Abstract
Patients with bone cancer metastasis suffer from unbearable pain and bone fractures due to bone remodeling. This is caused by tumor cells that disturb the bone microenvironment. Here, we have investigated the role of tumor-secreted sugar-binding protein, i.e., galectin-3, on osteoblast differentiation and report that it downregulates the expression of osteoblast differentiation markers, e.g., RUNX2, SP7, ALPL, COL1A1, IBSP, and BGLAP, of treated human fetal osteoblast (hFOB) cells. Co-culturing of hFOB cells with human breast cancer BT-549 and prostate cancer LNCaP cells harboring galectin-3 has resulted in inhibition of osteoblast differentiation by the secreted galectin-3 into culture medium. The inhibitory effect of galectin-3 was found to be through its binding to Notch1 in a sugar-dependent manner that has led to accelerated Notch1 cleavage and activation of Notch signaling. Taken together, our findings show that soluble galectin-3 in the bone microenvironment niche regulates bone remodeling through Notch signaling, suggesting a novel bone metastasis therapeutic target.
Abbreviations: hFOB, human fetal osteoblast; CRD, carbohydrate recognition-binding domain; ALP, alkaline phosphatase; pNPP, p-nitrophenol phosphate substrate; NICD, Notch intracellular domain; β-GP, β-glycerophosphate; AA, ascorbic acid; 1,25-(OH)2D3, 1α-25-dihydroxycholecalciferol
Introduction
Patients with malignant prostate and breast cancer are often afflicted by bone metastasis [1], [2]. The establishment of secondary tumor growth in the bone affects the bone microenvironment leading to bone remodeling, i.e., the balance between osteoclast-driven bone resorption and osteoblast-driven bone deposition [3], [4], [5], their differentiation statuses are essential for their functions [6], [7]. It was reported that bone metastatic cancer cells may affect the differentiation status of both osteoclasts and osteoblasts through the secretion of cytokines and growth hormones like PTH-rP, transforming growth factor–β, fibroblast growth factor (FGF), Wnt, and urokinase-type plasminogen activator (uPA) to name but few [3], [4], [5]. Thus, crosstalk between tumor cells and the bone microenvironment disrupts normal bone homeostasis, which leads to tumor growth and inappropriate bone remodeling [3], consequently affecting the quality of life in patients due to pain and fractures in metastatic lesions [8]. Therefore, understanding the regulation of bone remodeling is, without a doubt, a clinical priority and challenge to combat the cancer growth in the bone, since the arsenal of therapeutic tools available for treatment is thus far limited.
Galectin-3 is a β-galactoside–binding protein comprising 250 amino acid residues and binds to the carbohydrate portion of cell surface glycoproteins. It is a chimeric gene product composed of three distinct structural domains: a short NH2-terminal domain containing a phosphorylation site, a repeated collagen α-like sequence, and a C-terminal domain containing a single carbohydrate recognition-binding domain (CRD) composed of 140 amino acids [9]. Galectin-3 was shown to regulate proliferation and apoptosis and mediates cell-cell and cell–extracellular matrix adhesion for cancer progression and metastasis [9], [10]. Previous reports indicated that galectin-3 mediates bone metastasis through β-galactoside interactions on the tumor cell surface [11], [12]. In addition, we recently showed that patients with metastases arising from prostate cancer have a higher serum galectin-3 concentration [13]. Similarly, circulating galectin-3 levels in the serum of patients with metastatic breast cancer were higher compared to non-metastatic patients [14]. Nevertheless, the functional role of galectin-3 in the bone microenvironment of growing tumors has not yet been explored. On the basis of the fact that the circulating galectin-3 induces angiogenesis affecting tumor cell growth in the microenvironment [15], [16], [17], we examined its role in the bone.
Tumor-derived jagged-1, a membrane-associated protein inducing Notch signaling expression, was reported to be associated with galectin-3 [18] and causes bone remodeling through cell-to-cell interaction between cancer cells and osteoclasts or osteoblasts [19]. A recent study reported that jagged-1 overexpressed in bone metastatic lesions of patients with breast cancer, implicating the potential role of Notch signaling in bone metastasis [20]. A preclinical study indicates that activation of Notch signaling in both osteoclasts and osteoblasts promotes osteolytic bone metastasis [21]. In addition, it was previously reported that Deleted in Malignant Brain Tumors 1 protein interacts with galectin-3 and modulates the Notch signaling pathway [22]. These molecular mechanisms that underlie these painful and often incurable consequences of tumor metastasis to the bone have been suggested to be promising new molecular targets for therapy [3]. Current treatment for bone metastasis has two basic modalities: 1) treatment directed against the tumor with radiation and/or hormone therapy and/or chemotherapy and 2) osteoclast-suppressive drugs at the bone microenvironment such as using molecular targeted therapy [23], [24]. While these treatments fail to cure and have undesirable side effects, we have initiated a study to explore a novel molecular target(s) underling bone remodeling and focused on osteoblasts, the main cellular component in the bone microenvironment, and hypothesize that galectin-3 antagonists may be added to the therapeutic arsenal.
Materials and Methods
Cell Culture and Reagents
An immature pre-osteoblast cell line derived from human fetal bone, human fetal osteoblast (hFOB), was purchased from American Type Culture Collection (Manassas, VA) and cultured at 34°C in standard medium composed of 1:1 mixture of Ham’s F12 medium and Dulbecco's modified Eagle's medium supplemented with 10% FBS following the manufacturer’s protocol. The third passage cells were used in all experiments. The osteoblastic differentiation of hFOB cells was induced by 50 μg/ml ascorbic acid (AA) with 10 mM β-glycerophosphate (β-GP; Sigma-Aldrich, St Louis, MO) or 100 nM 1α-25-dihydroxycholecalciferol (1,25-(OH)2D3; ENZO, Farmingdale, NY) [25], [26]. The human breast cancer cell line BT-549 was a gift from Dr Eric W. Thompson (St. Vincent’s Institute of Medical Research and University of Melbourne, Melbourne, Australia). The human breast cancer cell line MDA-MB-231 was a gift from Dr Isaiah J. Fidler (University of Texas MD Anderson Cancer Center, Houston, TX). Human prostate cancer cell lines LNCaP and PC-3 were purchased from American Type Culture Collection. Customized polyclonal rabbit anti–Gal-3 antibody (HL31) was created by Invitrogen (Grand Island, NY). Rabbit anti-Notch1 polyclonal antibody was purchased from Abcam (Cambridge, United Kingdom) to detect the cleaved form of Notch1 named as Notch intracellular domain (NICD). Rabbit anti-Notch1 polyclonal antibody (H-131) was purchased from Santa Cruz Biotechnology (Dallas, TX) to detect Notch extracellular domain (NECD) of full-length Notch1. Mouse anti–β-actin monoclonal antibody was purchased from Sigma-Aldrich.
Transfection and Plasmid Constructs
The full-length (1-250) galectin-3 DNA fragments were ligated to p3xFLAG-MYC-CMV-25 expression vector (Sigma-Aldrich) containing a preprotrypsin leader sequence for secretion. It was transfected into BT549 and LNCaP cell lines using Lipofectamine LTX and Plus transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The stable clones were analyzed using reverse transcription–polymerase chain reaction (RT-PCR) using the following primer pair: forward, 5′-ATGTCTGCACTTCTGATCCTAGCTCT-3′; reverse, 5′-CAGATCCTCTTCTGAGATGAGTTTTT-3′ as described in our previous study [15]. The secreted protein was collected from serum-free culture media, and concentrated medium was analyzed by immunoblot. Small interfering oligonucleotides targeting human galectin-3 gene (5′-GATCCCGGGAAGAAAGACAGTCGGTTTCAAGAGAACCGACTGTCTTTCTTCCCTTTTTTGGAAA-3′) and its complement were synthesized and annealed by Thermo Scientific (Pittsburgh, PA). It was subcloned into pSilencer 3.1-H1 neo expression vector (Ambion, Austin, TX) between BamHI and HindIII sites to construct pSilencer 3.1-H1/siGal3-producing siRNA targeting galectin-3 mRNA as described in our previous study [27]. PC3 and MDA-MB-231 cells were transfected with this constructed vector and pSilencer 3.1-H1-negative control vector containing a random sequence insert using Lipofectamine LTX and Plus reagent (Invitrogen) according to the manufacturer’s instructions. To confirm downregulated mRNA expression of galectin-3, the following primers were used: 5′-GCCACTGATTGTGCCTTA-3′ (forward) and 5′-AACCGACTGTCTTTCTTCC-3′ (reverse) for human galectin-3 gene, and 5′-TCAACGGATTTGGTCGTATT-3′ (forward) and 5′-TTGGCAGGTTTTTCTAGACG-3′ (reverse) for human glyceraldehyde-3-phosphate dehydrogenase gene. The secreted protein was collected from serum-free culture media, and concentrated medium was analyzed by immunoblot.
Purification of Recombinant Galectin-3
1-250 (full-length, wild-type) and 1-107 and 108-250 (CRD) human galectin-3 were subcloned into the pET30as (modified pET30a) vector as a BamHI-XhoI fragment as described in our previous study [16] and overexpressed in Escherichia coli at 26°C. The expression construct introduced a His tag to the protein. The soluble protein was purified by nickel-agarose affinity chromatography. The protein was concentrated in a buffer containing 20 mM Tris (pH 7.9) and 150 mM NaCl with or without 10 mM DTT. His tags were not removed from the protein.
Alkaline Phosphatase Assay and Calcium Deposition
The expression of alkaline phosphatase (ALP), an early differentiation marker for osteoblast, was estimated by ALP staining kit (Biopioneer, San Diego, CA) 7 days after confluence according to the manufacturer’s instructions. The activity of ALP was measured by p-nitrophenol phosphate substrate (pNPP; Invitrogen), a substrate for ALP, 7 days after confluence. Culture medium was changed every 3 days with or without recombinant galectin-3. Briefly, after removing the culture medium and rinsing with phosphate-buffered saline, pNPP (1 mg/ml) diluted in buffer containing 0.1 M 2-amino-2-methyl-1-propanol (pH 10.3) was added and cells were incubated for 30 minutes. Then, the absorbance was determined at 410 nm. Calcium deposition was detected by Alizarin red S after culturing for 3 weeks. Particle size, particle number, and total area were quantitated by using ImageJ software.
Quantitative Real-Time PCR
Total RNA was isolated from hFOB cells using RNeasy mini kit (Qiagen, Valencia, CA); 0.5 μg of total RNA was used for RT reaction by using RT2 First Strand Kit (Qiagen). Quantitative real-time PCR was performed using RT2 SYBR Green qPCR master mix (Qiagen). Values were normalized to glyceraldehyde-3-phosphate dehydrogenase values as endogenous control. Data are presented as means + SD.
Immunofluorescence
hFOB cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked in 1% BSA/phosphate-buffered saline, and incubated with anti-Notch1 rabbit antibody as primary antibody to detect cleaved form of Notch1 (NICD) and then incubated with TRITC-conjugated secondary antibody. Next, cells were stained with 4′,6-diamidino-2-phenylindole, washed, and mounted on a glass slide with SlowFade Light Antifade kit (Molecular Probes, Eugene, OR). Immunofluorescence images were obtained using a Zeiss Confocal Laser Microscope LSM 780.
Indirect Co-Culture
hFOB cells were seeded in six-well plates and maintained until confluence. After confluence, cancer cells were seeded at a density of 5 × 105 cells in cell culture inserts (0.4 μm pore size) in a 1:1 mixture of Ham's F12 medium and Dulbecco's modified Eagle's medium containing 10% FBS at 34°C as an indirect co-culture method shown in Figure 4II-A. Culture medium was changed every 3 days. At 6 days after co-culture, total RNA of hFOB was extracted by using RNeasy kit (Qiagen) and subjected to quantitative real-time PCR.
Figure 4.
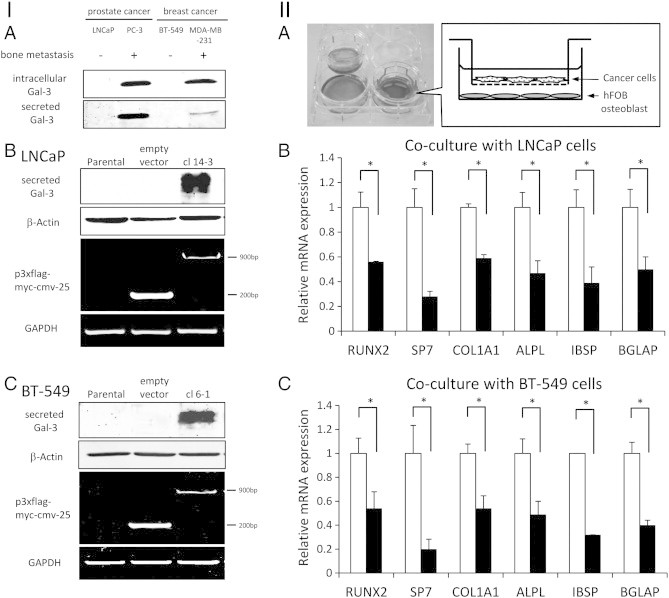
Cancer-secreted galectin-3 inhibits the gene expression for osteoblast differentiation. (I) Established clones for co-culture experiment. (A) Comparison of galectin-3 expression in cell lysate and secreted galectin-3 between bone metastatic cancer and non-bone metastatic cancer cell line. (B) LNCaP transfected p3xflag-myc-cmv-25. LNCaP clone 14-3 had potential to secrete galectin-3 by transfection of p3xflag-myc-cmv-25 inserted full-length galectin-3 sequence (1-250). (C) BT-549 transfected p3xflag-myc-cmv-25. BT-549 clone 6-1 had potential to secrete galectin-3 by transfection of p3xflag-myc-cmv-25 inserted full-length galectin-3 sequence (1-250). (II) Cancer-secreted galectin-3 inhibits osteoblast differentiation in a co-culture experiment. (A) Schematic representation of indirect co-culture using cell culture inserts. Osteoblast cells were cultured in a six-well plate until confluence, and after that, cancer cells were seeded on the inserts. On 6 days after co-culture with cancer cells, RNA samples of hFOB cells were extracted and expression levels were determined by quantitative real-time PCR. Results are presented as fold change compared to empty vector cells. (B) Results of co-culture with LNCaP. mRNA levels of osteoblast differentiation marker were compared between co-culture with empty vector cells and galectin-3 secretable cells, clone 14-3. White bars, co-culture with empty vector cells. Black bars, co-culture with galectin-3 transfected stable clone 14-3. (C) Results of co-culture with BT-549. mRNA levels of osteoblast differentiation marker were compared between co-culture with empty vector cells and galectin-3 secretable cells, clone 6-1. White bars, co-culture with empty vector clone. Black bars, co-culture with galectin-3 transfected stable clone 6-1. Data represent means ± SD. *P < .05 versus empty vector cells.
Pull-Down Assay
Purified His-tagged fusion proteins, galectin-3 (1-250), galectin-3 (1-107), and galectin-3 (108-250) were incubated with nickel–nitrilotriacetic acid (Qiagen) at 4°C for 2 hours with rotation and washed repeatedly. Next, the beads were incubated at 4°C for 2 hours with HEK293 cell lysate prepared by cell lysis buffer composed by 40 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, and protease inhibitor cocktail, complete EDTA-free (Roche Diagnostics, Indianapolis, IN). After incubation, the beads were washed repeatedly and then centrifuged; elution buffer including imidazole and sample buffer were added to the beads. The protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by immunoblot and Coomassie Brilliant Blue stain.
Luciferase Assay
Following the manufacturer’s protocol, HEK293 cells were seeded in duplicate at a density of 2.5 × 104 cells per well in 96-well plates and transfected with RBP-Jk responsive element containing luciferase reporter construct to monitor Notch signaling activation (Qiagen) by using Lipofectamine LTX and Plus reagent (Invitrogen). Twenty-four hours after transfection, cells were stimulated with galectin-3 (1-250) for another 12 hours. Then, luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Values were normalized with Renilla luciferase activity under control of a cytomegalovirus promotor.
Enzyme-Linked Immunosorbent Assay
Conditioned medium from cultured PC-3 cells was used to analyze the presence of galectin-3 in two replicates by ELISA. The concentration of galectin-3 secretion was determined using Galectin-3 ELISA kit (BG Medicine, Waltham, MA). For each sample, the mean of the two replicates was the level of galectin-3.
Statistical Analysis
Statistical differences were determined by analysis of variance or t test. P < .05 was considered as statistically significant.
Results
Galectin-3 Inhibits ALP Expression and Calcium Deposition in Osteoblast
To test the possible effect of cell-free galectin-3 on the differentiation of osteoblasts, we employed hFOB, an immature pre-osteoblast cell line derived from human fetal bone, which has the potential for spontaneous differentiation after reaching confluence [25]. In accordance with previous observations, we have verified the expression of ALP, which is an early differentiation marker for osteoblasts [28], [29] (Figure 1A), and noted that recombinant galectin-3 inhibited ALP expression under the inducing conditions for spontaneous differentiation (Figure 1B). To establish which domain of galectin-3 is involved in this process, lactose (75 mM), a sugar inhibitor for galectin-3 [30], [31], was added to the culture medium, leading to suppression of the effect of galectin-3 (Figure 1B), indicating that galectin-3 inhibits osteoblast differentiation through the CRD. Furthermore, the enzymatic activity of ALP was investigated by pNPP assay, which was also inhibited by galectin-3 in a dose-dependent manner (Figure 1C). Further, we tested the effect of galectin-3 on chemically induced differentiation using β-GP with either AA or 1,25-(OH)2D3 [25], [26]. We noted that although the cells were cultured with these potent osteoblast differentiation inducers, ALP enzyme activity was independently impaired by galectin-3 (Figure 1C). These results indicated that galectin-3 is a suppressor of osteoblast differentiation. Next, we examined whether the inhibitory effect of galectin-3 on osteoblast differentiation results from a transcriptional regulation of ALP or post-transcriptional regulation, such as inhibition of ALP enzyme activity. Quantitative real-time PCR analysis revealed that the mRNA level of ALP decreased in response to galectin-3 (Figure 1D), indicating the regulation of ALP expression at the transcriptional level. Calcium deposition, a marker for osteoblast differentiation, was detected by Alizarin Red stain. The number, size of particles, and total area were all reduced in response to galectin-3 (Figure 1, E and F). Taken together, these findings demonstrate that galectin-3 impairs the early differentiation pathway of osteoblasts, consequently resulting in the inhibition of calcium deposition.
Figure 1.
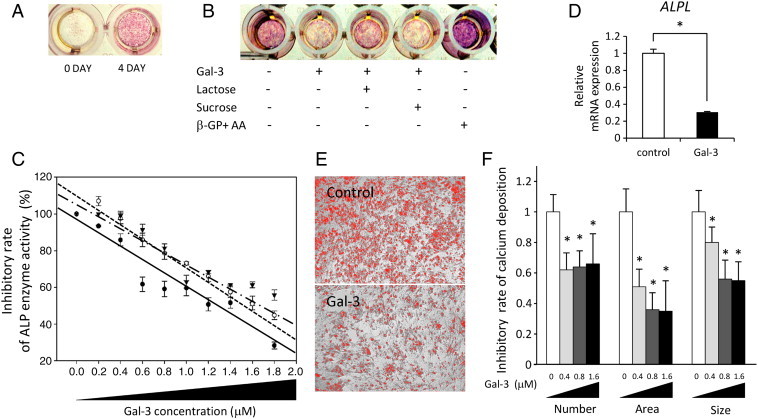
Galectin-3 inhibits ALP expression and calcium deposition. (A) Spontaneous ALP expression was identified in hFOB cell line after reaching confluence. Pink indicates the expression of ALP. 0 DAY or 4 DAY indicate the days after confluence, respectively. (B) Effect of galectin-3 on inhibition of ALP. Recombinant galectin-3 was added to culture medium in 1.6 μM as final concentration. The suppressive effect of galectin-3 on osteoblast differentiation was recovered by the treatment of lactose (75 mM), an inhibitor for galectin-3. Sucrose was used as a control disaccharide. AA with β-GP was used as positive control. (C) Galectin-3 inhibited ALP enzymatic activity in a dose-dependent manner even if cultured with AA with β-GP or 1,25-(OH)2D3 as inducers for osteoblast differentiation. Black filled circle and solid line indicate the culture with standard medium for spontaneous differentiation. White open circle and dotted line indicate the culture with AA and β-GP. Black triangle and dashed and dotted lines indicate the culture with 1,25-(OH)2D3. Data represent means ± SEM. (D) Galectin-3 suppressed mRNA expression level of ALP. The expression level was determined by quantitative real-time PCR compared to vehicle control. Data represent means ± SD. (E) Effect of galectin-3 on calcium deposition. The osteoblast differentiation was induced by 1,25-(OH)2D3. Calcium deposition was detected by Alizarin Red stain at 3 weeks after culture with galectin-3 or vehicle. Recombinant galectin-3 was added to culture medium every 3 to 4 days. Calcium deposition was selected by using ImageJ software. (F) Galectin-3 inhibited the calcium deposition. Number, size of particles, and total area of the calcium deposition were quantitated. Data represent means ± SD. *P < .05 versus vehicle control.
In contrast to recombinant galectin-3, we examined whether endogenous galectin-3 secreted from cancer cells inhibits osteoblast differentiation. First, we confirmed the presence of galectin-3 and measured the physiological concentration by immunoblot and ELISA using condition medium containing endogenous galectin-3 secreted by PC-3 cells, which is derived from bone metastasis lesion of a patient with prostate cancer. The results showed that cancer-secreted galectin-3 increased its concentration in a time-dependent manner, suggesting it could reach to higher concentration (Figure 2A). Next, to verify the inhibitory effect of galectin-3 on ALP expression, condition medium containing galectin-3 from cultured PC-3 was added into the osteoblast cell culture with or without lactose. The results of ALP stain and measurement of its enzymatic activity demonstrated that osteoblast differentiation was significantly inhibited by the condition medium containing endogenous galectin-3; it was recovered by lactose (Figure 2, B and C). Furthermore, to address the role of cancer-secreted galectin-3 under physiological status and its concentration, specific galectin-3 antagonist, e.g., modified citrus pectin (MCP), was used in indirect co-culture experiment with osteoblast cells. The results showed that ALP expression was inhibited by co-cultured PC-3 cells, and it was recovered by MCP. These results suggested that inhibitory effect of secretory factors from PC-3 cells on osteoblast differentiation is mediated by galectin-3 (Figure 2, D and E). Taken together, these findings showed that the results of recombinant galectin-3 (Figure 1) are similar to those of secreted endogenous galectin-3 (Figure 2).
Figure 2.
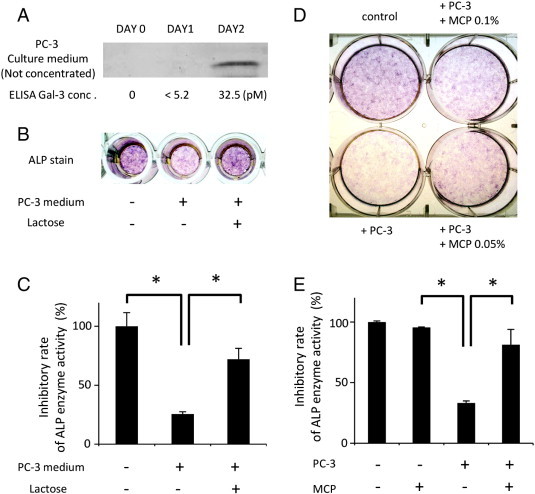
Endogenous galectin-3 inhibits ALP expression. (A) Cancer-secreted galectin-3 accumulated in condition medium in a time-dependent manner. PC-3 cells were cultured with condition medium for 0 to 2 days, and then it was subjected to immunoblot and ELISA without concentration. (B and C) Secretory factor by PC-3 cells inhibits ALP expression and its enzymatic activity in a sugar-dependent manner. Condition medium from PC-3 cell culture was concentrated to 20-fold, and 5 μl of concentrated medium was added into osteoblast cell culture in a 96-well plate. Culture medium was changed every 3 days. After 1 week, it was subjected to ALP stain and pNPP assay. The suppressive effect of cancer-secretory factor on osteoblast differentiation was recovered by the treatment of lactose (75 mM). (D and E) Inhibitory effect of secretory factors from PC-3 cells on osteoblast differentiation is mediated by galectin-3. Osteoblast cells were subjected to ALP stain and pNPP assay after co-culture with PC-3 cells for 1 week. MCP, a specific inhibitor of galectin-3, recovered the downregulated ALP expression and its activity. Data represent means ± SD. *P < .05 versus vehicle control.
Galectin-3 Inhibits Osteoblast Differentiation
Galectin-3 inhibits osteoblast differentiation at the transcriptional level of ALP (Figure 1D). We examined whether it also inhibits other differentiation markers, i.e., COL1A1, IBSP, and BGLAP, and transcriptional factors that were reported to regulate the osteoblast differentiation, i.e., RUNX2 and SP7 [6], [28], [29]. RUNX2 is an essential transcription factor for osteoblast differentiation, which leads to the promoter activation of COL1A1, ALPL, IBSP, BGLAP, or more [32]. SP7, known as osterix, functions downstream of RUNX2 and it also regulates the major osteoblast differentiation markers, i.e., COL1A1, ALPL, IBSP, and BGLAP [33], [34]. COL1A1 is known as type I collagen and an early marker of osteoblast differentiation because it is a primary product of osteoblast during bone formation [28], [29]. IBSP is known as bone sialoprotein, an intermediate stage marker [28], [29]. BGLAP, known as osteocalcin, is expressed in matured osteoblast and therefore is currently considered as the specific marker for late differentiation stage [28], [29]. Quantitative real-time PCR analysis demonstrated that the transcriptional levels of these genes are downregulated compared to the vehicle control (Figure 3), indicating that galectin-3 maintained osteoblasts in an undifferentiated state. These results imply that galectin-3 is a potent osteoblast differentiation inhibitor.
Figure 3.
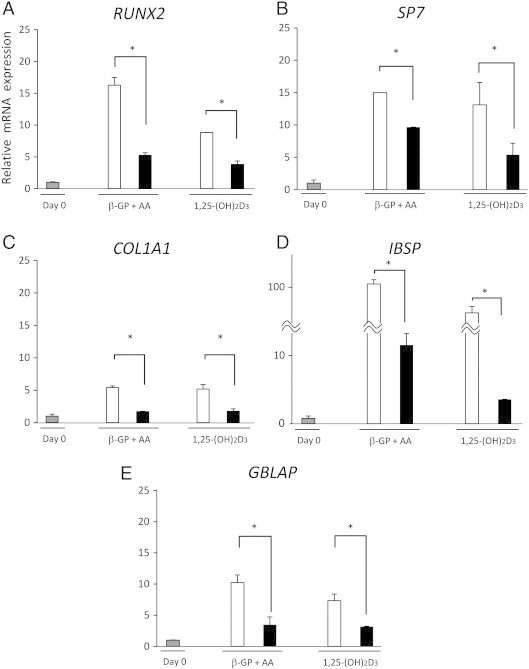
Galectin-3 inhibits the gene expressions for osteoblast differentiation. RNA samples were extracted at 1 week after the addition of recombinant galectin-3 with inducers for osteoblast differentiation. mRNA levels were determined by quantitative real-time PCR. Results are presented as fold changes compared to the sample before confluence. Data represent means ± SD. White bars, culture with vehicle control. Black bars, with galectin-3. *P < .05 versus vehicle control.
Cancer Cell Galectin-3 Inhibits Osteoblast Differentiation
The results described above documented that recombinant galectin-3 inhibited osteoblast differentiation, and since it may be argued that it may have a different three-dimensional conformation or affinity to endogenous galectin-3, we next tested whether galectin-3 secreted by cancer cells affect osteoblast differentiation. Galectin-3 is secreted from tumor cells by a non-conventional secretory pathway [35]. Thus, PC-3 and LNCaP prostate cancer cell lines and MDA-MB-231 and BT-549 breast cancer cell lines were used in a co-culture experiment. PC-3 is derived from a bone metastatic site in patients with prostate cancer and causes osteolytic lesions [36]. Both intracellular and secreted galectin-3 were detectable in PC-3 cells and culture medium (Figure 4I-A). MDA-MB-231 also has high metastatic potential to bone in an intra-cardiac injection model and causes osteolytic lesions [4], and the cells both expressed endogenous galectin-3 and secreted it (Figure 4I-A). LNCaP and BT-549 cells were devoid of galectin-3 (Figure 4I-A) and were transfected with p3xflag-myc-cmv-25 plasmid containing the preprotrypsin leader sequence as a secretory signal peptide for secretion of expressed protein into the culture medium. Transfection was confirmed by RT-PCR and identification of galectin-3 in culture medium by immunoblot. It was observed that indeed the transfected cell lines express and secrete galectin-3 (Figure 4, I-B and C). Moreover, galectin-3 downregulated PC-3 and MDA-MB-231 lines were established by using sh-RNA. Decreased expression was confirmed by RT-PCR and immunoblot for intracellular expression and secretion (data not shown). These established cell lines were subjected to a co-culture experiment with hFOB human osteoblasts (Figure 4II-A).
In the sample of hFOB co-cultured with LNCaP and BT-549 cells transfected with plasmid containing galectin-3, RUNX2, SP7, COL1A1, ALPL, IBSP, and BGLAP were decreased compared to control vector clone (Figure 4, II-B and C) as determined by quantitative real-time PCR. However, in the sample of co-culture with downregulated galectin-3, PC-3 and MDA-MB-231, SP7, and ALPL expression levels were increased compared to scrambled vector clone (data not shown). These findings confirm the results obtained with recombinant galectin-3 and support the conclusion that galectin-3 secreted by cancer cells inhibits osteoblast differentiation.
Galectin-3 Inhibits Osteoblast Differentiation through Notch Signaling
Osteoblast differentiation is regulated by various pathways such as Hedgehog, Wnt, bone morphogenetic protein (BMP), FGF signaling, and Notch signaling [6]. Wnt, BMP, and FGF signal pathways were reported to positively regulate osteoblast differentiation. In contrast, Notch signaling pathway inhibits osteoblast differentiation [37], [38], [39], [40], [41], [42]. Since the results depicted here show that galectin-3 suppresses osteoblast differentiation (Figure 1, Figure 2, Figure 3, Figure 4), it was necessary to examine the possible relationship between galectin-3 and Notch signaling pathway. To address thus, we tested HEY-1 expression, a target gene of Notch signaling in osteoblasts [41], [42]. HEY-1 was upregulated by the addition of recombinant galectin-3 to the cell cultures (Figure 5A). Consistently, co-culture with BT-549 transfected with galectin-3 plasmid upregulated HEY-1 expression (Figure 5B), suggesting that the osteoblast differentiation inhibitory effect of galectin-3 is mediated by Notch signaling. Previous reports suggested that Notch1 impairs osteoblast differentiation through NICD, a cleavage form of Notch1 [37], [38]. NICD releases from cell membranes and translocates into the nucleus to activate the target genes after the binding with ligands [6], [43], [44]. Therefore, we next tested whether NICD induction is affected by galectin-3. Immunoblot analysis showed that galectin-3 strongly induced NICD expression in a time-dependent manner in hFOB cells (Figure 5C). However, full-length Notch1 was not affected (Figure 5C). Interestingly, galectin-3–induced NICD was inhibited by lactose (Figure 5D) and antibodies against galectin-3 (Figure 5E). Moreover, immunofluorescence analysis has confirmed that galectin-3 induces NICD in the cytoplasm and its translocation into the nucleus (Figure 5F). These results suggested that the inhibitory effect of galectin-3 on osteoblast differentiation is mediated by Notch signaling.
Figure 5.
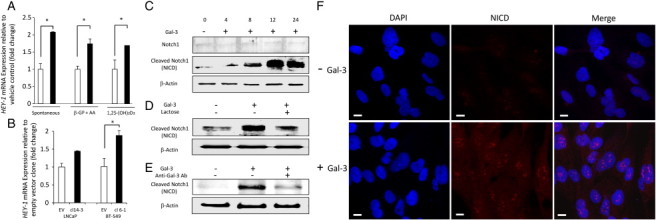
Galectin-3 upregulates HEY-1 and induces NICD in hFOB cells. (A) Recombinant galectin-3 induced mRNA expression level of HEY-1. RNA samples were extracted at 1 week after addition of recombinant galectin-3 (1.6 μM) with or without inducers for osteoblast differentiation. mRNA level was determined by quantitative real-time PCR. Results are presented as fold changes compared to the vehicle control. White bars, culture with vehicle control. Black bars, with galectin-3. (B) Cancer-secreted galectin-3 induced mRNA expression level of HEY-1 in co-culture experiment. RNA samples were extracted at 6 days after co-culture with transfected cancer clones with or without secretion of galectin-3. Results are presented as fold changes compared to the empty vector cells. Data represent means ± SD. White bars, co-culture with empty vector cells. Black bars, co-culture with galectin-3 transfected stable clone. EV, empty vector cells. *P < .05 versus vehicle or empty vector cells. (C) Galectin-3 induced NICD in hFOB cells in a time-dependent manner. Recombinant galectin-3 was added to culture medium in 1.6 μM as final concentration, respectively. (D) Suppression study using lactose; 75 mM of lactose or sucrose was used as final concentration. Cell lysates were extracted 12 hours after treatment. (E) Suppression study using HL31 anti–galectin-3 rabbit polyclonal antibody; 15 μg/ml HL31 was used as final concentration. Cell lysates were extracted 12 hours after treatment. The same concentration of rabbit isotype IgG was used as control (data were not shown). (F) Immunofluorescence images of hFOB with or without galectin-3 stimulation. Recombinant galectin-3 was added to culture medium in 1.6 μM as final concentration. Cells were fixed 24 hours after treatment. Images were shown for NICD (TRITC, red) and 4′,6-diamidino-2-phenylindole (nuclear stain, blue). White bar indicates 10 μm as scale.
Galectin-3 Induction of Notch Signaling Is Carbohydrate Dependent
Next, to delineate the underlying mechanism whereby galectin-3 enhances Notch signaling pathways, we examined the effect of galectin-3 on Notch signaling in HEK293 cells treated with the deletion mutants of galectin-3. Our previous results showed that the CRD plays a crucial role for inhibition of osteoblast differentiation (Figures 1D and 6B). Therefore, we purified the CRD of galectin-3 (108-250) and domain 1-107 by using the same plasmid (pET30) as full-length galectin-3 (1-250) to compare the potential of galectin-3 for induction of Notch signaling (Figure 6A). In HEK293, full-length galectin-3 (1-250) induced NICD in a time-dependent manner. The CRD of galectin-3 (108-250) also increased NICD, whereas other portion of galectin-3 (1-107) did not (Figure 6B). In addition, according to the results of a pull-down assay, full-length (1-250) and carbohydrate recognition domain (108-250) of galectin-3 bound Notch1, whereas galectin-3 (1-107) did not (Figure 6C). Lastly, we have monitored the activity of Notch signal transduction pathway using HEK293 cells with luciferase reporter containing responsive element to Notch activation and noted that full-length galectin-3 induces Notch signaling, and this induction was mediated by the CRD (Figure 6D).
Figure 6.
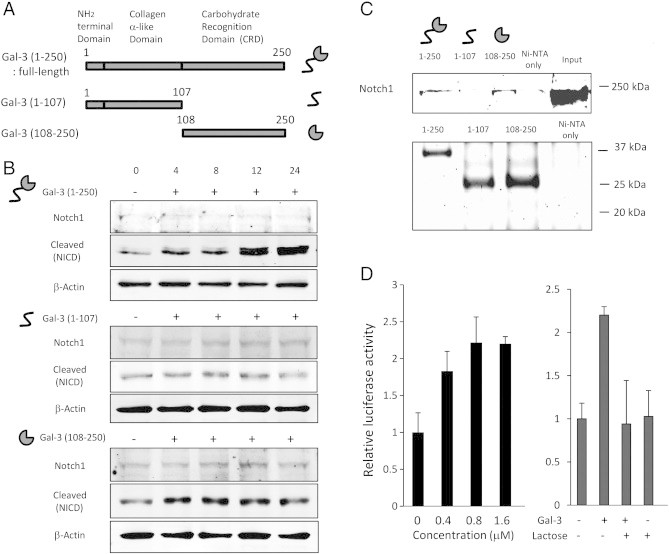
Full-length galectin-3 induces Notch signaling in HEK293 cells. (A) Schematic representation of deletion mutants of galectin-3 and its illustration. (B) Galectin-3 induced NICD in HEK293 cells in a time-dependent manner. Recombinant galectin-3 was added to culture medium in 1.6 μM as final concentration. (C) Result of pull-down assay. Full-length galectin-3 (1-250) and the CRD of galectin-3 (108-250) bound Notch1, whereas other portion of galectin-3 (1-107) did not bind. Total cell lysate of HEK293 was used as input sample. Coomassie stain was used to show the presence of recombinant galectin-3 in each sample. (D) Results of luciferase assay. HEK293 cells were transfected with the luciferase reporter construct containing RBP-Jk transcriptional response element to monitor the Notch signaling activation. Then, HEK293 cells were treated with recombinant full-length galectin-3 (1-250). Data represent means ± SD.
Discussion
Here, we presented a novel view of the inhibition of osteoblast differentiation by cancer-secreted galectin-3, not previously described. We report that following galectin-3–Notch1 interaction results in the appearance of NICD leading to up-regulation of HEY-1 and down-regulation of SP7 and RUNX2 expression. These expression changes manifested themselves in the inhibition of osteoblast differentiation. To date, it is widely accepted that, in the bone, Notch signaling mediates the communication between neighboring cells by direct cell-cell contact of membrane-associated jagged-1, 2 and delta like-1, 3, 4 [6], [43], [44], [45]. The data presented here reiterate this central role of Notch signaling and that its induction by a secretory factor, e.g., galectin-3, suppresses osteoblast differentiation. Because patients with breast and prostate cancer metastases have a higher serum concentration of circulated galectin-3 [13], [14], we explored its putative role in bone metastasis lesions. The results showed that galectin-3 inhibits osteoblast differentiation, it was apparent from the co-culturing experiments with LNCaP and BT-549 expressing galectin-3 following their transfection with human galectin-3 cDNA with a secretory leader peptide (Figure 4, II-B and C). Furthermore, the results indicated that SP7 and ALPL are the main galectin-3 differentiation suppression targets of downstream of Notch signaling. Similarly to galectin-3, previous studies have indicated that tumor necrosis factor-alpha (TNF-α), epidermal growth factor, sclerostin, and Dickkopf1 can impair osteoblast differentiation [46], [47], [48], [49], [50]. Bone metastasis is clinically classified into three types: osteolytic, osteoblastic, and mixed lesions [51]. Bone remodeling results from the activation statuses of osteoblasts and osteoclasts or the summation of secretory/non-secretory factors derived from cellular components of the bone microenvironment, bone matrix, and cancer; we thus speculate that the potent function of tumor-associated galectin-3 on osteoblasts should be considered as a new therapeutic target for patients with osteolytic bone metastasis.
Activation of Notch signaling in the bone microenvironment of metastatic lesions plays a critical role in the promotion of bone metastasis through tumor-derived jagged-1, a Notch ligand [21]. Although Notch signaling is thought to be activated by cell-to-cell contact, our data suggest an additional function whereby cancer-secreted galectin-3 directly induces Notch signaling activation leading to the inhibition of osteoblast differentiation. Mammals possess four different Notch transmembrane receptors, referred to as Notch 1-4. Among them, Notch1 is most important for osteoblast differentiation [37], [38], [52]. After binding their ligands, Notch receptors undergo a proteolytic cleavage that is catalyzed by the γ-secretase complex. As a result, NICD is released from the plasma membrane and translocates to the nucleus and activates the transcription of target gene HEY-1, which in turn controls the expression of RUNX2 and SP7 as crucial transcriptional factors for osteoblast differentiation [6], [43], [45]. In this study, we confirmed the above and showed that galectin-3 also induces the appearance of NICD following galectin-3–Notch interaction in hFOB cells (Figure 3, Figure 4, Figure 5). The extracellular domain of Notch1 contains 36 tandem epidermal growth factor-like repeats, many of which are modified with O-fucose (6-deoxy-galactose) [53], and O-fucose glycan plays an important role in ligand-mediated Notch activation and embryogenesis [54], [55]. Furthermore, the presence of galactose on O-fucose glycans differentially affects the intensity of Notch signaling [56], clarifying the sugar-dependent Notch–galectin-3 binding (Figure 6).
Our study depicts a new molecular mechanism in which inhibition of osteoblast differentiation by cancer-secreted galectin-3 may lead to bone destruction. In conclusion, we hypothesize that galectin-3 secreted by tumor cells growing in bone inhibits osteoblast differentiation through Notch signaling (Figure 7), and therefore, it is necessary to consider galectin-3 expression during treatment, which make this study highly significant.
Figure 7.
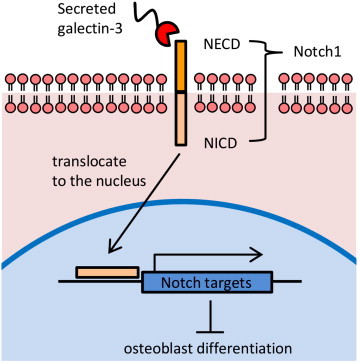
Proposed model for the regulation of Notch signaling by cancer-secreted galectin-3. Bone metastatic cancer cells secrete galectin-3 in the bone microenvironment. Secreted galectin-3 binds to Notch1 through the CRD. After the interaction, NICD translocates to the nucleus, and consequently, it upregulates Notch target genes such as HEY-1. These events suppress osteoblast differentiation and cause bone remodeling in bone metastatic lesions.
Footnotes
This work was supported by NIH/National Cancer Institute (R37 CA46120; A.R.).
Conflict of interest: There are no potential conflicts to declare.
References
- 1.Berruti A, Dogliotti L, Bitossi R, Fasolis G, Gorzegno G, Bellina M, Torta M, Porpiglia F, Fontana D, Angeli A. Incidence of skeletal complications in patients with bone metastatic prostate cancer and hormone refractory disease: predictive role of bone resorption and formation markers evaluated at baseline. J Urol. 2000;164:1248–1253. [PubMed] [Google Scholar]
- 2.Koizumi M, Yoshimoto M, Kasumi F, Iwase T. An open cohort study of bone metastasis incidence following surgery in breast cancer patients. BMC Cancer. 2010;21:381. doi: 10.1186/1471-2407-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–497. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 5.Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 6.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2011;7:235–243. doi: 10.1038/nrrheum.2011.23. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Nangia-Makker P, Balan V, Raz A. Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron. 2008;1:43–51. doi: 10.1007/s12307-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Balan V, Kho DH, Hogan V, Nangia-Makker P, Raz A. Galectin-3 regulates p21 stability in human prostate cancer cells. Oncogene. 2013;32:5058–5065. doi: 10.1038/onc.2012.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–527. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glinskii OV, Sud S, Mossine VV, Mawhinney TP, Anthony DC, Glinsky GV, Pienta KJ, Glinsky VV. Inhibition of prostate cancer bone metastasis by synthetic TF antigen mimic/galectin-3 inhibitor lactulose-l-leucine. Neoplasia. 2012;14:65–73. doi: 10.1593/neo.111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balan V, Wang Y, Nangia-Makker P, Kho DH, Bajaj M, Smith D, Heilbrun L, Raz A, Heath E. Galectin-3: a possible complementary marker to the PSA blood test. Oncotarget. 2013;4:542–549. doi: 10.18632/oncotarget.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–1393. [PubMed] [Google Scholar]
- 15.Nangia-Makker P, Wang Y, Raz T, Tait L, Balan V, Hogan V, Raz A. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer. 2010;127:2530–2541. doi: 10.1002/ijc.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balan V, Nangia-Makker P, Kho DH, Wang Y, Raz A. Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem. 2012;287:5192–5198. doi: 10.1074/jbc.C111.331686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orgaz JL, Benguria A, Sanchez-Martinez C, Ladhani O, Volpert OV, Jimenez B. Changes in the gene expression profile of A375 human melanoma cells induced by overexpression of multifunctional pigment epithelium-derived factor. Melanoma Res. 2011;21:285–297. doi: 10.1097/CMR.0b013e32834495c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao J, Erez A, Lee B. One NOTCH further: jagged 1 in bone metastasis. Cancer Cell. 2011;19:159–161. doi: 10.1016/j.ccr.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massagué J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller H, Hu J, Popp R, Schmidt MH, Müller-Decker K, Mollenhauer J, Fisslthaler B, Eble JA, Fleming I. Deleted in malignant brain tumors 1 is present in the vascular extracellular matrix and promotes angiogenesis. Arterioscler Thromb Vasc Biol. 2012;32:442–448. doi: 10.1161/ATVBAHA.111.239830. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damião R. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett-Lee P, Casbard A, Abraham J, Hood K, Coleman R, Simmonds P, Timmins H, Wheatley D, Grieve R, Griffiths G. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non-inferiority phase 3 trial. Lancet Oncol. 2014;15:114–122. doi: 10.1016/S1470-2045(13)70539-4. [DOI] [PubMed] [Google Scholar]
- 25.Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10:178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- 26.Eichner A, Brock J, Heldin CH, Souchelnytskyi S. Bone morphogenetic protein-7 (OP1) and transforming growth factor-β1 modulate 1,25(OH)2-vitamin D3-induced differentiation of human osteoblasts. Exp Cell Res. 2002;275:132–142. doi: 10.1006/excr.2002.5488. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, Raz A. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174:1515–1523. doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Malaval L, Gupta AK, Aubin JE. Simultaneous detection of multiple bone-related mRNAs and protein expression during osteoblast differentiation: polymerase chain reaction and immunocytochemical studies at the single cell level. Dev Biol. 1994;166:220–234. doi: 10.1006/dbio.1994.1309. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Malaval L, Aubin JE. Global amplification polymerase chain reaction reveals novel transitional stages during osteoprogenitor differentiation. J Cell Sci. 2003;116:1787–1796. doi: 10.1242/jcs.00376. [DOI] [PubMed] [Google Scholar]
- 30.Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010;207:1981–1993. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 32.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;30:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 34.Peng Y, Shi K, Wang L, Lu J, Li H, Pan S, Ma C. Characterization of Osterix protein stability and physiological role in osteoblast differentiation. PLoS One. 2013;8:e56451. doi: 10.1371/journal.pone.0056451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straube T, von Mach T, Hönig E, Greb C, Schneider D, Jacob R. pH-dependent recycling of galectin-3 at the apical membrane of epithelial cells. Traffic. 2013;14:1014–1027. doi: 10.1111/tra.12086. [DOI] [PubMed] [Google Scholar]
- 36.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 37.Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639. doi: 10.1210/en.2003-0463. [DOI] [PubMed] [Google Scholar]
- 38.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/β-catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. doi: 10.1074/jbc.M508370200. [DOI] [PubMed] [Google Scholar]
- 39.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149:3890–3899. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamurovic N, Cappellen D, Rohner D, Susa M. Coordinated activation of Notch, Wnt, and transforming growth factor-β signaling pathways in bone morphogenic protein 2-induced osteogenesis. Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. J Biol Chem. 2004;279:37704–37715. doi: 10.1074/jbc.M403813200. [DOI] [PubMed] [Google Scholar]
- 42.Salie R, Kneissel M, Vukevic M, Zamurovic N, Kramer I, Evans G, Gerwin N, Mueller M, Kinzel B, Susa M. Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone. 2010;46:680–694. doi: 10.1016/j.bone.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Canalis E. Notch signaling in osteoblasts. Sci Signal. 2008;1:pe17. doi: 10.1126/stke.117pe17. [DOI] [PubMed] [Google Scholar]
- 44.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-α. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NFκB pathways. J Biol Chem. 2006;281:6297–6306. doi: 10.1074/jbc.M507804200. [DOI] [PubMed] [Google Scholar]
- 48.Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, Keller ET, Li Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer. 2008;123:1034–1042. doi: 10.1002/ijc.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Shimizu E, Zhang X, Partridge NC, Qin L. EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. J Cell Biochem. 2011;112:1749–1760. doi: 10.1002/jcb.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendoza-Villanueva D, Zeef L, Shore P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13:R106. doi: 10.1186/bcr3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 52.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 53.Rampal R, Arboleda-Velasquez JF, Nita-Lazar A, Kosik KS, Haltiwanger RS. Highly conserved O-fucose sites have distinct effects on Notch1 function. J Biol Chem. 2005;280:32133–32140. doi: 10.1074/jbc.M506104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge C, Stanley P. The O-fucose glycan in the ligand-binding domain of Notch1 regulates embryogenesis and T cell development. Proc Natl Acad Sci U S A. 2008;105:1539–1544. doi: 10.1073/pnas.0702846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moloney DJ. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem. 2000;275:9604–9611. doi: 10.1074/jbc.275.13.9604. [DOI] [PubMed] [Google Scholar]
- 56.Hou X, Tashima Y, Stanley P. Galactose differentially modulates lunatic and manic fringe effects on Delta1-induced NOTCH signaling. J Biol Chem. 2012;287:474–483. doi: 10.1074/jbc.M111.317578. [DOI] [PMC free article] [PubMed] [Google Scholar]


