Abstract
Crosstalk between cancer cells and carcinoma-associated fibroblasts (CAFs) has earned recognition as an interaction that plays a pivotal role in carcinogenesis. Thus, we attempted to clarify whether increase in the level of CAFs promotes cancer progression by proportionally enhancing the interaction between cancer cells and CAFs. We first analyzed clinical correlation between the levels of fibroblasts and cancer progression and found that the level of CAFs made a noticeable difference on the prognosis of patients with oral squamous cell carcinoma (OSCC). In vivo animal study also demonstrated that tumor volume depended on the dose of CAFs that was co-injected with OSCC cells. The same tendency was observed in an in vitro study. We also found that interleukin-1α (IL-1α) secreted from OSCC cells had dual effects on CAFs: IL-1α not only promoted the proliferation of CAFs but also upregulated the secretion of cytokines in CAFs such as CCL7, CXCL1, and IL-8. The induction activity of cytokine secretion by IL-1α surpassed that of proliferation in OSCC cells. In summary, we unraveled an important interactive mechanism of carcinogenesis: IL-1α released from carcinoma stimulates the proliferation of CAFs and the simultaneous increase in cytokine secretion from CAFs promotes cancer progression in human OSCC. On the basis of these findings, we propose that the level of CAFs is eligible for being selected as a prognostic factor that will be useful in routine diagnosis. We also propose that blockage of reciprocal interaction between cancer cells and CAFs will provide an insight for developing novel chemotherapeutic strategy.
Keywords: cancer progression, carcinoma-associated fibroblasts, interleukin-1 alpha, oral squamous cell carcinoma, reciprocal interaction
Introduction
Accumulating evidence suggests that cancer treatment targeting cancer cells alone is insufficient to completely remove cancer because cancer progression is governed by a complex microenvironment composed of fibroblasts and their soluble factors, extracellular matrix, various immune competent cells and their soluble factors, and angiogenic factors [1], [2], [3]. Thus, it is widely acknowledged that tumor microenvironment is a significant contributor to cancer initiation and progression [4], [5], [6].
Carcinoma-associated fibroblasts (CAFs) have emerged as one of the most important components of tumor microenvironment that plays an active role in carcinogenesis [7], [8], [9]. Fibroblasts adjacent to transformed epithelial cells were reported to have modified characteristics compared to fibroblasts in normal stroma [10]. While the nature of CAFs has not been clearly identified as yet, CAFs are known to have characteristics of myofibroblasts [11], [12]. It is also widely recognized that CAFs are composed of increased fraction of senescent cells along with decreased fraction of proliferative cells [13]. However, the histologic observations of human cancer that the level of fibrosis supporting cancer cells was deeply related to poor prognosis in several human cancers have been reported [14], [15], [16].
The interaction between cancer cells and CAFs has been investigated as a main carcinogenic mechanism. As communication tools between cancer cells and CAFs, CAF-derived growth factors and their receptors have been studied. Up to date, diverse growth factors and their cognate receptors derived from cancer cells or CAFs have been reported in several human cancers [17], [18], [19]. Because the interaction between cancer cells and CAFs is considered to be an intercellular dialogue, cytokines and chemokines have been investigated as another communication tool. Co-culture of cancer cells with CAFs induced the production of cytokines such as interleukin-1α (IL-1α), IL-6, IL-8, vascular endothelial growth factor A, CCL20, and cyclooxygenase 2. As cancer cell–associated IL-1α could bind to IL-1R1 expressed predominantly in CAFs to increase the production of cytokines listed above, it was established as the major initiator of enhanced cytokine production in CAFs [20], [21]. Our previous study also found that a reciprocal interaction between oral squamous cell carcinoma (OSCC) cells and CAFs promoted cancer invasion: IL-1α released from OSCC cells stimulated CAFs to secrete CCL7, CXCL1, and IL-8, thereby facilitating cancer invasion [22].
Desmoplasia refers to the growth of dense connective tissue or stroma [23]. Because desmoplasia is a typical sign of wound healing and cancer progression, it has been focused on as a potentially effective prognostic factor for survival. Nevertheless, previous studies have been carried out using whole stroma around cancer rather than focusing on a specific component of stroma such as CAFs [23], [24], [25]. As the evidence that a large portion of stroma around cancer comprises myofibroblasts accumulates [12], [26], enrichment of CAFs in the cancer-associated stroma has been spotlighted as a potentially useful indicator for cancer prognosis in recent studies [27], [28]. A close relationship between CAFs and cancer progression has been found [8], [9], [16], but complete understanding of the underlying carcinogenic mechanism remains elusive.
We believed that a study on relational mechanism between the level of CAFs and cancer cells would contribute to the more accurate assessment of cancer prognosis and development of a new therapeutic strategy. Therefore, this study attempted to clarify whether proportional increase in the number of CAFs correspondingly promotes interactive response between cancer cells and CAFs during carcinogenesis. We revealed reciprocal interaction between cancer cells and CAFs that IL-1α released from cancer cells stimulated the proliferation of CAFs and the simultaneous increase in cytokine secretion from CAFs. In turn, a proportional increase in the cytokine secretion from CAFs promoted cancer progression in OSCC.
Materials and Methods
Patient Selection
We selected 100 patients who experienced OSCC and were diagnosed by Yonsei Dental Hospital from 1995 to 2007. The following data were available from the patients: age, degree of differentiation, TNM stage, locoregional/distant recurrence, and overall survival period. Overall survival was calculated from the date of initial surgery to the date of death. The last date of examination was April 30, 2011. All procedures were approved by the Institutional Review Board of Yonsei University College of Dentistry (IRB 2-2012-0041) in advance.
Histomorphometry
To calculate stromal proportion, three tissue sections containing both tumor and stromal portion were randomly selected from each of the 100 patients with OSCC. Hematoxylin and eosin (H&E)–stained tissue sections were observed to measure the stromal area. Immunohistochemical staining using anti–α-smooth muscle actin antibody (α-SMA, 1:100, human monoclonal; Dako, Glostrup, Denmark) was also carried out to examine the presence of myofibroblasts. Moreover, IL-1α antibody (1:50, rabbit polyclonal; Abcam, Cambridge, UK) was employed to assess the relationship between IL-1α expression and desmoplasia. Details about immunohistochemistry procedures are described in the Supplementary Materials and Methods.
Image-Pro plus V 3.0 software was employed to determine the target area. Bone, tooth, muscle, salivary gland, and fat tissues were excluded from the measurement of stromal area. The stromal portion was calculated as the ratio of whole stromal area to whole tumoral area (low < 1.0 vs high ≥ 1.0). Proportion of α-SMA–expressing myofibroblasts was examined in three randomly selected fields per slide and calculated as the ratio of α-SMA–positive area to whole area. Because the mean value of α-SMA–positive proportion was 22.97%, we selected the value as a cutoff criterion for distinguishing between low and high α-SMA expression. For examining the expression level of IL-1α, the weighted histoscore method was employed in this study [29].
Cell Culture
Three kinds of CAFs (CAFs 1-3) and four kinds of OSCC cell lines (YD-10B, YD-32, YD-38, and HSC-2) were used for this study. Three kinds of normal fibroblasts (NFs 1-3) were also used as controls. Details about the procedures of cell culture and identification are described in the Supplementary Materials and Methods.
Chemokines and Antibodies
Human recombinant protein CCL7 (FIC/MCP-3), human recombinant protein CXCL1 (FSP/GRO1), human recombinant protein IL-8 (CXCL8/GCP-1), and human recombinant protein IL-1α (IL-1A/IL-1F1) were products of R&D Systems. Anti-human CCL7, anti-human CXCL1, anti-human IL-8, anti-human IL-1α, and isotype control antibodies (mouse IgG1, IgG2A, and IgG2B) were also products of R&D Systems, Minneapolis, MN.
Mouse Orthotopic Xenograft Model
Animal studies were approved by the Animal Ethics Committee of Yonsei University College of Dentistry. BALB/c male mice (16 ± 2 g, 4 weeks of age) were provided by Central Lab Animal, Inc. (Seoul, South Korea) Animal studies were performed with six different experimental groups, and each group consisted of five mice. As a negative control (Media), the cell culture medium was injected alone. Groups of cells composed of different OSCC-to-CAF ratios were injected into the dorsal tongue as follows: in group CAFs, 5 × 104 CAFs were injected; in group OSCC, 5 × 104 HSC-2 were injected; in groups OSCC:CAFs/1:1, OSCC:CAFs/1:2, and OSCC:CAFs/1:3, 5 × 104 HSC-2 were injected with the corresponding numbers of CAFs. After 4 weeks, the mice were sacrificed and the dorsal tongues of mice were stained with H&E for morphologic analysis. Tumor volume of OSCC was assessed by two-dimensional measurements [30].
For analyzing the proliferative activity of xenografted tumor tissues, proliferating cell nuclear antigen (PCNA) was employed. PCNA index was accomplished for quantitative analysis by counting PCNA-positive cells in five randomly selected microscopic fields of each slide and calculating the percentage of PCNA-positive cells. Human vimentin was also immunostained to assess the level of human myofibroblasts that remained in the stroma of mouse OSCC specimens. Human vimentin–positive cells were counted in five randomly selected microscopic fields and the percentage of vimentin-positive cells was calculated. Result for each sample was obtained by averaging the data from three different persons.
Cell Proliferation Assay
Cell proliferation was determined by using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay [31]. In monoculture system, CAFs or OSCC cells (5 × 104 per well) were seeded and incubated overnight before treatment. Cells were treated with either recombinant proteins or neutralizing antibodies and subjected to MTT assay after 48 hours (OSCC cells) or 1 week (CAFs). In co-culture system, transwell system was used to examine the proliferation of CAFs or OSCC cells; 5 × 104 of either CAFs or OSCC cells were seeded per well. In transwell system, cells that need to be measured were placed in the lower chamber. That is, 5 × 104 OSCC cells were placed in the lower chamber for counting OSCC cells, while multiples of OSCC cells or CAFs were placed in the upper chamber, as shown in Figure 3A; 5 × 104 CAFs were seeded in the lower chamber for counting CAFs, as shown in Figure 4C, 4D. After incubation, only the cells in the lower chamber were used for the MTT assay.
Figure 3.
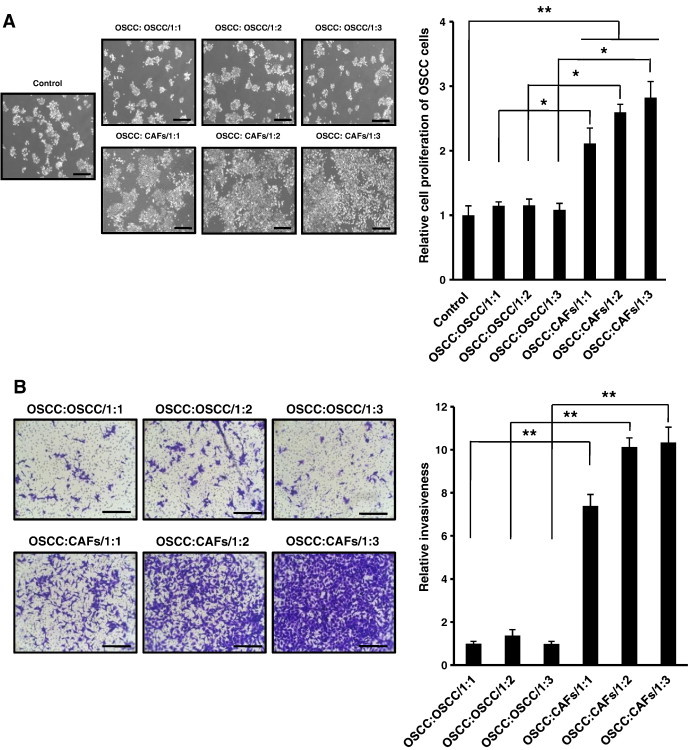
Proliferation and invasiveness of OSCC cells under various co-culture conditions. OSCC cells were co-cultured with either OSCC cells or CAFs at different doses. (A) Microscopic morphology was obtained (original magnification, × 100; scale bar, 500 μm), and cell proliferation rate after 48 hours was determined by the MTT assay. (B) Invasion assay was performed under various co-culture conditions. After incubation at 37°C for 48 hours, microscopic morphology was obtained (original magnification, × 100; scale bar, 100 μm) and cells that penetrated the upper filter were counted manually under a light microscope. The micrographs shown in this figure are representative of three independent experiments that showed similar results. Quantitative results indicate average values of three independent experiments, each of which was conducted in triplicate (n = 9). The results are shown as mean values ± SD (n = 9) and were analyzed by the Mann-Whitney U test (*P < .05 and **P < .01).
Figure 4.
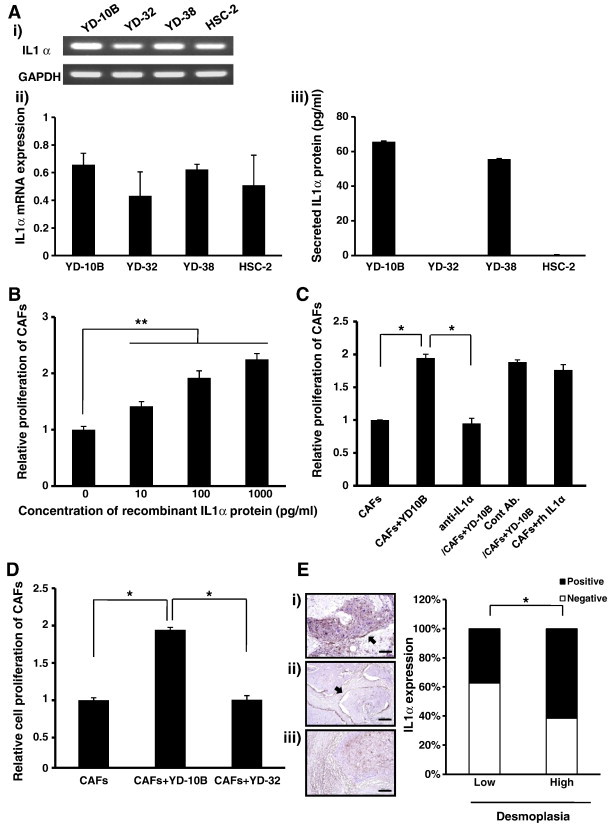
Proliferation of CAFs under various IL-1α-expressing conditions. (A) (i, ii) Expression levels of IL-1α mRNAs in OSCC cells were determined by RT-PCR. (i) Micrographs shown in this figure are representative of three independent experiments that showed similar results. (ii) Results were quantitatively analyzed and normalized to GAPDH expression. (iii) Secretion levels of IL-1α proteins were determined by ELISA. (B) Proliferation of CAFs was measured after treatment with or without IL-1α human recombinant protein at different doses (0, 10, 100, and 1000 pg/ml). (C) Cell proliferation of CAFs treated with or without 1 μg/ml neutralizing antibody against IL-1α. Mouse IgG2A and human recombinant IL-1α protein were used as controls. Treatment concentration of human recombinant IL-1α protein was 50 pg/ml. (D) Proliferation of CAFs co-cultured with YD-10B or YD-32. Proliferation of CAFs was measured by the MTT assay after 1-week incubation for all experiments. Quantitative results indicate average values of three independent experiments, each of which was conducted in triplicate (n = 9). The results are shown as mean values ± SD (n = 9) and were analyzed by the Mann-Whitney U test (⁎P < .05 and ⁎⁎P < .01). (E) Histomorphometry of IL-1α expression was performed in 100 human OSCC surgical specimen slides. (i) IL-1α expression in tumoral portion of OSCC. (ii, iii) IL-1α expression in stromal portions of OSCC. The micrographs shown in this figure are representative of 100 different tissues that showed similar results. All sections were viewed at × 100 magnification (scale bar, 200 μm). Relationship between IL-1α expression and the level of desmoplasia. IL-1α expression in each group was compared by the χ2 test (*P < .05).
Transwell Invasion Assay
Membrane invasion culture system (Corning, Tewksbury, MA) was used to quantify invasive activity as previously described [32]. CAFs or OSCC cells (2 × 104 for standard protocol and OSCC:OSCC or CAFs/1:1, 4 × 104 for OSCC:OSCC or CAFs/1:2, and 6 × 104 for OSCC:OSCC or CAFs/1:3) were seeded on the lower chamber; 2 × 104 OSCC cells were placed in the upper chamber of the transwell. After 48 hours of incubation, the cells that penetrated the upper filter were counted manually using a light microscope.
Enzyme-Linked Immunosorbent Assay
Cells were incubated under various culture conditions and the conditioned media were harvested after 48 hours. Concentration of cytokines (CCL7, CXCL1, IL-8, and IL-1α) was evaluated by ELISA according to the manufacturer’s protocol (R&D Systems).
Reverse Transcription–Polymerase Chain Reaction
Reverse transcription–polymerase chain reaction (RT-PCR) was employed to examine mRNA expression of IL-1α in YD-10B, YD-32, YD-38, and HSC-2 cells. Details of the RT-PCR procedure are described in the Supplementary Materials and Methods.
Statistical Analysis
Kaplan-Meier method was employed to estimate the overall survival of patients with OSCC. Analysis of Pearson correlation was employed to assess the correlation between the staining data of H&E and the staining data of α-SMA in 100 patients with OSCC. IL-1α expression in each group was compared by the χ2 test. All variables of the in vitro study were tested in three independent experiments, and each experiment was performed in triplicate. The results are reported as mean ± SD. Mann-Whitney U test was performed to analyze differences between groups. The value of P < .05 was considered statistically significant.
Results
Overall Survival Period of Patients with OSCC Is Inversely Correlated with the Levels of Stromal Proportion and α-SMA Expression
Selected examples of microscopical field from stroma-rich and stroma-poor tumors for quantification of tumor-to-stroma ratio are shown in Figure 1A (upper panel). Histologic staining with H&E allowed a clear-cut discrimination between stromal and epithelial components. Of 100 patients with OSCC, 49 patients were found to have low stromal proportion, while 51 patients were found to have high stromal portion. From the results of survival analysis, we observed that the survival rate of patients with OSCC was closely associated with the stromal proportion. Patients who had rich stromal areas showed poor survival rate in Kaplan-Meier analysis (P < .05; Figure 1Bi). We also evaluated myofibroblast composition of OSCC tissues by performing histomorphometry with α-SMA (Figure 1A, lower panel). In a preliminary study, we first examined the expression of three proteins, α-SMA, vimentin, and fibroblast activation protein, that are widely recognized as markers of CAFs. Because α-SMA is the most distinguishable marker of CAFs (Figure W1A), we used α-SMA as the marker of CAFs. Results of α-SMA expression corresponded with those of stroma in that overall survival of patients with high level of α-SMA expression was significantly decreased compared to that of patients with low level of α-SMA expression (P < .05; Figure 1Bii). These results suggested that the overall survival period of patients with OSCC correlated negatively with the levels of stromal proportion and α-SMA expression. We also examined the correlation between the staining data of H&E and the staining data of α-SMA in 100 patients with OSCC. Expression level of α-SMA was directly proportional to stromal proportion (P < .05; Figure W2).
Figure 1.
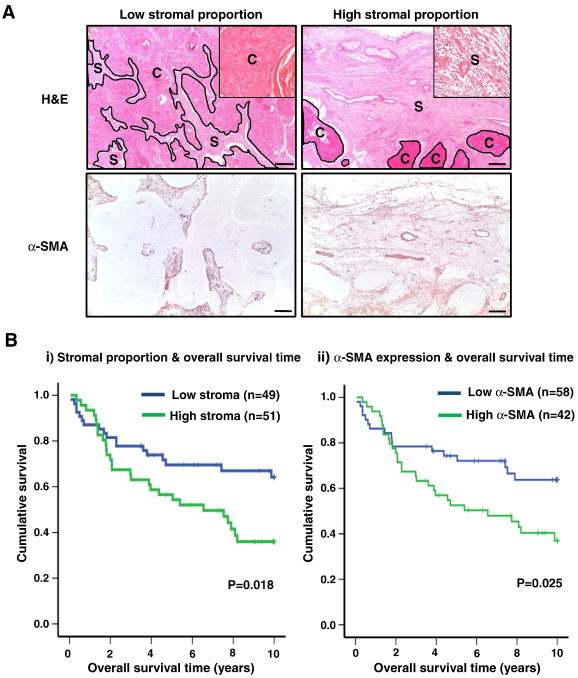
Overall survival period of patients with OSCC. (A) Two histologic sections of OSCC, stained with H&E (upper) and α-SMA (lower) (C, cancer region; S, stromal region). Each column is representative of low-level stromal proportion/α-SMA expression (left) and high-level stromal proportion/α-SMA expression (right) (original magnification, × 40; scale bar, 200 μm). Higher magnification (× 200) views of cancer and stromal regions are presented in the inset micrograph. The micrographs shown in this figure are representative of 100 independent tissues that showed similar results. (B) Overall survival of 100 patients with OSCC classified into (i) low (blue)/high (green) stromal proportion and (ii) low (blue)/high (green) α-SMA expression was analyzed by Kaplan-Meier method.
Tumor Progression Depends on the Dose of Co-injected CAFs In Vivo
To examine the effect of CAFs on cancer progression in vivo, CAFs were co-injected with OSCC cells into the dorsal tongues of nude mice at different doses. One mouse in group OSCC:CAFs/1:3 did not wake up after the injection of the anesthetic. Because two mice in group OSCC:CAFs/1:2 died during the experiment due to the development of obstructive tongue mass, necropsies were performed on them. After 4 weeks, no observable tumor growth (0%) was noticed in group Media and group CAFs. Four of five mice (80%) in group OSCC showed tumor formation. Tumors developed in all of the mice (100%) in group OSCC:CAFs/1:1, group OSCC:CAFs/1:2, and group OSCC:CAFs/1:3 (Figure W3A). Tumors from the co-injected groups showed more than 16-fold increase in volume compared to those from group OSCC in which mice were injected with OSCC cells alone (group OSCC:CAFs/1:1: P < .01, group OSCC:CAFs/1:2: P < .01, and group OSCC:CAFs/1:3: P < .05). Tumor volume derived from OSCC cells was increased in accordance with the dose of co-injected CAFs (Figure 2A).
Figure 2.
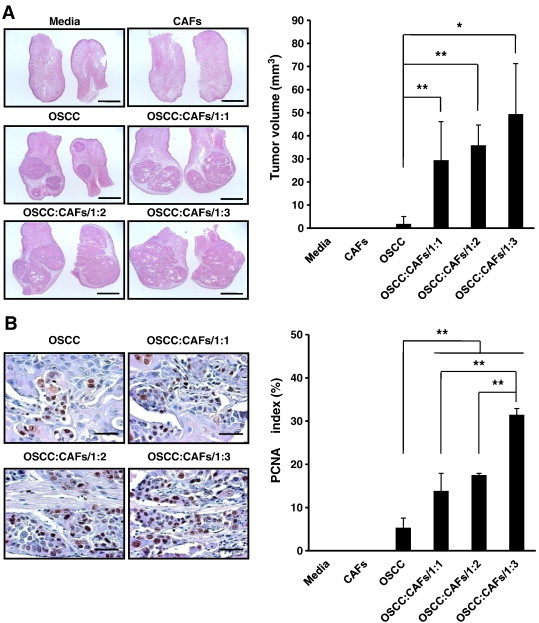
Tumor progression in a mouse orthotopic xenograft model. (A) Dorsal tongue of mouse was stained with H&E (original magnification, × 40; scale bar, 2 mm). Tumor volume of OSCC was assessed by two-dimensional measurements. (B) Dorsal tongue of mouse was stained with PCNA (original magnification, × 500; scale bar, 200 μm). PCNA index was established to quantitatively analyze the proliferative activity of xenografted tumor tissues. The micrographs shown in this figure are representative of five independent tissues that showed similar results. The results are shown as mean values ± SD (n = 5) and were analyzed by the Mann-Whitney U test (*P < .05 and **P < .01).
Mouse OSCC tissues were stained with human vimentin to exclude the voluminal effect of injected CAFs. Human vimentin was not found in the OSCC cell mono-injected group. Human vimentin–positive cells were detected in less than 2% of all the CAF-injected groups and the intergroup difference was not statistically significant (Figure W3B), suggesting that the increased tumor volume by higher doses of co-injected CAFs was not due to higher volume of the remaining CAFs.
PCNA index was increased in proportion to the dose of co-injected CAFs, and the PCNA indices of co-injected groups were significantly higher compared with that of the OSCC mono-injected group [group OSCC: 5.31%, group OSCC:CAFs/1:1: 13.83% (P < .01), group OSCC:CAFs/1:2: 17.5% (P < .01), and group OSCC:CAFs/1:3: 31.43% (P < .001); Figure 2B]. These results agreed well with those of tumor volume, indicating that the co-injected CAFs act in a dose-dependent manner to promote the tumor progression induced by OSCC cells in vivo.
CAFs Co-cultured with OSCC Cells Increase the Proliferation and Invasiveness of OSCC Cells in a Dose-Dependent Manner
Cell proliferation assay showed that CAFs stimulated the proliferation of OSCC cells. As a control, OSCC cells were monocultured without any treatment. Proliferation rate of OSCC cells co-cultured with CAFs was significantly higher than that of control (OSCC:CAFs/1:1: P < .01, OSCC:CAFs/1:2: P < .001, and OSCC:CAFs/1:3: P < .001). Additionally, OSCC cells were co-cultured with OSCC cells in conditions identical to CAF co-culture to confirm our assumption that the crosstalk between OSCC cells and CAFs is a unique interaction. No significant difference was found between the co-cultured groups that consisted only of OSCC cells and the control. Proliferation rate of OSCC cells co-cultured with CAFs was at least twice higher than that of OSCC cells co-cultured with OSCC cells under the same doses (P < .05 for all comparisons; Figure 3A).
To study the effects of CAFs on invasion of OSCC cells, we employed a modified transwell invasion assay. Under the same doses, the invasiveness of OSCC cells co-cultured with CAFs was at least seven times higher than that of OSCC cells co-cultured with OSCC cells (P < .01 for all comparisons). Invasiveness of OSCC cells co-cultured with CAFs was increased in proportion to the dose of co-cultured CAFs, while the invasiveness of OSCC cells co-cultured with OSCC cells was not affected by the dose of co-cultured OSCC cells (Figure 3B).
We also investigated the effect of NFs on cell proliferation and invasion of OSCC cells in comparison with the effect of CAFs. Even though cell proliferation and invasiveness of OSCC cells co-cultured with NFs were slightly increased compared to monocultured OSCC cells or OSCC cells co-cultured with OSCC cells, a significant difference was not found. However, cell proliferation and invasiveness of OSCC cells co-cultured with CAFs were significantly increased compared to OSCC cells co-cultured with NFs and other controls (P < .05; Figure W4). These results suggested that CAFs induced the proliferation and invasion of OSCC cells through unique interaction with OSCC cells.
Proliferation Rate of CAFs Depends on the Level of IL-1α
On the basis of the report that IL-1α secreted from OSCC stimulates CAFs for cancer progression [22], we examined whether IL-1α regulates the proliferation of CAFs in OSCC. First, we screened for IL-1α expression in several human OSCC cell lines (YD-10B, YD-32, YD-38, and HSC-2). mRNA (Figure 4, Ai and Aii) and secreted protein (Figure 4Aiii) of IL-1α were detected in all of the OSCC cell lines. Among the OSCC cell lines we examined, YD-10B showed the highest IL-1α, while YD-32 showed the lowest expression in both assays (Figure 4A). We also screened for expression of IL-1β, another type of IL-1, in several human OSCC cell lines. Secreted protein of IL-1β was not detected in any human OSCC cell lines subjected to this study (data not shown). Because this study emphasized reciprocal reaction between cancer cells and CAFs regulated by cytokines, we focused on the role of IL-1α during carcinogenesis. Western blot analysis was also employed to investigate IL-1R1 protein expression of CAFs. IL-1R1 protein was detected in all three CAFs. CAFs co-cultured with OSCC cells showed even higher expression of IL-1R1 than monocultured CAFs (P < .05; Figure W5A). To evaluate whether IL-1α induces the proliferation of CAFs, CAFs were monocultured with different doses (0, 10, 100, and 1000 pg/ml) of human recombinant IL-1α protein and the proliferation rate was measured. The proliferation rate of CAFs was significantly increased compared to non-treated control in proportion to the dose of IL-1α (P < .01 for all comparisons; Figure 4B). Subsequently, the proliferation rate of co-cultured CAFs with or without the treatment of IL-1α neutralizing antibody was examined. Neutralizing concentration (1 μg/ml) was determined on the basis of 50% neutralizing dose (ND50) listed in the data sheet. Proliferation rate of CAFs co-cultured with YD-10B was significantly (P < .05) higher than that of monocultured CAFs, but the treatment with IL-1α neutralizing antibody resulted in marked (P < .05) reduction of proliferation rate down to the level of monocultured CAFs. No significant difference was found between the CAFs co-cultured with YD-10B and other control groups treated with control antibody or human recombinant IL-1α protein (Figure 4C). Through CAF-OSCC co-culture experiments, we could once again confirm that IL-1α induces the proliferation of CAFs. CAFs were co-cultured with YD-10B and YD-32, respectively. Proliferation rate of CAFs co-cultured with YD-10B was significantly higher than that of monocultured CAFs and CAFs co-cultured with YD-32 (P < .05 for both comparisons). Additionally, statistical significance was not found between the proliferation rate of monocultured CAFs and CAFs co-cultured with YD-32 (Figure 4D). Taken together, our results suggest that the proliferation of CAFs is affected by the level of IL-1α released from the carcinoma cells.
To confirm these results in human OSCC tissues, we first examined the histologic features of the surgical specimens of two cell lines, YD-10B and YD-32. The level of CAFs was parallel to the level of IL-1α of each cell line (Figure W5B). Further, we examined the relationship between IL-1α expression and desmoplasia in 100 human OSCC surgical specimen slides. Membrane and cytoplasmic expressions of IL-1α were frequently detected in fibroblasts from OSCC tissue samples as well as in carcinoma cells. IL-1α expression in human OSCC tissues was closely associated with the level of desmoplasia, supporting in vitro data (P < .05; Figure 4E). However, no statistical correlation was found between IL-1α expression in cancer tissue and overall survival period (data not shown).
Cytokine Secretion from CAFs Depends on the Level of IL-1α
In our previous study, gene expression profiles of monocultured and co-cultured CAFs were analyzed by microarray and complete data were deposited in the Gene Expression Omnibus (Accession No. GSE18532) [22]. On the basis of the microarray data, five cytokines that were most upregulated in co-cultured CAFs compared to monocultured CAFs were selected and then the top three, CCL7, CXCL1, and IL-8, were singled out by real-time PCR.
We first examined the secretion level of three cytokines from CAFs stimulated by OSCC cells. All three cytokines were rarely detected in the supernatants of monocultured CAFs and co-cultured groups that consisted only of OSCC cells. However, CCL7, CXCL1, and IL-8 were detected at high levels in the supernatants of CAFs co-cultured with OSCC cells (Figure W6, A–C). We confirmed that an increase in cytokine secretion also occurs through the unique interaction between CAFs and OSCC.
To examine the direct effect of IL-1α on the secretion level of these three cytokines from CAFs, monocultured CAFs were treated with human recombinant IL-1α protein. Treatment concentration of human recombinant IL-1α protein was 50 pg/ml, where 50 pg/ml was an approximate concentration released from OSCC cells in our study (Figure 4Aiii). While the secretion of CCL7, CXCL1, and IL-8 was almost undetectable in the non-treated CAFs, the secretion of these three cytokines was significantly upregulated in CAFs after IL-1α treatment (CCL7 and CXCL1: P < .001 and IL-8: P < .05; Figure 5A). To confirm cytokine secretion from CAFs on blocking IL-1α secreted from OSCC cells, neutralizing antibody against IL-1α (1 μg/ml) was added to the serum-free conditioned medium of CAFs co-cultured with OSCC cells. Treatment with IL-1α neutralizing antibody to conditioned medium of CAFs–YD-10B co-culture resulted in marked (P < .05 for all comparisons) reduction of CCL7, CXCL1, and IL-8 secretion (CCL7: 94%, CXCL1: 89%, and IL-8: 34% reduction) compared to both non-treated controls and controls treated with isotype antibody (Figure 5B). As shown in Figure 4A, it was confirmed that IL-1α expression was the highest in YD-10B and the lowest in YD-32. To test whether the cytokine secretion from CAFs is regulated by IL-1α expression in OSCC, we compared the secretion levels of three cytokines (CCL7, CXCL1, and IL-8) in CAFs co-cultured with each of the two OSCC cell lines mentioned above. Secretion of the three cytokines was rarely detected in monocultured CAFs and CAFs co-cultured with YD-32. However, secretion of all three cytokines was significantly increased in CAFs co-cultured with YD-10B (P < .05 for all comparisons). Cytokine secretion levels in CAFs co-cultured with YD-10B were elevated from 21-fold (CCL7) to 165-fold (CXCL1) compared with the CAFs co-cultured with YD-32 (Figure 5C). Thus, a conclusion could be drawn that IL-1α stimulated cytokine secretion from CAFs.
Figure 5.
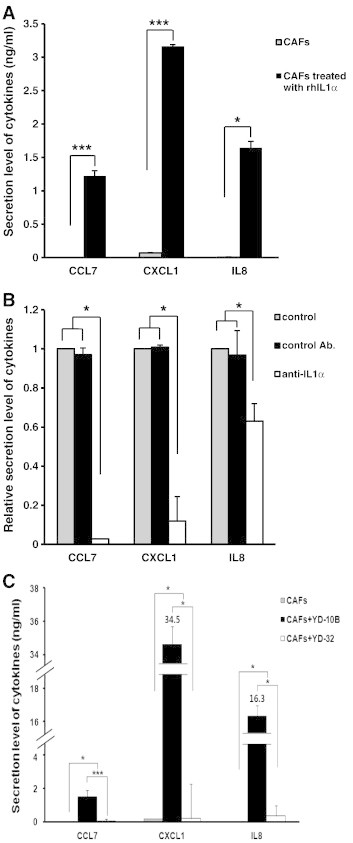
Cytokine secretion from CAFs under various IL-1α expression conditions. (A) Cytokine secretions from CAFs that were treated with or without 50 pg/ml IL-1α human recombinant protein. (B) Cytokine secretions from CAFs that were treated with or without 1 μg/ml neutralizing antibody against IL-1α. Mouse IgG2A was used as a control. (C) Secretion levels of cytokines from CAFs co-cultured with YD-10B or YD-32. Monocultured CAFs was used as a control. In all the experiments, ELISA was employed to measure the secretion levels of three cytokines (CCL7, CXCL1, and IL-8) in conditioned media after 48 hours. Quantitative results indicate average values of three independent experiments, each of which was conducted in triplicate (n = 9). The results are shown as mean values ± SD (n = 9) and were analyzed by the Mann-Whitney U test (*P < .05 and ***P < .001).
Cytokines Secreted from CAFs Induce Proliferation of Cancer Cell
We then studied whether the cytokines secreted from CAFs induced the proliferation of OSCC cells. To evaluate whether three cytokines from CAFs induces the proliferation of OSCC cells in several human OSCC cell lines (YD-10B, YD-32, YD-38, and HSC-2), OSCC cells were monocultured with different doses (0, 1, 10, and 100 ng/ml) of human recombinant protein of each cytokine and the proliferation rate was measured. Treatment doses of cytokines were determined on the basis of the secretion level of three cytokines from co-cultured CAFs (CCL7: 2.6-7.0 ng/ml, CXCL1: 34.4-60.3 ng/ml, IL-8: 15.5-32.3 ng/ml; Figure W6, A–C). The proliferation rate of all OSCC cell lines treated with each cytokine was significantly increased compared to non-treated control in proportion to the dose of cytokines (Figure 6, A–C). Our results suggest that the proliferation of OSCC cells is affected by the cytokines secreted from CAFs.
Figure 6.
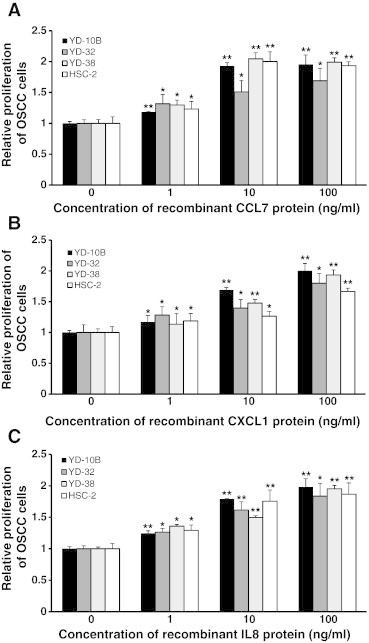
Effect of cytokines on proliferation of OSCC cells in several human OSCC cell lines. (A) Proliferation of OSCC cells in several human OSCC cell lines (YD-10B, YD-32, YD-38, and HSC-2) was measured after treatment with or without CCL7 human recombinant protein at different doses (0, 1, 10, and 100 ng/ml). (B) Proliferation of OSCC cells in several human OSCC cell lines (YD-10B, YD-32, YD-38, and HSC-2) was measured after treatment with or without CXCL1 human recombinant protein at different doses (0, 1, 10, and 100 ng/ml). (C) Proliferation of OSCC cells in several human OSCC cell lines (YD-10B, YD-32, YD-38, and HSC-2) was measured after treatment with or without IL-8 human recombinant protein at different doses (0, 1, 10, and 100 ng/ml). After incubation at 37°C for 48 hours, the rate of cell proliferation was determined by the MTT assay. Non-treated cells of each cell line were used as controls. Quantitative results indicate average values of three independent experiments, each of which was conducted in triplicate (n = 9). The results are shown as mean values ± SD (n = 9) and were analyzed by the Mann-Whitney U test (*P < .05 and **P < .01).
Discussion
The interaction between cancer cells and stromal fibroblasts plays a critical role in carcinogenesis [17], [18], [19], [20], [21], [22]. In this study, fibroblasts that were adapted to tumor microenvironment were denominated as CAFs. Identity of CAFs was verified with the expression of α-SMA and vimentin, which are recognized as markers of CAFs (Figure W1B).
In light of the previous investigations that the growth of dense connective tissue or stroma stimulates cancer progression [14], [15], [16], we first resorted to clinical evaluation to confirm that the level of CAFs could serve as an important criterion for predicting cancer prognosis in this study. On the basis of the correlation data that stromal proportion was directly proportional to expression level of α-SMA, we suggested that CAFs are the primary contributors to stromal component. Results of an animal study also revealed the increase of tumor volume paralleled to the level of CAFs. To rule out voluminal effect by co-injected CAFs, we examined human fibroblasts in xenografted tumor mass and found no significant difference of retained human fibroblasts among experimental groups. Given the data that normal human fibroblasts showed no transplantability, we could extrapolate that the co-injected CAFs could not survive while cancer cells were colonized in mouse tongue tissue [33]. While the proliferation of CAFs turned out to be correlated with cancer progression, its carcinogenic factor is not unveiled. Thus, a detailed study was carried out to examine the specific factor that underlies CAF-mediated cancer progression.
Results of the co-culture in vitro study showed that the increased ratio of CAFs induced the proliferation and invasiveness of OSCC cells in a dose-dependent manner. However, there was no noticeable difference in cell proliferation and invasiveness of OSCC cells co-cultured with NFs or co-cultured groups that consisted only of OSCC cells, indicating that tumor-stromal crosstalk can be considered to be a peculiar interaction. We also found that CAFs were stronger inducers of cell proliferation and invasion in OSCC cells than NFs.
Prior studies, including ours, demonstrated that IL-1α is a regulator of cytokine secretion in CAFs [20], [21], [22]. To study a direct relationship between the level of IL-1α expression in OSCC and the level of CAFs, we examined the effects of IL-1α expression on the proliferation of CAFs that can serve as a contributing factor to the proliferation of CAFs. Exposure to recombinant IL-1α protein or treatment with IL-1α neutralizing antibody led to considerable changes in the proliferation of CAFs. For further clinical verification of our results, we examined IL-1α expression in OSCC tissue samples. Boundary area between OSCC and stroma stained strongly with IL-1α in immunohistochemistry. Another recent study also reported that a regulatory factor of reciprocal interaction between cancer and stroma was strongly immunostained in the stromal region proximal to cancer epithelium [34]. IL-1α expression in human OSCC tissues was strongly associated with the level of CAFs. Our findings in the in vitro study was also supported by the results of histomorphometry that histologic feature of surgical tissue in YD-10B cell line showed invasive squamous cell carcinoma with abundant stromal reaction, whereas the surgical specimen of YD-32 cell line revealed scanty stromal reaction. Therefore, it could be concluded that IL-1α plays an important role in cancer progression by mediating the crosstalk between tumor and stroma. We presumed that IL-1R1 expression of CAFs co-cultured with OSCC cells would be increased when CAFs take IL-1α signal from OSCC cells. Expression level of IL-1R1 was increased substantially when CAFs are co-cultured with OSCC cells. The results suggest that CAFs are responsive to IL-1α released from OSCC cells. Further study silencing IL-1α receptor on CAFs and seeing downstream inhibition of cytokine production would be helpful to clarify unrevealed mechanism underlying the interaction between carcinoma and CAFs.
Our previous study showed that remarkable changes were found in cytokine expressions of CAFs when they were exposed to cancer cells [22]. In this study, we reconfirmed that an increase in cytokine secretion occurs through the unique interaction between CAFs and OSCC.
All results of cell proliferation assay in this study showed that proliferation rate of CAFs were not increased more than three times under various experimental conditions. However, the effect of CAFs including tumor volume, invasive activity, and induction of cytokines was more powerful at least 7 times or even up to 165 times. The secretion level of cytokines was gradually in direct proportion to the level of CAFs (Figure W6, A–C). Thus, a conclusion could be drawn that CAFs contribute extensively to cancer progression beyond only mere proliferative effect.
CAFs are known to have characteristics of senescent cells [13]. Thus, senescence-associated secretory phenotype factors of senescent cells can render the tissue microenvironment favorable for cancer progression [35], [36]. Given our data that the induction activity for cytokine secretion through IL-1α (more than 47-fold increase) surpassed the induction activity for cell proliferation of CAFs (less than three times increase), we could presume that a certain fraction of CAFs is obliged to undergo a senescent process. Taken together, a further study would be needed to clarify whether both proliferative CAFs and senescent CAFs harboring different distribution of receptors differently responded to IL-1α coexist in tumor microenvironment or one type of CAFs merely shows sequential response to IL-1α.
We then evaluated whether these cytokines released from CAFs stimulated by IL-1α might induce proliferation of OSCC cells. All three cytokines induced proliferation of OSCC cells, confirming that cytokines secreted from CAFs are immediate causes of tumor growth. Our findings are supported by previous studies that CXCL1 and IL-8 stimulated the proliferation of cancer cells. CXCL1 was reported to modulate tumor growth and proliferation of cancer cells [37], [38]. A close relationship between IL-8 and proliferation of cancer cells has been found in several human cancers including OSCC [39], [40], [41]. CCL7 also induced the proliferation of cancer cells; however, the induction activity of invasion surpassed proliferative activity [22]. We further suggest that other regulatory factors might also be responsible for the increased tumor cell proliferation because an increase in tumor volume that paralleled the higher proportion of CAFs in co-culture (more than 16-fold increase) was more definitely changed than proliferation of OSCC cells treated with these cytokines (less than 2.5-fold increase). For example, CXCL2 (growth-regulated oncogene 2 (GRO2)/GROβ) and CXCL3 (GRO3/GROγ) might be involved in the proliferation of cancer cells. In fact, a plethora of previous studies has reported that CXCL2 and CXCL3 stimulated the proliferation of cancer cells [42], [43], [44]. Microarray and real-time PCR results from our previous research, which showed that the expression of CXCL2 and CXCL3 was increased in co-cultured CAFs, could support our assumption. Moreover, regarding the increased tumor volume of CAF-OSCC co-injected groups in a xenograft study, the angiogenic effect of cytokines such as IL-1α and IL-8 might also have contributed to the growth of tumors [45], [46], [47], [48].
Here, we attempted to investigate reciprocal reaction between cancer cells and CAFs regulated by cytokines: IL-1α, released from carcinoma, stimulated CAFs resulting in the proliferation of CAFs and the simultaneous increase of cytokine secretion promoted in the proliferation of OSCC cells. Further research is needed to examine autocrine effect of cytokines released from CAFs or OSCC cells that might be also involved in cancer progression. This study unraveled one of the mechanisms underlying the interaction between carcinoma and CAFs, especially confined to IL-1α–secreting cancer. Further study to elucidate the crosstalk between cancer cells and CAFs may provide a new insight for the development of effective diagnostics and therapeutics to treat OSCC and other types of cancers.
Footnotes
This article refers to supplementary materials, which are designated by Figures S1 to S6 and are available online at www.neoplasia.com.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2009-0094027). Conflict of interest: The authors declare that they have no conflict of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neo.2014.09.003.
Appendix A. Supplementary data
Supplementary Materials and Methods
Supplementary figures
References
- 1.Oskarsson T., Massague J. Extracellular matrix players in metastatic niches. EMBO J. 2012;31:254–256. doi: 10.1038/emboj.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedl P., Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Allen M., Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol. 2011;223:162–176. doi: 10.1002/path.2803. [DOI] [PubMed] [Google Scholar]
- 6.Wu S.D., Ma Y.S., Fang Y., Liu L.L., Fu D., Shen X.Z. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38:218–225. doi: 10.1016/j.ctrv.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmick N.A., Neilson E.G., Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer I.G., Sood A.K., Mok S., Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia. 2011;13:393–405. doi: 10.1593/neo.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haviv I., Polyak K., Qiu W., Hu M., Campbell I. Origin of carcinoma associated fibroblasts. Cell Cycle. 2009;8:589–595. doi: 10.4161/cc.8.4.7669. [DOI] [PubMed] [Google Scholar]
- 11.Polanska U.M., Orimo A. Carcinoma-associated fibroblasts: non-neoplastic tumour-promoting mesenchymal cells. J Cell Physiol. 2013;228:1651–1657. doi: 10.1002/jcp.24347. [DOI] [PubMed] [Google Scholar]
- 12.Orimo A., Weinberg R.A. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 13.Dean J.P., Nelson P.S. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98:245–249. doi: 10.1038/sj.bjc.6604087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Novoa J.M., Nieto M.A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh D., Suchak K., Moutasim K.A., Vallath S., Hopper C., Jerjes W., Upile T., Kalavrezos N., Violette S.M., Weinreb P.H. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470–481. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- 16.Kadaba R., Birke H., Wang J., Hooper S., Andl C.D., Di Maggio F., Soylu E., Ghallab M., Bor D., Froeling F.E. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol. 2013;230:107–117. doi: 10.1002/path.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo M., Liu W., Serra S., Asa S.L., Ezzat S. FGFR2 isoforms support epithelial-stromal interactions in thyroid cancer progression. Cancer Res. 2012;72:2017–2027. doi: 10.1158/0008-5472.CAN-11-3985. [DOI] [PubMed] [Google Scholar]
- 18.Schauer I.G., Zhang J., Xing Z., Guo X., Mercado-Uribe I., Sood A.K., Huang P., Liu J. Interleukin-1β promotes ovarian tumorigenesis through a p53/NF-κB–mediated inflammatory response in stromal fibroblasts. Neoplasia. 2013;15:409–420. doi: 10.1593/neo.121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai K.P., Yamashita S., Huang C.K., Yeh S., Chang C. Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines. EMBO Mol Med. 2012;4:791–807. doi: 10.1002/emmm.201101140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjomsland V., Spångeus A., Välilä J., Sandström P., Borch K., Druid H., Falkmer S., Falkmer U., Messmer D., Larsson M. Interleukin 1α sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011;13:664–675. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogan-Sakin I., Cohen M., Paland N., Madar S., Solomon H., Molchadsky A., Brosh R., Buganim Y., Goldfinger N., Klocker H. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis. 2009;30:698–705. doi: 10.1093/carcin/bgp043. [DOI] [PubMed] [Google Scholar]
- 22.Jung D.W., Che Z.M., Kim J., Kim K., Kim K.Y., Williams D., Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer. 2010;127:332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 23.Ayala G., Tuxhorn J.A., Wheeler T.M., Frolov A., Scardino P.T., Ohori M., Wheeler M., Spitler J., Rowley D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–4801. [PubMed] [Google Scholar]
- 24.Halvorsen T.B., Seim E. Association between invasiveness, inflammatory reaction, desmoplasia and survival in colorectal cancer. J Clin Pathol. 1989;42:162–166. doi: 10.1136/jcp.42.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jass J.R., Atkin W.S., Cuzick J., Bussey H.J., Morson B.C., Northover J.M., Todd I.P. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawashiri S., Tanaka A., Noguchi N., Hase T., Nakaya H., Ohara T., Kato K., Yamamoto E. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1346–1353. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 27.Tsujino T., Seshimo I., Yamamoto H., Ngan C.Y., Ezumi K., Takemasa I., Ikeda M., Sekimoto M., Matsuura N., Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- 28.Fuyuhiro Y., Yashiro M., Noda S., Matsuoka J., Hasegawa T., Kato Y., Sawada T., Hirakawa K. Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells. Cancer Sci. 2012;103:797–805. doi: 10.1111/j.1349-7006.2012.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witton C.J., Hawe S.J., Cooke T.G., Bartlett J.M. Cyclooxygenase 2 (COX2) expression is associated with poor outcome in ER-negative, but not ER-positive, breast cancer. Histopathology. 2004;45:47–54. doi: 10.1111/j.1365-2559.2004.01898.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.Y., Oh S.H., Woo J.K., Kim W.Y., Van Pelt C.S., Price R.E., Cody D., Tran H., Pezzuto J.M., Moriarty R.M. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Cancer Inst. 2005;97:1695–1699. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 31.Berridge M.V., Tan A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 32.Kim E.J., Che Z.M., Park Y.J., Hwang Y.S., Kim K.Y., Jung da W., Jeon N.K., Choi Y.W., Lee E.J., Kim J. Morphogenesis and biological significance of spindle cell transformation in a spindle cell carcinoma. Cancer Lett. 2009;275:61–71. doi: 10.1016/j.canlet.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Namba M., Nishitani K., Fukushima F., Kimoto T., Yuasa Y. Multi-step neoplastic transformation of normal human fibroblasts by Co-60 gamma rays and Ha-ras oncogenes. Mutat Res. 1988;199:415–423. doi: 10.1016/0027-5107(88)90218-7. [DOI] [PubMed] [Google Scholar]
- 34.Murata T., Mizushima H., Chinen I., Moribe H., Yagi S., Hoffman R.M., Kimura T., Yoshino K., Ueda Y., Enomoto T. HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells with cancer-associated fibroblasts to support progression of uterine cervical cancers. Cancer Res. 2011;71:6633–6642. doi: 10.1158/0008-5472.CAN-11-0034. [DOI] [PubMed] [Google Scholar]
- 35.Davalos A.R., Coppe J.P., Campisi J., Desprez P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velarde M.C., Demaria M., Campisi J. Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol. 2013;38:17–27. doi: 10.1159/000343572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bandapalli O.R., Ehrmann F., Ehemann V., Gaida M., Macher-Goeppinger S., Wente M., Schirmacher P., Brand K. Down-regulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine. 2012;57:46–53. doi: 10.1016/j.cyto.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Bolitho C., Hahn M.A., Baxter R.C., Marsh D.J. The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr Relat Cancer. 2010;17:929–940. doi: 10.1677/ERC-10-0107. [DOI] [PubMed] [Google Scholar]
- 39.Luppi F., Longo A.M., de Boer W.I., Rabe K.F., Hiemstra P.S. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Christofakis E.P., Miyazaki H., Rubink D.S., Yeudall W.A. Roles of CXCL8 in squamous cell carcinoma proliferation and migration. Oral Oncol. 2008;44:920–926. doi: 10.1016/j.oraloncology.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y.M., Webster S.J., Flower D., Woll P.J. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer. 2004;91:1970–1976. doi: 10.1038/sj.bjc.6602227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess M., Cheung C., Chambers L., Ravindranath K., Minhas G., Knop L., Mollee P., McMillan N.A., Gill D. CCL2 and CXCL2 enhance survival of primary chronic lymphocytic leukemia cells in vitro. Leuk Lymphoma. 2012;53:1988–1998. doi: 10.3109/10428194.2012.672735. [DOI] [PubMed] [Google Scholar]
- 43.Van Sweringen H.L., Sakai N., Tevar A.D., Burns J.M., Edwards M.J., Lentsch A.B. CXC chemokine signaling in the liver: impact on repair and regeneration. Hepatology. 2011;54:1445–1453. doi: 10.1002/hep.24457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong Q., Zhang J., Hendricks D.T., Zhao X. GROβ and its downstream effector EGR1 regulate cisplatin-induced apoptosis in WHCO1 cells. Oncol Rep. 2011;25:1031–1037. doi: 10.3892/or.2011.1163. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.J., Uehara H., Karashima T., McCarty M., Shih N., Fidler I.J. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3:33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto J., Sakaguchi H., Aoki I., Tamaya T. Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–2635. [PubMed] [Google Scholar]
- 47.Matsuo Y., Sawai H., Ma J., Xu D., Ochi N., Yasuda A., Takahashi H., Funahashi H., Takeyama H. IL-1α secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1α release and tumor cells' potential for liver metastasis. J Surg Oncol. 2009;99:361–367. doi: 10.1002/jso.21245. [DOI] [PubMed] [Google Scholar]
- 48.Matsuo Y., Sawai H., Ochi N., Yasuda A., Takahashi H., Funahashi H., Takeyama H., Guha S. Interleukin-1alpha secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J Surg Res. 2009;153:274–281. doi: 10.1016/j.jss.2008.04.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Supplementary figures


