Abstract
Background
Patient retention in antiretroviral therapy (ART) programs remains a major challenge in sub-Saharan Africa. We examined whether and why retention in ART care has changed with increasing access.
Methods
Retrospective cohort study combining individual data from ART registers and interview data, enabling us to link patients across different ART clinics in Karonga District, Malawi.
We recorded information on all adults (≥15 years) starting ART between July 2005 and August 2012, including those initiating due to pregnancy and breastfeeding (Option B+).
Retention in care was defined as being alive and receiving ART at the end of study. Predictors of attrition were assessed using a multi-variable Cox-proportional hazards model.
Results
The number of clinics providing ART increased from one in 2005 to 16 in 2012. Six month retention increased from 73% (95%CI 71-76) to 93% (92-94) when comparing the 2005-06 and 2011-12 cohorts, and 12-month retention increased from 70% (67-73) to 92% (90-93). Over the study period, the proportion of patients starting ART at WHO stage 4 declined from 62% to 10%.
Being a man, younger than 35 years, having a more advanced WHO stage and being part of an earlier cohort were all independently associated with attrition. Women starting ART for Option B+ experienced higher attrition than women of child-bearing age starting for other reasons.
Conclusions
In this area retention in care has increased dramatically. Improved health in patients starting ART and decentralization of ART care to peripheral health centres appear to be the major drivers for this change.
Keywords: HIV, ART, retention, sub-Saharan Africa
Introduction
At the end of 2012 an estimated 35 million people were living with HIV, two thirds of them in sub-Saharan Africa.1 The number of people receiving antiretroviral therapy (ART) reached about 10 million, including 7.5 million in sub-Saharan Africa.2
However, retention of patients in ART care remains a major concern in ART programs. A meta-analysis of 32 studies from sub-Saharan Africa in 2007-9 showed that only 80% of patients started on ART were still attending clinic after 1 year, and about 77% and 72% after 2 and 3 years respectively.3 Loss to follow-up and death were the major causes of attrition, and up to half of those lost to follow-up may have died.4 Retention may improve over time, as healthier cohorts are started on ART and ART services move closer to patients.5 Retention can therefore be used as a key indicator of current programme quality.6 Moreover with the rapid ART scale-up and decentralization of ART services, true retention in care is likely to be substantially higher than existing estimates of retention in single clinics, as people “silently” transfer to closer (often lower-level) facilities for convenience.7;8
Malawi has been one of the pioneers of the public health approach, with simplified clinical decision making, standardized ART regimens, non-physician care, limited laboratory support, and centralized monitoring and evaluation.9;10 This, together with the decentralization of ART services from district hospitals to rural hospitals and health centres, has been one of the driving factors for increased access to ART,5;11 which is now estimated at 70% of those in need.2
We examined whether retention of adults in ART care in Karonga District, northern Malawi, changed over the period 2005-2012, and explored potential drivers for this change. Importantly this study included all ART clinics in an entire district from the time of introduction of ART services and was able to follow a substantial proportion of patients across ART clinics over a long period.
Methods
Setting
We conducted a retrospective cohort study of all adults started on ART between July 2005 and August 2012 in Karonga District, northern Malawi. Karonga District has a population of about 270,000 people, and a moderate HIV prevalence: 9% in women and 7% in men in 2009.12 The first ART clinic in the District opened in July 2005 at the district hospital, followed by ART services in the two rural hospitals in 2006 and 2008, and in rural health centres from 2008 onwards. In 2008 there were four clinics, in 2010 six, and by August 2012, 16 clinics were certified to initiate and provide ART.
During the study period HIV-positive individuals were eligible for ART initiation if they were in WHO clinical stages 3 or 4, or had an absolute CD4 cell count below 250 (2005 - 2005) or below 350 cells/μL (2011 onwards). From July 2011, HIV infected pregnant and breastfeeding women were universally eligible for lifelong ART, irrespective of clinical or immunological stage (“Option B+”).
After a 2-week lead-in period with half dose nevirapine, patients were seen at one and later two month intervals unless they had poor adherence, in which case the visits were required to be more frequent.
Data collection
During three rounds of data collection (2009, 2011 and August-September 2012), we photographed all ART clinic registers in Karonga District (after obscuring the names) to obtain anonymised individual level baseline and outcome data on all patients. From 2011 we performed a yearly check of the ART registers to update the outcomes of patients who were alive and on ART, and of patients who were lost to follow-up during the previous update and re-engaged in care.
The Malawi ART register records age, sex, village, start date, ART regimen and outcome and a clinic-specific registration number, the ART registration number. This number is written in the patient-held health passport (which captures details of clinical consultations and other health service contacts) and treatment card (a clinic held record which is specific for ART care and captures ART initiation date and type of regimen). Although the card is used in formal inter-clinic transfers, the number is not recorded at the new clinics so there is no linkage between registers held at different clinics.
In order to link the data from the ART registers and to allow us to collect additional information, we interviewed patients, following written consent, and assigned unique study identifiers to patients starting ART at the two main ART centers (Karonga district hospital and Chilumba rural hospital) from the start of the ART program. From 2009 onwards, we also interviewed patients collecting medication at all the ART clinics to assign unique identifiers and to record ART treatment history, including the start date and ART registration number at previous ART clinics. Data were anonymised and double entered in an Access database.
We used global positioning system (GPS) coordinates of villages, ART clinics and roads to estimate the travel distance between the patient’s village and the ART clinic where they started ART, using previously described methods.13
The data from the patient interviews were linked to the data from the clinic registers as far as possible. For patients with a unique identifier seen at multiple clinics we used the baseline characteristics from their first ART clinic and the outcome data from their last ART clinic.
Study population
We included patients who were at least 15 years of age at ART initiation during the study period (2005-2012). To avoid double counting we excluded from the analysis patients for whom it was not clear where they had started ART.
For our main analysis we focused on patients initiating ART for clinical reasons or CD4 cell count criteria, and excluded pregnant and breastfeeding women initiating ART under Option B+. As a separate analysis we compared retention rates of women initiating ART under Option B + and women of childbearing age (15-49 years) initiating ART based on clinical grounds or CD4 cell count criteria in the period in which Option B+ was implemented (July 2011-August 2012).
Statistical analysis
We defined retention in care as being alive and on ART at the end of the study (31st of August 2012). The time to discontinuation was analyzed using survival analysis. Non-retention or attrition was defined as recorded date of ART stop, loss to follow-up (“defaulted”) or death. Patients who were lost to follow-up immediately after starting ART were discontinued at day 1 in the analysis. In line with national guidelines, 2 months after dispensed drugs would have run out was used as the date of the event for patients lost-to follow-up.6 Patients who transferred out of the district, or who could not be linked to a destination clinic, were censored at the date of transfer. Attrition rates were estimated using Kaplan-Meier methods.
We constructed 2-year cohorts of patients initiating ART in 2005-06, 2007-08, 2009-10, and 2011-12. To compare the cohorts we examined: the WHO clinical stage at presentation; the mortality rate at 6 months after ART initiation; the accessibility of clinics (proportion of patients initiating at the district hospital, and median distance to the initiating ART clinic); and retention of these cohorts over time. We compared survival curves using the log-rank test.
For the analysis of factors associated with attrition we used Cox-proportional hazards regression. We also examined the factors associated with mortality separately. As only a limited number of variables were available and as the dataset contained a large number of observations, we included all variables in the multivariable analysis. All associations were tested using likelihood-ratio tests. All analyses were performed using Stata 12.1 (Stata Corporation, College Station, TX, USA).
Ethical review
The research was approved by the National health Sciences Research Committee in Malawi (NHSRC#424 and #448) and the Ethics Committee of the London School of Hygiene and Tropical Medicine (LSHTM#5067 and #5214). Informed consent to link data was obtained from patients on ART during the interview. As ART clinic register data were obtained in an anonymised way, no individual patient consent was needed. Permission to use the anonymised patient registers was given by national, regional and clinic ART coordinators.
Results
ART roll-out in Karonga District, 2005-2012
By August 2012, 16 ART clinics were providing ART. The average number of patients initiating ART in the district increased from 49 per month in 2005 to about 140 per month in 2012 or 186 per month including women started on ART under Option B+.
Study population
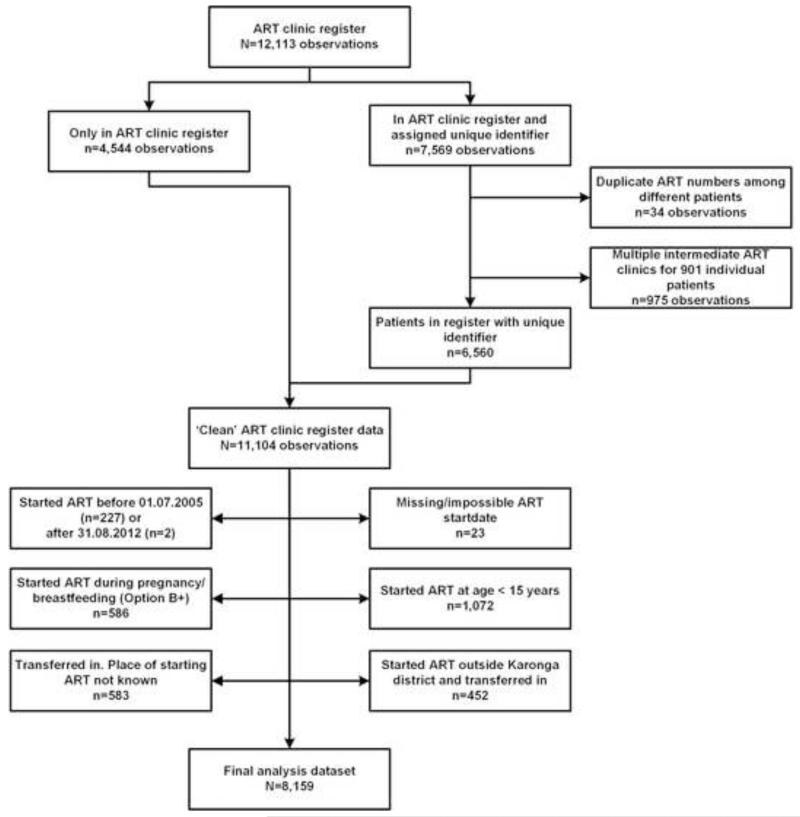
At the end of August 2012, the ART clinic registers from Karonga District contained 12,113 entrants. We assigned a unique identifier for 6,560 patients (representing 7,569 observations, of which 975 were from multiple clinics for 901 patients). Together with the other 4,544 observations in the ART clinic registers for which we did not obtain unique identifiers, this resulted in 11,104 patients (Figure 1).
Figure 1. Study flowchart.
23 out of 11,104 patients had a missing or implausible ART start date, 229 initiated ART outside the study period (July 2005-August 2012), 1072 started ART below the age of 15, and 586 were women who started ART because of Option B+. 452 initiated ART outside Karonga District and 583 were transferred in after being started on ART without documentation of the originating ART clinic, leaving us with 8,159 patients in the final analysis set. This included 5,432 (67%) for whom we had assigned a unique identifier.
Baseline characteristics of study population
The median age at ART initiation was 36 years (inter quartile range (IQR): 30-44), and 4,731 (58%) were female. Over half of the patients (55%) initiated ART at the district hospital, about a third (30%) at one of the 2 rural hospitals, and the remainder (15%) at one of the 13 rural health centres. Other characteristics are presented in Table 1.
Table 1. Baseline characteristics of patients starting ART in Karonga District, 2005-2012.
| Characteristic | Total number of patients (N=8,159) |
|---|---|
| Demographics | |
| Age (years): median (IQR) | 36 (30-44) |
| Age 15-34 years: n (%) | 3,369 (41.3) |
| Age ≥ 35 years: n (%) | 4,775 (58.5) |
| Gender: n (%) | |
| Male | 3,426 (42.0) |
| Female | 4,731 (58.0) |
| Distance from clinic: n (%) | |
| <5km | 3,884 (47.6) |
| 5 - <10km | 1,797 (22.0) |
| 10 - <25km | 1,752 (21.5) |
| ≥25km | 705 (8.6) |
| Missing | 21 (0.3) |
| Clinical characteristics | |
| Reason for starting ART | |
| CD4 cell count criteria | 1,198 (14.7) |
| WHO stage 3 | 5,015 (61.5) |
| WHO stage 4 | 1,939 (23.8) |
| ART related characteristics | |
| Year of ART initiation: n (%) | |
| 2005 | 292 (3.6) |
| 2006 | 793 (9.7) |
| 2007 | 1,000 (12.3) |
| 2008 | 1,244 (15.2) |
| 2009 | 1,194 (14.6) |
| 2010 | 1,264 (15.5) |
| 2011 | 1,255 (15.4) |
| 2012¶ | 1,117 (13.7) |
| Clinic related characteristics | |
| Health facility level: n (%) | |
| District Hospital | 4,456 (54.6) |
| Rural Hospital | 2,440 (29.9) |
| Rural Health Centre | 1,263 (15.5) |
Data are missing when the total number of patient was less than 8,159.
Data for 2012 only includes patients up the end of August 212.
The proportion of patients initiating ART in WHO stage 4 declined from 62% in the 2005-06 cohort to 10% in the 2011-12 cohort. Mortality within 6 months after ART initiation decreased from 23% to 3% (test for trend, p<0.001) (Table 2). The proportion of patients initiating ART at the district hospital decreased from 92% to 39% (p<0.001), and the median distance to the ART clinic decreased from 11 km (IQR: 3.8 −30.1) to 5 km (3.0-8.5) (p<0.001).
Table 2. Severity, composition and accessibility of consecutive 2-year cohorts starting ART in Karonga District, 2005-2012.
| Cohort | Number of patients (N=8,159) | Severity | Composition | Accessibility | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Initiated because of CD4 cell count | Initiated in WHO stage 3 (n, %) | Initiated in WHO stage 4 (n, %) | Mortality at 6 months (n, %) | Number of female patients (n, %) | Number of patients ≥ 35 years (n, %) | Number started in district hospital (n, %) | Distance (km) to initiating ART clinic (median, IQR) | ||
| 2005-2006 | 1,085 (13.3) | 16 (1.5) | 401 (37.0) | 668 (61.6) | 244 (22.5) | 653 (60.2) | 636 (58.6) | 996 (91.8) | 10.8 (3.8-30.1) |
| 2007-2008 | 2,244 (27.5) | 151 (6.7) | 1,410 (62.8) | 682 (30.4) | 205 (9.1) | 1,309 (58.3) | 1,282 (57.2) | 1,342 (59.8) | 5.2 (3.3-12.9) |
| 2009-2010 | 2,458 (30.1) | 622 (25.3) | 1,485 (60.4) | 351 (14.3) | 122 (5.0) | 1,439 (58.5) | 1,388 (56.5) | 1,190 (48.4) | 5.3 (3.1-11.3) |
| 2011-2012 | 2,372 (29.1) | 409 (17.2) | 1,719 (72.5) | 238 (10.0) | 70 (3.0) | 1,330 (56.1) | 1,469 (62.3) | 928 (39.1) | 4.6 (3.0-8.5) |
Retention
The total period of follow-up for 8,159 patients was 17,808 person-years, with a median follow-up time of 1.6 years per person (IQR: 0.4-3.6).
Among the 1,541 non-retained patients, 781 (51%) were known to have died (after a median of 51 days [IQR: 23-129]), 8 patients (0.5%) were known to have stopped treatment and the remaining 752 (49%) were lost to follow-up. Of the 6,618 retained patients, 5,067 (77%) were assigned a unique identifier. 621 observations (8%) were censored at the time of transfer if this was out of the district or if they could not be linked to a destination clinic.
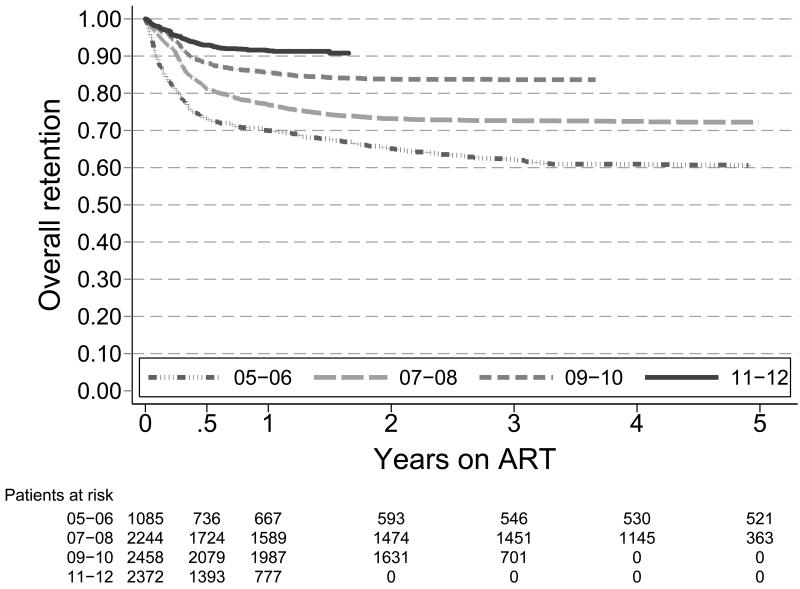
Six-month retention increased from 73% (95% Confidence Interval (95%CI): 71-76) to 93% (95%CI: 92-94) when comparing the 2005-06 and 2011-12 cohort, and 12-month retention increased from 70% (95%CI: 67-73) to 92% (95%CI: 90-93) (Figure 2a).
Figure 2a. Kaplan-Meier survival estimates for retention in ART care by year of starting ART, 2005-2012.
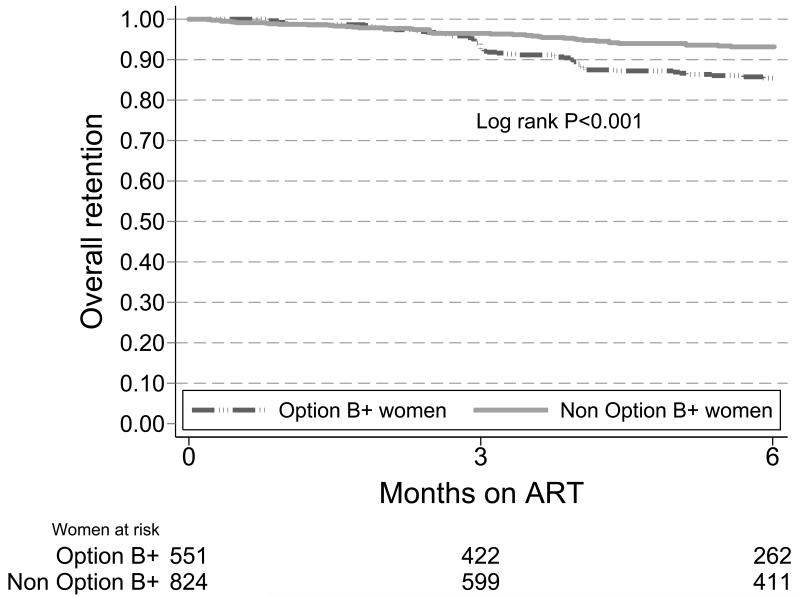
The retention rate at 6 months among women initiated on ART because of Option B+ was 85% (95%CI: 82-88) compared to 93% (95%CI: 91-95) among women of childbearing age initiated on ART because of clinical or CD4 cell count criteria in the same time period (Log rank P<0.001). This difference became apparent within 3 months of ART initiation (Figure 2b).
Figure 2b. Kaplan-Meier survival estimates for retention in ART care among women initiating ART because of Option B+ versus women of childbearing age initiating ART on clinical grounds or CD4 cell count criteria, 2011-2012.
Predictors of attrition (Table 3)
Table 3. Risk factors for attrition and mortality of patients starting ART in Karonga District, 2005-2012*.
| Attrition | Mortality | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk factor | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | P-value | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | P-value |
| Age | <0.001 | NS | ||||
| 15-34 years | 1.24 (1.12-1.37) | 1.32 (1.19-1.46) | 1.05 (0.91-1.21) | 1.12 (0.97-1.29) | ||
| ≥35 years | 1 | 1 | 1 | 1 | ||
| Gender | <0.001 | <0.001 | ||||
| Male | 1.39 (1.26-1.54) | 1.49 (1.34-1.65) | 1.54 (1.34-1.77) | 1.61 (1.39-1.85) | ||
| Female | 1 | 1 | 1 | 1 | ||
| Distance from clinic | NS | NS | ||||
| <5km | 1 | 1 | 1 | 1 | ||
| 5 - <10km | 0.95 (0.83-1.08) | 1.04 (0.91-1.20) | 0.85 (0.70-1.04) | 0.98 (0.80-1.19) | ||
| 10 - <25km | 1.09 (0.96-1.24) | 1.05 (0.92-1.19) | 1.13 (0.94-1.35) | 1.04 (0.86-1.24) | ||
| ≥25km | 1.64 (1.40-1.92) | 1.04 (0.88-1.23) | 1.95 (1.58-2.41) | 0.98 (0.79-1.22) | ||
| Reason for starting ART | <0.001 | <0.001 | ||||
| CD4 cell count criteria | 1 | 1 | 1 | 1 | ||
| WHO stage 3 | 1.91 (1.56-2.35) | 1.60 (1.30-1.97) | 2.23 (1.60-3.12) | 1.69 (1.20-2.37) | ||
| WHO stage 4 | 3.98 (3.23-4.91) | 2.51 (2.02-3.13) | 6.65 (4.76-9.27) | 3.27 (2.31-4.63) | ||
| Year of ART initiation | <0.001 | <0.001 | ||||
| Per increasing calendar year | 0.77 (0.75-0.79) | 0.82 (0.79-0.84) | 0.68 (0.66-0.71) | 0.74 (0.71-0.77) | ||
Complete data for 8,116 patients.
HR: Hazard Ratio; CI: Confidence Interval; NS: non-significant
In the univariable analysis attrition was more likely for the following baseline characteristics: younger age (<35 years), male sex, increasing distance to the ART clinic, WHO stage 3 and WHO stage 4 compared to CD4 cell count threshold for initiating ART, and earlier calendar period of ART initiation.
As the number of patients with missing values was minimal (43/8,159=0.5%), and no significant difference was observed in the primary outcome between those with missing and those with complete information, we used the 8,116 complete cases for the multivariable analysis. The final Cox-regression model revealed that younger patients (<35 years) [adjusted Hazard Ratio (aHR) (95%CI) = 1.32 (1.19-1.46)] and men [aHR (95%CI) = 1.48 (1.34-1.64)] were at higher risk of attrition. Patients with WHO clinical stage of 3 or 4 had higher proportions of attrition compared to patients initiating ART because of CD4 cell count criteria [aHR (95%CI) = 1.60 (1.30-1.97) and 2.51 (2.02-3.13) respectively]. Patients initiating ART in later calendar periods were more likely to stay in care [aHR/year (95%CI) = 0.82 (0.79-0.84)]. Distance was not retained as an independent predictor of attrition when year was included in the model; we found a negative correlation between distance and calendar year (r=−0.29, p <0.001). When we did not adjust for calendar year in the model, distance remained as an independent predictor for attrition (p<0.001). We explored plausible interactions but none were found.
Predictors of mortality (Table 3)
During multivariable analysis we found that male patients experienced a higher risk of mortality [aHR (95%CI) = 1.61 (1.39-1.85)], as did patients with WHO clinical stage of 3 or 4 [aHR (95%CI) = 1.69 (1.20-2.37) and 3.27 (2.31-4.63) respectively]. Patients initiating ART in later calendar periods were less likely to die [aHR/year (95%CI) = 0.74 (0.71-0.77)]. Distance and age were not independent predictors for mortality. However, as above, when not adjusting for calendar year in the model, distance remained as an independent predictor.
Discussion
To date, most studies describe program retention for individual ART clinics. However due to the rapid scale-up of ART and decentralisation, many patients transfer themselves (silent transfers) to closer (often lower-level) facilities. They may thus be reported lost to follow-up in their initial ART clinic, which results in an underestimation of retention on ART.7;8
Importantly, this study included all ART clinics in an entire district from the time of introduction of ART services, and was able to follow a substantial proportion of patients across ART clinics over a long period. This study therefore adds considerably to our understanding of ART retention by examining retention in ART care in a complete district over a seven year period, accounting for the majority of “silent” transfers.
ART retention rates in the early period of the treatment program in Karonga were very similar to retention rates reported elsewhere in sub-Saharan Africa.3;15 However, we observed a dramatic improvement of retention in care during a period of rapid expansion and decentralisation of ART services, from 70% at 12 months in the 2005-2006 cohort to 92% during the most recent cohort (2011-2012).
This increase in retention appears to have been driven by better health status of patients when starting ART, suggesting patients initiate ART earlier in their disease course, presumably because of easier access to ART clinics and the relaxing of ART eligibility criteria in the National ART Program. Over the study period a decreasing proportion of patients started ART in WHO stage 4 (from 62% to 10%), and 6-month mortality decreased from 22% to 3%, indicating that patients are accessing ART earlier.
The decentralization of ART services, bringing ART services to lower level health centres and closer to patients might have facilitated this, demonstrated by a lower proportion of patients starting in the district hospital (from 92% to 39%) and by the reduction in median distance to ART clinics in the district (from 11 to 5km).
The high attrition and mortality rates in the cohort of 2005-2006 can be explained by the backlog of patients with advanced disease and the presence of waiting lists for ART during that period; only a limited number of slots for ART initiation were available at the first clinic providing ART, leading to delayed access, late initiation and high mortality. Following decentralisation of ART services to the current 16 clinics, there are no longer any waiting lists.
The impact of decentralization on improved retention was confirmed during the risk factor analysis for attrition. We found that the risk for attrition decreased by 18% per increasing calendar year. The association between increased ART coverage and decreasing mortality and loss to follow-up rates has been described in other settings16;17, however some programs in sub-Saharan Africa report the opposite: an increased risk of attrition with increasing calendar year.18;19 This observed difference might be explained by our ability to capture most of the silent transfers in Karonga District; these would be considered lost to follow-up when examining retention in an individual ART program/clinic. This is an important bias in cohort reporting that is likely to affect all national ART Programs in comparable settings.
Distance to the ART clinic was not retained as an independent risk factor in our study, contrary to other studies20 but this is probably due to strong correlation between distance and calendar year. Over the study period and over the course of decentralisation, the distance patients had to travel to their ART clinic consistently dropped, and the effect of distance was most likely captured by calendar year. This was confirmed in the multivariable analysis excluding calendar year.
The other baseline clinical characteristics predictive of attrition reinforce findings from other studies in sub-Saharan Africa, including younger age (< 35 years), having a more advanced WHO clinical stage and being male.21-24 Except for younger age, the same baseline characteristics were found predictive for mortality as for attrition, a finding which is confirmed by other studies.22-23
The implementation of the current WHO guidelines aims to increase ART coverage and retention to save lives and to decrease HIV transmission.25 This paper shows how decentralizing HIV care to the smallest clinics increases uptake, and thereby coverage, while keeping retention in care high. But in order to achieve the ambitious goal of universal coverage in rural Africa, treatment will need to expand even further to serve communities beyond the reach of current clinics. The potential of further decentralization of ART delivery (through mobile clinics and community pharmacies) and community participation (through community health workers and the patients and their families) needs to be explored further.
The group of women who started ART because of Option B+ experienced lower retention at 6 months (85% versus 93%) than women of childbearing age started on ART for their own health (WHO stage 3 or 4, or based on CD4 cell count criteria). These results are similar to those found across Malawi, where retention rates of 82% at 6 months were reported among women started because of Option B+.26 In our study most of the attrition among women started on ART because of Option B+ occurred within 3 months of ART initiation. It appears that these women are lost to-follow up at the moment of transferring from antenatal care clinic (ANC) services to the routine ART clinic services. This demonstrates that even with integration of HIV services in antenatal care, there is still need to improve the transitioning of patients across different points of care.
Our study relied on routine operational data (the ART clinic registers). These are filled by registry clerks and health care providers from Karonga district during normal service delivery. We augmented this through interviews to get individual identifiers; this only started in 2009, but still captured 77% of those still in care or transferred. Our use of operational data limited the number of risk factors we could evaluate, but adding distance data demonstrated the importance of bringing ART services closer to the patients. Although these data are regularly monitored, the manual registering and recording is very labour intensive, time consuming and prone to human error. Recently electronic data capturing systems have been introduced in the district to strengthen the data collection systems.27 This should facilitate linking patients across different ART clinics and improve timely monitoring of indicators including retention in clinic and retention in care.
Conclusion
Given our findings, it appears that while initial high rates of loss to follow-up should remain a motivator for strengthening care, this should not deter programmes from expanding ART availability. This is likely to enable patients to access care sooner and thus improve outcomes further.
In Karonga District retention in care has increased dramatically. Improved health in patients starting ART and decentralization of ART care to peripheral health centres appear the major drivers for this change. Earlier ART initiation and decentralization of services is likely to yield the greatest public health impact in national ART Programs. Centralized registration will allow the impact to be monitored.
Acknowledgments
This work was supported by the Wellcome Trust [079828/Z/06/Z and 096249/Z/11/A]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RH was partially supported by a Gordon Smith Travelling Scholarship. TVB was supported by the Belgian Fonds de la Recherche Scientifique.
Conflicts of Interest/Sources of Funding: This work was supported by the Wellcome Trust [079828/Z/06/Z and 096249/Z/11/A]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. RH was partially supported by a Gordon Smith Travelling Scholarship. TVB was supported by the Belgian Fonds de la Recherche Scientifique.
Footnotes
Presented in part at the 20th Conference on Retroviruses and Opportunistic Infections 2013, 3-6 March, Atlanta, USA.
References
- (1).UNAIDS [Accessed January 31, 2014];Global report: UNAIDS report on the global AIDS epidemic 2013. 2013 Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- (2).WHO. UNICEF. UNAIDS [Accessed January 31, 2014];Global update on HIV treatment 2013: results, impact and opportunities. 2013 Available at http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf.
- (3).Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Chan AK, Mateyu G, Jahn A, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Trop Med Int Health. 2010;15(Suppl 1):90–97. doi: 10.1111/j.1365-3156.2010.02503.x. [DOI] [PubMed] [Google Scholar]
- (6).Giordano TP, Gifford AL, White AC, Jr., et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- (7).Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV /AIDS Rep. 2010;7:234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tweya H, Feldacker C, Estill J, et al. Are They Really Lost? “True” Status and Reasons for Treatment Discontinuation among HIV Infected Patients on Antiretroviral Therapy Considered Lost to Follow Up in Urban Malawi. PLoS ONE. 2013;8:e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lowrance DW, Makombe S, Harries AD, et al. A public health approach to rapid scale-up of antiretroviral treatment in Malawi during 2004-2006. J Acquir Immune Defic Syndr. 2008;49:287–293. doi: 10.1097/QAI.0b013e3181893ef0. [DOI] [PubMed] [Google Scholar]
- (10).Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- (11).Weigel R, Estill J, Egger M, et al. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS. 2012;26:365–373. doi: 10.1097/QAD.0b013e32834ed814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Floyd S, Molesworth A, Dube A, et al. Underestimation of HIV prevalence in surveys when some people already know their status, and ways to reduce the bias. AIDS. 2013;27:233–242. doi: 10.1097/QAD.0b013e32835848ab. [DOI] [PubMed] [Google Scholar]
- (13).Houben RM, Van Boeckel TP, Mwinuka V, et al. Monitoring the impact of decentralised chronic care services on patient travel time in rural Africa--methods and results in Northern Malawi. Int J Health Geogr. 2012;11:49. doi: 10.1186/1476-072X-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- (15).Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).McGuire M, Pinoges L, Kanapathipillai R, et al. Treatment initiation, program attrition and patient treatment outcomes associated with scale-up and decentralization of HIV care in rural Malawi. PLoS ONE. 2012;7:e38044. doi: 10.1371/journal.pone.0038044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Labhardt ND, Keiser O, Sello M, et al. Outcomes of antiretroviral treatment programmes in rural Lesotho: health centres and hospitals compared. J Int AIDS Soc. 2013;16:18616. doi: 10.7448/IAS.16.1.18616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002-2007. AIDS. 2010;24:2263–2270. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Koole O, Kalenga L, Kiumbu M, et al. Retention in a NGO supported antiretroviral program in the Democratic Republic of Congo. PLoS ONE. 2012;7:e40971. doi: 10.1371/journal.pone.0040971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Charurat M, Oyegunle M, Benjamin R, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS ONE. 2010;5:e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- (22).Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- (23).Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20:1163–1169. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- (24).Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).WHO [Accessed: 2014 April 1];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach 2013. 2013 Available: http://www.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- (26).Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS. 2014;28:589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Douglas GP, Gadabu OJ, Joukes S, et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]