gisvold JJ, Goellner JR, Grant CS, Donohue JH, Sykes MW, Karsell PR, Coffey SL, Jung SH. Breast biopsy: a comparative study of stereotaxically guided core and excisional techniques. AJR Am J Roentgenol. 1994;162:815–820. doi: 10.2214/ajr.162.4.8140997. [DOI] [PubMed] [Google Scholar]
- 24.Oyama T, Koibuchi Y, McKee G. Core needle biopsy (CNB) as a diagnostic method for breast lesions: comparison with fine needle aspiration cytology (FNA) Breast Cancer. 2004;11:339–342. doi: 10.1007/BF02968040. [DOI] [PubMed] [Google Scholar]
- 25.Retsky M, Demicheli R, Hrushesky WJ, Forget P, De Kock M, Gukas I, Rogers RA, Baum M, Sukhatme V, Vaidya JS. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem. 2013;20:4163–4176. doi: 10.2174/09298673113209990250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Pract Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- 27.Hansen NM, Ye X, Grube BJ, Giuliano AE. Manipulation of the primary breast tumor and the incidence of sentinel node metastases from invasive breast cancer. Arch Surg. 2004;139:634–639. doi: 10.1001/archsurg.139.6.634. [discussion 639–640] [DOI] [PubMed] [Google Scholar]
- 28.Chao C, Torosian MH, Boraas MC, Sigurdson ER, Hoffman JP, Eisenberg BL, Fowble B. Local recurrence of breast cancer in the stereotactic core needle biopsy site: case reports and review of the literature. Breast J. 2001;7:124–127. doi: 10.1046/j.1524-4741.2001.007002124.x. [DOI] [PubMed] [Google Scholar]
- 29.Hofer SO, Shrayer D, Reichner JS, Hoekstra HJ, Wanebo HJ. Wound-induced tumor progression: a probable role in recurrence after tumor resection. Arch Surg. 1998;133:383–389. doi: 10.1001/archsurg.133.4.383. [DOI] [PubMed] [Google Scholar]
- 30.Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–768. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 31.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–1828. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 32.Al-Sahaf O, Wang JH, Browne TJ, Cotter TG, Redmond HP. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann Surg. 2010;252:1037–1043. doi: 10.1097/SLA.0b013e3181efc635. [pii] [DOI] [PubMed] [Google Scholar]
- 33.Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17(Suppl. 1):S27–S36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 34.Diaz LK, Wiley EL, Venta LA. Are malignant cells displaced by large-gauge needle core biopsy of the breast? AJR Am J Roentgenol. 1999;173:1303–1313. doi: 10.2214/ajr.173.5.10541110. [DOI] [PubMed] [Google Scholar]
- 35.Perez RO, Habr-Gama A, Proscurshim I, Campos FG, Kiss D, Gama-Rodrigues J, Cecconello I. Local excision for ypT2 rectal cancer—much ado about something. J Gastrointest Surg. 2007;11:1431–1438. doi: 10.1007/s11605-007-0271-3. [discussion 1438–1440] [DOI] [PubMed] [Google Scholar]
- 36.Chagpar AB, Scoggins CR, Sahoo S, Martin RC, II, Carlson DJ, Laidley AL, El-Eid SE, McGlothin TQ, Noyes RD, Ley PB. Biopsy type does not influence sentinel lymph node status. Am J Surg. 2005;190:551–556. doi: 10.1016/j.amjsurg.2005.06.009. [S0002-9610(05)00543-X [pii]] [DOI] [PubMed] [Google Scholar]
- 37.Knight R, Horiuchi K, Parker SH, Ratzer ER, Fenoglio ME. Risk of needle-track seeding after diagnostic image-guided core needle biopsy in breast cancer. JSLS. 2002;6:207–209. [PMC free article] [PubMed] [Google Scholar]
- 38.Liebens F, Carly B, Cusumano P, Van Beveren M, Beier B, Fastrez M, Rozenberg S. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas. 2009;62:113–123. doi: 10.1016/j.maturitas.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Diaz NM, Vrcel V, Centeno BA, Muro-Cacho C. Modes of benign mechanical transport of breast epithelial cells to axillary lymph nodes. Adv Anat Pathol. 2005;12:7–9. doi: 10.1097/01.pap.0000151267.34438.a1. [DOI] [PubMed] [Google Scholar]
- 40.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [0008-5472.CAN-08-4619 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;(30 Suppl.):S32–S40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiwari N, Tiwari VK, Waldmeier L, Balwierz PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schubeler D, van Nimwegen E, Christofori G. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–783. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Juratli MA, Sarimollaoglu M, Siegel ER, Nedosekin DA, Galanzha EI, Suen JY, Zharov VP. Real-time monitoring of circulating tumor cell release during tumor manipulation using in vivo photoacoustic and fluorescent flow cytometry. Head Neck. 2013 doi: 10.1002/hed.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Flaherty JD, Gray S, Richard D, Fennell D, O'Leary JJ, Blackhall FH, O'Byrne KJ. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer. 2012;76:19–25. doi: 10.1016/j.lungcan.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Predina J, Eruslanov E, Judy B, Kapoor V, Cheng G, Wang LC, Sun J, Moon EK, Fridlender ZG, Albelda S. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc Natl Acad Sci U S A. 2013;110:E415–E424. doi: 10.1073/pnas.1211850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE., III Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat. 2013;140:13–21. doi: 10.1007/s10549-013-2618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 51.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Baruch A. The tumor-promoting flow of cells into, within and out of the tumor site: regulation by the inflammatory axis of TNFα and chemokines. Cancer Microenviron. 2012;5:151–164. doi: 10.1007/s12307-011-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karavitis J, Hix LM, Shi YH, Schultz RF, Khazaie K, Zhang M. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012;7:e46342. doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashida T, Jinno H, Kitagawa Y, Kitajima M. Cooperation of cancer stem cell properties and epithelial-mesenchymal transition in the establishment of breast cancer metastasis. J Oncol. 2011;2011:591427. doi: 10.1155/2011/591427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasportas LS, Gambhir SS. Imaging circulating tumor cells in freely moving awake small animals using a miniaturized intravital microscope. PLoS One. 2014;9:e86759. doi: 10.1371/journal.pone.0086759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi N, Nakamura S, Tokuda Y, Shimoda Y, Yagata H, Yoshida A, Ota H, Hortobagyi GN, Cristofanilli M, Ueno NT. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol. 2012;17:96–104. doi: 10.1007/s10147-011-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DuPré SA, Redelman D, Hunter KW., Jr. The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88:351–360. doi: 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11:816–826. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao X, Feng J, Zhang Y, Gao H, Liu DX. SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression. Cancer Res. 2012;72:4597–4608. doi: 10.1158/0008-5472.CAN-12-1045. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt M, Böhm D, von Torne C, Steiner E, Puhl A, Pilch H, Lehr HA, Hengstler JG, Kolbl H, Gehrmann M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 62.Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102:1327–1334. doi: 10.1038/sj.bjc.6605651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burgess DJ. Breast cancer: circulating and dynamic EMT. Nat Rev Cancer. 2013;13:148. doi: 10.1038/nrc3475. [DOI] [PubMed] [Google Scholar]
- 64.Playfair JHL, Chain BM. 9th edn. Wiley-Blackwell; Chichester, United Kingdom; Hoboken, NJ: 2009. Immunology at a Glance. [Google Scholar]
- 65.Todd I, Spickett G. Immunology. 6th edn. Wiley-Blackwell; Chichester, West Sussex; Hoboken, NJ: 2010. Lecture notes. [Google Scholar]
- 66.Janeway's Immunobiology. Choice: Current Reviews for Academic Libraries. 2008;45:1793–1794. [Google Scholar]
- 67.Sompayrac L. 3rd edn. Blackwell Publishing; Malden, MA: 2008. How the Immune System Works. [Google Scholar]
- 68.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, De Baetselier P, Van Ginderachter JA. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 69.Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol. 2010;32:153–158. [PubMed] [Google Scholar]
- 70.Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—a new mechanism of impaired antitumor immunity. Int J Cancer. 2014 doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 71.Hirbe AC, Morgan EA, Weilbaecher KN. The CXCR4/SDF-1 chemokine axis: a potential therapeutic target for bone metastases? Curr Pharm Des. 2010;16:1284–1290. doi: 10.2174/138161210791034012. [DOI:BSP/CPD/E-Pub/00048 [pii]] [DOI] [PubMed] [Google Scholar]
- 72.Huang M, Li Y, Zhang H, Nan F. Breast cancer stromal fibroblasts promote the generation of CD44+CD24 − cells through SDF-1/CXCR4 interaction. J Exp Clin Cancer Res. 2010;29:80. doi: 10.1186/1756-9966-29-80. [1756-9966-29-80 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009;16:664–673. doi: 10.1038/cdd.2008.190. [cdd2008190 [pii]] [DOI] [PubMed] [Google Scholar]
- 74.Haas S, Park TW, Hahne JC, Fischer HP. Influence of preoperative core biopsies on uPA/PAI-1 expression in breast cancer tissue. Virchows Arch. 2008;452:277–283. doi: 10.1007/s00428-007-0563-8. [DOI] [PubMed] [Google Scholar]
- 75.Kim SJ, Shiba E, Taguchi T, Watanabe T, Tanji Y, Kimoto Y, Izukura M, Takai SI. Urokinase type plasminogen activator receptor is a novel prognostic factor in breast cancer. Anticancer Res. 1997;17:1373–1378. [PubMed] [Google Scholar]
- 76.Conese M, Blasi F. The urokinase/urokinase-receptor system and cancer invasion. Baillieres Clin Haematol. 1995;8:365–389. doi: 10.1016/s0950-3536(05)80273-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene-Specific qPCR Primer List: Sequence Information for Primers Used for Real-Time qPCR Gene Expression Analysis
(A) Flow cytometry analysis was used to quantify frequencies of macrophages (CD45 +, CD11blow, F4/80 +), MDSCs (CD45 +, CD11b +, Gr-1 +), CD4 + T cells, CD8 + T cells, and NK cells (Pan-NK, CD3 +). (B) Immune cellular population in the axillary lymph node showed no significant difference between biopsied and non-biopsied mice frequencies for CD4 + T cells, CD8 + T cells, MDSCs, macrophages, or NK cells over the 10-day period (n = 10, t test, *P ≤ .05; error bars represent SEM).


